Abstract
Aims
Coronary artery occlusion resulting in ischaemia/reperfusion (I/R) injury is a major cause of mortality in the western world. Circulating natural IgM has been shown to play a significant role in reperfusion injury, leading to the notion of a pathogenic response against self by the innate immune system. A specific self-antigen (non-muscle myosin heavy chain II) was recently identified as the major target of pathogenic natural IgM. Therefore, we hypothesized that a synthetic peptide mimetope (N2) or monoclonal antibodies directed against the self-antigen would prevent specific IgM binding to the self-antigen and reduce reperfusion injury in the heart.
Methods and results
We find that treatment with N2 peptide reduces infarct size by 47% and serum cardiac troponin-I levels by 69% following 1 h ischaemia and 24 h reperfusion. N2 peptide or an anti-N2 F(ab′)2 (21G6) is also effective at preventing IgM and complement deposition. Additionally, N2 peptide treatment significantly reduces monocyte and neutrophil infiltration at 24 h and collagen deposition at 5 days. Finally, we show that human IgM (hIgM) also includes specificity for the highly conserved self-antigen and that myocardial injury in antibody-deficient mice reconstituted with hIgM is blocked by treatment with N2 peptide or 21G6 F(ab′)2.
Conclusion
The findings in this study identify potential therapeutics [i.e. N2 peptide or 21G6 F(ab′)2] that prevent specific IgM binding to ischaemic antigens in the heart, resulting in a significant reduction in cardiac I/R injury.
Keywords: Myocardial infarction, Peptides, Lymphocytes, Antibodies, Reperfusion
1. Introduction
Ischaemia/reperfusion (I/R) injury represents an acute inflammatory response following trauma, surgery, stroke, heart attack, or other events in which circulation is transiently blocked.1 Although the mechanism responsible for injury remains unclear, one general model is that injury occurs in two stages. The first stage represents intrinsic cell injury which can be due to various processes including hypoxia-induced increased levels of intracellular reactive oxygen species (ROS),2–5 energy depletion, cell swelling, and opening of the mitochondrial permeability transition pore.6 A second pathway of injury is based on the induction of acute inflammation possibly by neutrophil contact with the hypoxia-induced modified cell surface.7 A related hypothesis is that recognition of the modified surface is specific and results in activation of the innate immune system via the lectin complement pathway.8–12 Thus, according to this model, intrinsic up-regulation of non-lethal levels of ROS leads to the exposure of a ‘neo’ self-determinant on the cell surface and activation of the innate immune system. Using a rat model of myocardial infarction, Hill and Ward13 identified a role for complement component 3 (C3) in induction of injury. Subsequently, it was found that specific inhibition of C3b with a soluble complement receptor 1 (CR1) reduced myocardial injury in a rat model of transient I/R.14 A similar approach was used to block I/R injury in other models such as the central nervous system,15 skeletal muscle,16 and intestine.17 The finding that complement receptor 2-deficient mice (Cr2−/−), which have a limited repertoire of natural IgM,18 or recombination activation gene-deficient (RAG-1−/−) mice, which are antibody deficient,19,20 were protected in an intestinal I/R model first suggested that injury was initiated by circulating natural IgM; a single clone, i.e. IgMcm-22, was identified that restored injury.21 This led to the identification of non-muscle myosin heavy chain II (NMHC-II) as the target self-antigen for pathogenic IgM.8 In addition, a phage-display library of random 12-mer amino acid sequences was screened with IgMcm-22 and the phage-displayed peptides that were identified shared conserved sequence homology with NMHC-II.8 A synthetic peptide, referred to as N2, which represents the highly conserved epitope within the myosin heavy chain and to which IgMcm-22 has specific affinity as demonstrated by surface plasmon resonance, was shown to reduce I/R injury in both an intestinal and skeletal muscle model when injected prior to reperfusion.8,22
In order to determine whether a similar pathway of natural IgM and N2 antigen plays a role in acute myocardial infarction (AMI), we examined whether a peptide mimetope or blocking antibody specific for the N2 epitope was protective in a closed chest model of coronary artery occlusion.
2. Methods
2.1. Animals
Both WT C57BL/6 and RAG-1−/− mice on a C57BL/6 genetic background were purchased from The Jackson Laboratory and housed under specific pathogen-free conditions at the Immune Disease Institute. Animal use in this investigation conforms with the guidelines of the Committee on Animals of Harvard Medical School as well as with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Myocardial model of I/R injury
A previously described ‘closed chest’ model for myocardial I/R injury was used.23 The experimental procedure is described in detail in the Supplementary material online. Unless specified otherwise, the left anterior descending coronary artery (LAD) was occluded for 60 min (referred in the text as I/R) and allowed to reperfuse for 24 h at which time tissues were harvested and serum samples taken. For the ‘mock-treated’ group, animals were subjected to the medial sternotomy, but the LAD was not occluded. All injections were administered intravenously (iv) into the tail vein prior to I/R unless specified otherwise.
2.3. Triphenyltetrazolium chloride staining
Following I/R, mouse hearts were harvested and infarct size was determined by triphenyltetrazolium chloride (TTC) staining which is described in detail in the Supplementary material online.
2.4. Masson trichrome staining
Mouse hearts were harvested 5 days post-occlusion and collagen was visualized by Accustain Trichrome staining (Sigma, St Louis, MO, USA) on acetone-fixed cryosections. The method used to quantify the Masson trichrome (MT) staining is described in the Supplementary material online.
2.5. Immunohistochemistry and histopathology
Immunofluorescence staining was performed as previously described11 with minor modifications as described in the Supplementary materials online. Quantification of IgM and C3d staining was performed by using Photoshop software version CS2 as described previously.24 For histopathology, cryosections of heart tissues were stained with haematoxylin and eosin (H&E) and images were taken with a Leica DMLB digital imaging system (Leica Microsystems, Bannockburn, IL, USA).
2.6. Cardiac troponin-I measurements
Following I/R, serum was collected and measured by using a high-sensitivity mouse cardiac troponin-I (cTnI) ELISA kit as recommended by the manufacturer (Life Diagnostics, West Chester, PA, USA).
2.7. Human IgM purification
IgM was purified as described previously.25
2.8. Generation of monoclonal antibodies
BALB/c mice were first immunized by intraperitoneal injection using N2 peptide coupled to keyhole limpet haemocyanin (KLH) with complete Freund's adjuvant. Splenocytes were fused with myeloma partners (P3Ag8) and cells were selected in HAT media (Sigma). Clones were screened by ELISA against N2 BSA and positive clones were subcloned. Selected hybridomas were maintained in IMDM media and the monoclonal antibodies (mAbs) were then purified using a protein G column (GE Healthcare, Piscataway, NJ, USA).
2.9. F(ab′)2 production
21G6 (IgG1) was digested with ficin using a mouse IgG1 F(ab′)2 preparation kit as recommended by the manufacturer (Pierce, Rockford, IL, USA).
2.10. Flow cytometry
Hearts were harvested after I/R and perfused with phosphate-buffered saline (PBS). After weighing tissues, hearts were minced and digested in two rounds at 37°C: PBS/0.25% trypsin (Invitrogen) and 0.2% collagenase B (Sigma) for 45 min followed by PBS/0.25% trypsin, 0.2% collagenase B, and 0.1% DNase I (Sigma) for 45 min. Tissues were gently triturated into single-cell suspensions and stained for flow cytometry in Hank's buffered saline solution (Invitrogen, Carlsbad, CA, USA) for 30 min on ice. Combinations of antibodies used included PE anti-CD11b, FITC anti-Gr-1, FITC anti-Ly-6G, PercP anti-CD45, Biotin anti-Ly-6C (1:200 each, BD Biosciences, San Jose, CA, USA), Alexa 633 anti-7/4 (1:200, AbD Serotec, Raleigh, NC, USA). For biotin staining, Streptavidin-APC (1:500, BD Biosciences) was also used. Cells were then subjected to flow cytometry on a four-colour, FACSCalibur machine (BD Biosciences) and data analysed by FlowJo software (Tree Star, Ashland, OR, USA).
2.11. Peptide sequences
The N2 peptide sequence is LMKNMDPLNDNV and the scrambled N2 peptide sequence is NVLMNKDDMLPN.
2.12. Statistical analysis
Data are presented as means ± SEM. Comparisons between two groups only were performed using a Student's t-test and comparisons between three or more groups were performed using a one-way analysis of variance with the Tukey–Kramer post hoc test for unequal sample sizes using GraphPad Prism v4.03 software. Differences were considered statistically significant at P < 0.05.
2.13. Animal exclusion
Animals subjected to the I/R protocol were only excluded from the study if they did not fully recover from the initial surgery or had complications during the I/R protocol.
3. Results
3.1. N2 peptide prevents myocardial I/R injury
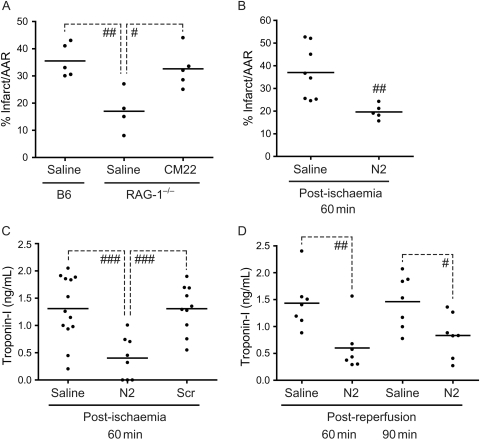
Previous studies in RAG-1−/− mice demonstrated that I/R injury following LAD occlusion was IgM-dependent.26 To test whether IgM specific for NMHC-II could initiate injury, RAG-1−/− mice were reconstituted with clone IgMcm-22 and subjected to I/R. Infarct size was significantly increased in RAG-1−/− mice reconstituted with IgMcm-22 when compared with the RAG-1−/− normal saline (NS) control (32.6 ± 3.2 vs. 17.0 ± 3.9% infarct/AAR, n = 5 and n = 4, respectively; Figure 1A). Hence, the data suggest that a single pathogenic IgM clone (IgMcm-22) is capable of restoring myocardial I/R injury to a level similarly seen in C57BL/6 mice (Figure 1A).
Figure 1.
Effect of N2 peptide on myocardial I/R injury. (A) IgM from a single hybridoma clone (IgMcm-22) restores myocardial I/R injury in RAG-1−/− mice. RAG-1−/− mice were injected iv with 200 µg of IgMcm-22 and then subjected to I/R. Infarct sizes were determined by TTC staining. (B and C) Effect of N2 peptide on infarct size (B) and cTnI levels (C). N2 or N2 scrambled (Scr) peptide (200 µg) was injected iv into C57BL/6 mice following 60 min ischaemia (post-ischaemia). Infarct sizes were determined after 24 h reperfusion by TTC staining (B) and serum cTnI levels were also measured (C). (D) Effect of N2 peptide during early stages of reperfusion. C57BL/6 mice were first subjected to 60 min ischaemia after which N2 peptide (200 µg) was injected at various times during reperfusion (post-reperfusion). After 24 h of total reperfusion, serum cTnI levels were analysed (#P < 0.05; ##P < 0.01; ###P < 0.001).
IgMcm-22 binds a highly conserved region of NMHC-II, referred to as N2, and previous studies found that pre-treatment of WT mice with a 12-amino acid synthetic peptide or mimetope representing this sequence was protective in two models of I/R injury.8,22 Therefore, to evaluate whether N2 peptide is protective in the myocardial I/R model at a clinically relevant time frame (i.e. at the initiation of reperfusion), N2 peptide was administered following 60 min ischaemia (post-ischaemia). C57BL/6 mice were also analysed for infarct size (see Supplementary material online, Figure S1) and cTnI release 24 h post-reperfusion. When N2 peptide was administered 60 min post-ischaemia, infarct size was reduced by 47% when compared with the NS control (37.0 ± 4.2 vs. 19.8 ± 1.6% infarct/AAR, n = 8 and n = 5, respectively; Figure 1B) and cTnI levels were reduced by 69% when compared with the NS control (1.3 ± 0.2 vs.0.4 ± 0.1 ng/mL, n = 13 and n = 8, respectively; Figure 1C). As expected, treatment with a scrambled N2 peptide sequence was not protective as determined by serum cTnI levels (1.3 ± 0.1 ng/mL, n = 10; Figure 1C). However, although both mean infarct size and cTnI release tended to decrease following administration of N2 peptide at extended ischaemia times (i.e. 90 min ischaemia), this reduction was not statistically significant (data not shown).
To test whether injury could be blocked at specific time points after reperfusion was initiated, N2 peptide was administered 60, 90, or 120 min post-reperfusion following 60 min ischaemia. N2 peptide significantly reduced cTnI levels when administered 60 min post-reperfusion compared with NS controls (1.43 ± 0.18 vs. 0.59 ± 0.17 ng/mL, n = 7 for each group; Figure 1D). Interestingly, administration of the peptide within 90 min of reperfusion was also significantly protective based on a 43% reduction in cTnI release when compared with saline controls (1.46 ± 0.19 vs. 0.83 ± 0.15 ng/mL, n = 7 for each group; Figure 1D). However, the window is limited as treatment at 120 min post-reperfusion was not significantly protective (data not shown). Therefore, the protective effect of N2 peptide, whether administered during ischaemia, at the initiation of reperfusion, or at early times post-reperfusion, creates an extended therapeutic window during which N2 peptide can effectively block circulating IgM and limit overall injury.
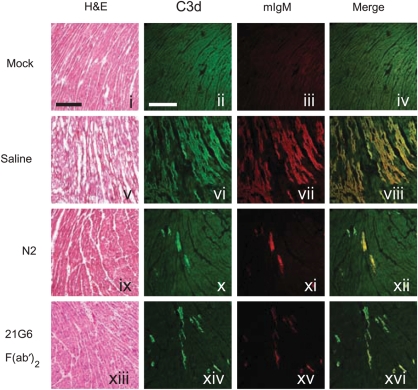
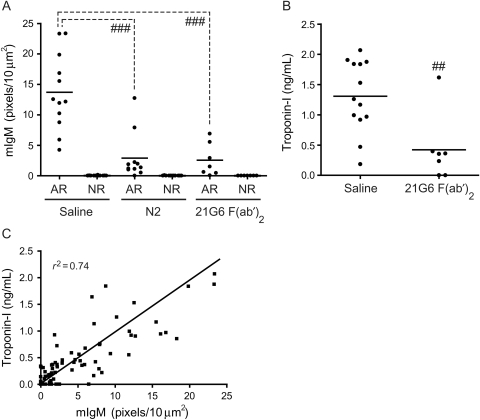
Although N2 peptide significantly reduced infarct size and cTnI release, we further investigated the direct effect on natural antibody binding and activation of complement (C) following I/R injury. C57BL/6 mice were subjected to I/R and cryosections from hearts were stained with antibodies to either IgM or C3d. Notably, IgM and C3d deposition within the left ventricle (LV) were significantly reduced in mice treated with N2 peptide (Figure 2, panels x–xii). Quantitation of IgM deposition in N2-treated animals when compared with NS controls (13.7 ± 1.8 vs. 2.9 ± 1.1 pixels/10 µm2, n = 12 and n = 11, respectively) within the LV (area at risk) and the right ventricle (non-risk area) is shown in Figure 3A. Additionally, the amount of IgM deposition strongly correlated to the serum levels of cTnI (r2 = 0.74; Figure 3C).
Figure 2.
Effect of N2 peptide and 21G6 F(ab′)2 on myocardial I/R injury. Representative cryosections of mock-, NS-, N2-, or 21G6 F(ab′)2-treated C57BL/6 mice subjected to I/R. Cryosections were stained with H&E, anti-C3d-FITC, and anti-IgM-568. Scale bars, 200 µm.
Figure 3.
(A) Quantitation of IgM staining in the area of risk (AR) and non-risk (NR) area of NS-, N2-, and 21G6 F(ab′)2-treated C57BL/6 mice. (B) Effect of 21G6 F(ab′)2 on cTnI levels following I/R. 21G6 F(ab′)2 (150 µg) was injected iv into C57BL/6 mice following 60 min ischaemia and serum cTnI levels were measured 24 h post-ischaemia. Each filled circle represents a single mouse (##P < 0.01; ###P < 0.001). Images were collected with a Zeiss/BioRad Radiance 2000MP system attached to an Olympus BX50WI upright microscope, ×20 UPlanapo N.A. 0.7 objective, controlled by the Lasersharp 2000 software. (C) Correlation between serum cTnI levels and IgM deposition in C57BL/6 mice.
3.2. Anti-N2 antibodies are protective in cardiac I/R model
The finding that N2 peptide is protective suggests that it acts as a mimetope and inhibits the action of pathogenic IgM. To test this hypothesis further, mAbs were prepared against N2 peptide coupled to KLH. We proposed that mAbs could be used as an inhibitor to block specific recognition of N2 epitope by circulating pathogenic IgM in the I/R model. One clone, 21G6, was selected for use in vivo. To limit possible binding and activation of C or FcγR-dependent events, the Fc piece was removed enzymatically to generate F(ab′)2 fragments. Treatment with 21G6 F(ab′)2 reduced serum cTnI levels 68% when compared with the NS control (1.3 ± 0.2 vs. 0.4 ± 0.2 ng/mL, n = 13 and n = 7, respectively; Figure 3B). Further, a marked reduction in IgM deposition was observed in the area at risk of mice pre-treated with 21G6 F(ab′)2 when compared with the NS control (13.7 ± 1.8 vs. 2.6 ± 1.0 pixels/10 µm2, n = 12 and n = 7, respectively; Figures 2 and 3A). Thus, either N2 peptide or anti-N2 21G6 F(ab′)2 block circulating pathogenic IgM resulting in significant cardiac protection from I/R injury.
3.3. N2 peptide reduces monocyte and neutrophil infiltration in ischaemic hearts
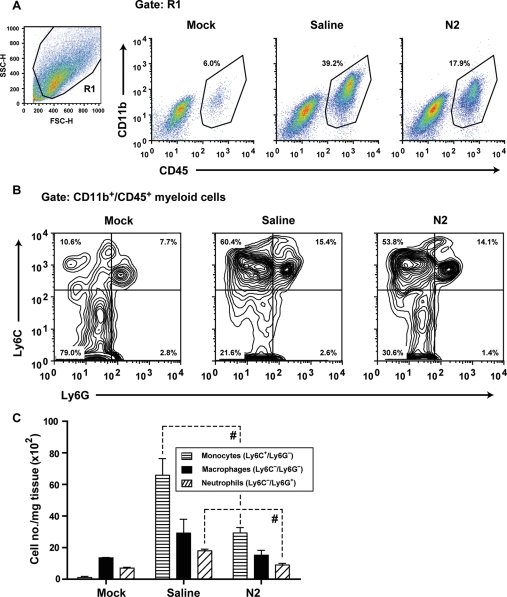
One possible effect of N2 peptide treatment is to reduce downstream leucocyte-induced inflammation following cardiac I/R injury. In order to quantify immune cell populations present in infarcted hearts, hearts were harvested after 24 h reperfusion and single-cell suspensions were stained and characterized by flow cytometry. CD11b+/CD45+ myeloid cells were significantly increased in NS-treated hearts following I/R injury (Figure 4A), whereas N2 peptide treatment significantly decreased myeloid cells in the heart. Within the CD11b+/CD45+ pool, three major populations were identified: infiltrating monocytes (Ly6C+/Ly6G−), neutrophils (Ly6C−/Ly6G+), and resident macrophages (Ly6C−/Ly6G−) (Figure 4B). These populations were further confirmed to be neutrophils and monocytes by Gr-1 and 7/4 antigen staining27 (see Supplementary material online, Figure S2). The frequency of inflammatory monocytes and neutrophils (expressed as cells/mg tissue) increased significantly after 24 h reperfusion compared with mock-treated hearts (Figure 4C). With N2 peptide treatment, however, we observed significant reductions in both infiltrating monocyte and neutrophil populations, but not in resident macrophages (Figure 4C).
Figure 4.
Effect of N2 treatment on neutrophil and monocyte infiltration of injured myocardium. C57BL/6 mice were injected with NS or N2 peptide and subjected to I/R. Inflammatory cell populations were analysed by FACS of mock-, NS-, or N2-treated hearts. (A) Representative FACS plots show myeloid cells in the heart, defined by expression of CD45 and CD11b. The live-cell gate is shown on the left (forward vs. side scatter). (B) Gating on myeloid cells (CD11b+/CD45+), three distinct populations were defined: infiltrating monocytes (Ly6C+/Ly6G−), resident macrophages (Ly6C−/Ly6G−), and neutrophils (Ly6C−/Ly6G+). Corresponding percentages of each population are indicated on plots. (C) Cumulative analyses of heart inflammation. N2 peptide treatment significantly decreases both infiltrating monocyte and neutrophil populations. Absolute leucocyte numbers were calculated from total cell count, relative population proportions within each heart, and tissue weight (#P < 0.05).
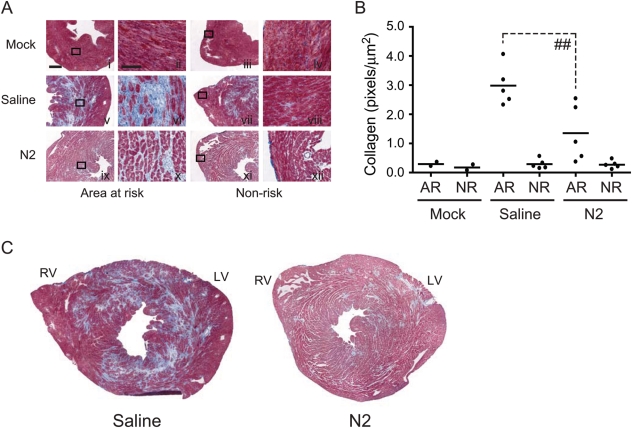
3.4. Collagen formation is diminished in N2-treated mice
Following myocardial infarction and resultant cell death, an inflammatory response is normally triggered to clear tissue debris and necrotic cells ultimately leading to the formation of a collagen-based scar in the affected region. To evaluate the functional correlation of mimetope protection to later stages of reperfusion, we examined the effect of N2 peptide on collagen deposition at 5-day reperfusion. As predicted, C57BL/6 mice injected with NS had significant amounts of collagen formation in the LV (area at risk) when compared with mock-treated controls (Figure 5A, panel v vs. i). However, the amount of collagen deposition in the LV of N2-treated mice was significantly reduced when compared with NS-treated controls (3.0 ± 0.3 vs. 1.4 ± 0.4 pixels/µm2, n = 5 for each group; Figure 5A, panel ix vs. v, and B). In all treatment groups, the non-risk area (right ventricle), which is not affected by LAD occlusion and remains viable, showed no resultant collagen deposition. Further, it is well established that after several weeks following infarction, a mature scar consisting of cross-linked collagen has formed. The reduction in collagen deposition seen in N2-treated animals at 5-day reperfusion is proportional to the size of the mature scar seen in N2-treated animals at 3-week reperfusion, which has the characteristic loss of cellularity and a clearly delineated infarct zone (see Supplementary material online, Figure S3). Thus, blocking of acute inflammation by N2 peptide correlates with reduced leucocyte infiltration and subsequent scar formation.
Figure 5.
Effect of N2 treatment on collagen deposition in C57BL/6 mice. (A) C57BL/6 mice were injected iv with NS or N2 peptide and subjected to 1 h ischaemia and 5-day reperfusion. Hearts were collected after 5-day reperfusion and cryosections were stained with MT to evaluate collagen content. (B) Quantitation of collagen in the AR and NR area of NS- and N2-treated C57BL/6 mice. Each filled circle represents a single mouse. (C) Representative composite MT-stained cryosections from either NS- or N2-treated C57BL/6 mice. Scale bar shown in panel i, 500 µm; panel ii, 100 µm (##P < 0.01). Images were collected with a Leica DMLB, ×20 AR N.A. 0.5 Leica objective and captured with a Leica DFP480 camera. Photoshop Version 7 software was used for quantitation.
3.5. Human IgM bears specificity for N2 self-antigen
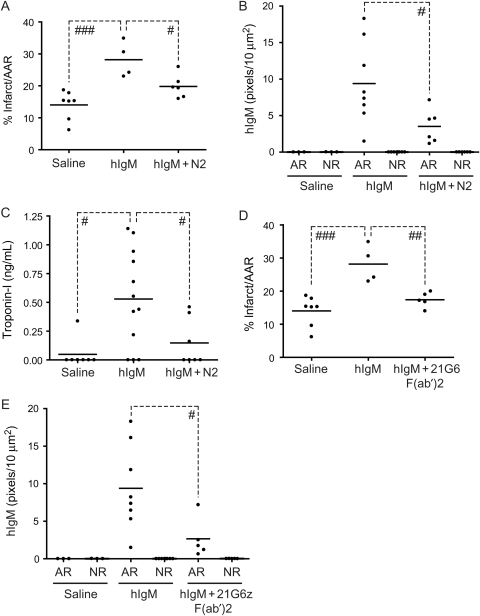
It is generally held that humans express similar specificities of circulating natural IgM. Recent results reveal that human IgM (hIgM) mediates injury in an intestinal model of I/R.28 To examine whether hIgM includes specificity for the N2 epitope in cardiac tissue, antibody-deficient RAG-1−/− mice were reconstituted with hIgM in the presence or absence of N2 peptide. hIgM in mock-treated mice had a background level of injury as expected (data not shown). However, mice receiving hIgM and subjected to I/R developed infarct sizes that were significantly increased relative to NS controls (28.2 ± 2.8 vs. 14.0 ± 1.7% infarct/AAR, n = 4 and n = 7, respectively; Figure 6A). Administration of N2 peptide resulted in a 30% reduction in infarct size (28.2 ± 2.8 vs. 19.8 ± 1.5% infarct/AAR, n = 4 and n = 6, respectively; Figure 6A) as well as the amount of hIgM deposition in the area at risk when compared with mice receiving hIgM alone (9.4 ± 2.0 vs. 3.5 ± 0.9 pixels/10 µm2, n = 8 and n = 6, respectively; Figure 6A and B). Importantly, the reduction in infarct size and hIgM deposition seen in N2-treated animals also correlated with a 72% reduction in serum cTnI levels when compared with mice receiving hIgM alone (0.7 ± 0.2 vs. 0.15 ± 0.08 ng/mL, n = 13 and n = 7, respectively; Figure 6C).
Figure 6.
Reconstitution of RAG-1−/− mice with hIgM. (A) RAG-1−/− mice were injected iv with pooled individual hIgM (400 µg) from 10 donors followed by NS or N2 peptide and subjected to I/R. Infarct sizes were determined by TTC staining. (B and C) RAG-1−/− mice were treated as in (A) and analysed for hIgM deposition (B) or serum cTnI levels (C). (D and E) RAG-1−/− mice were injected iv with hIgM as in (A) and then NS or 21G6 F(ab′)2 and subjected to I/R. Infarct sizes were determined by TTC staining (D) or analysed for hIgM deposition (E) (#P < 0.05; ##P < 0.01; ###P < 0.001).
A further test of the specificity of hIgM is whether injury is blocked with anti-N2 mAbs, which serve to compete for binding of the ischaemic antigen. RAG-1−/− mice were reconstituted with hIgM and treated with 21G6 F(ab′)2 prior to I/R. Treatment with 21G6 F(ab′)2 resulted in a 38% reduction in infarct size (28.2 ± 2.8 vs. 17.4 ± 1.0% infarct/AAR, n = 4 and n = 5, respectively; Figure 6D) and a significant reduction in deposition of hIgM (9.4 ± 2.0 vs. 2.7 ± 1.2 pixels/10 µm2, n = 8 and n = 5, respectively; Figure 6E) when compared with mice receiving hIgM alone. Thus, hIgM is not only pathogenic in the mouse coronary model, but the N2 epitope (NMHC-II) is the major target, suggesting that this specificity is conserved between the two species.
4. Discussion
It is estimated that more than 1.2 million people in the USA will suffer from a new or recurrent coronary attack in 2010.29 Although restoration of blood flow to ischaemic tissue is essential for survival, it leads to substantial injury to the myocardium due to activation of both intrinsic (myocardial cell death)30 and extrinsic (acute inflammation) pathways.8 This secondary injury, which has been estimated to account for over 25% of myocardial damage following AMI, is referred to as ‘lethal reperfusion injury’.31 The current study used a murine model of coronary artery occlusion to test whether blocking of natural antibody recognition of NMHC-II could reduce lethal reperfusion injury. We found that treatment of mice with a peptide mimetope or a blocking antibody specific for NMHC-II substantially reduces infarct size, IgM and C deposition, neutrophil and monocyte infiltration, and collagen deposition within the LV. Moreover, we find that hIgM bears specificity for the N2 self-antigen similar to that of rodents.
The findings presented here are consistent with previous observations in animal models of intestinal8 and skeletal muscle I/R injury.22 Thus, our current general model is that reperfusion of ischaemic tissue induces exposure or release of the N2 epitope of NMHC-II on the surface of vascular endothelium. Recognition of the self-antigen by natural IgM activates the lectin pathway of C10,11,32 leading to proteolytic cleavage of C3 and complement component 5 (C5). Release of C3a and C5a anaphylatoxins activates mast cells via specific G-protein-coupled receptors resulting in degranulation and direct tissue injury.33–35 Our finding of a correlation between deposition of IgM within the LV and infarct size supports the general model of C3 activation and induction of injury in the cardiac I/R model.
The observation that treatment with either the N2 mimetope or blocking antibody was protective in RAG-1−/− mice reconstituted with hIgM was important for several reasons. First, it suggests that natural antibody bearing N2 specificity may have a common beneficial role in vertebrates. For example, given the highly conserved nature of the N2 region of NMHC-II, natural antibody specific to this epitope could be protective against certain N2-expressing pathogens. Secondly, these results suggest that release or exposure of the N2 epitope within the vasculature of reperfused tissues could trigger activation of acute inflammation in humans. Thus, blocking circulating anti-N2 natural IgM could provide a potential therapy in AMI or other I/R-dependent injuries.
Although a number of inhibitors of lethal reperfusion have shown promise in preclinical trials over the past decade, their general failure to limit infarct size in clinical trials of AMI patients has led to a negative outlook on the development of new approaches.36 For example, inhibitors designed to block C5 (Pexelizumab)10,37 and leucocyte integrins CD11/CD11b (LeukArrest)38,39 appeared protective in animal models of AMI but both failed to show benefit in clinical trials.40,41 With respect to the current study, it is important to note that blocking of C5 would not be expected to inhibit injury identified in our current model. Thus, activation of C3 and release of C3a anaphylatoxin alone would be sufficient to activate mast cells via C3a receptor (C3aR) and induce local injury. Specifically, blocking of C5 with the mAb BB5.1 had negligible effect on cTnI release in our cardiac I/R model (see Supplementary material online, Figure S4). Therefore, the apparent failure of anti-C5 treatment in humans is not a predictor of a possible therapy to block N2 recognition by circulating natural IgM. Similarly, the failure of humanized anti-integrin antibody in clinical trials such as ‘HALT MI’ is not a predictor of the outcome of N2 inhibition because blocking of CD11/CD11b would not prevent mast cell activation via C3aR.41 Thus, the current study provides support for a novel pathway of inflammation that circumvents the requirement for both C5 and neutrophils.
Inflammation following AMI is known to be a double-edged sword as there are beneficial effects in recruitment of inflammatory cells into sites of injury. Recent studies identify specific populations of monocytes that participate in a bi-phasic response to myocardial I/R injury; a Ly-6Chi population that accumulates during the initial inflammatory phase via chemokine (C–C motif) receptor 2 (CCR2) and an Ly-6Clo population via chemokine (C–X3–C motif) receptor 1 (CX3CR1) that is involved in later reparative events such as granulation tissue formation.42,43 In our model, characterization of heart cell extracts by flow cytometry identified a dramatic influx of monocytes and neutrophils at 24 h reperfusion. After the initial inflammatory phase, granulation tissue begins to appear supported by newly formed vessels while myofibroblasts synthesize interstitial collagens. Although collagen can be harmful as it limits myocardial function, it stabilizes the infarcted area and minimizes the expansion of the infarct protecting against events such as ventricular rupture or aneurysm.44 Examination of collagen deposition following 5-day reperfusion identified significant amounts of collagen in the infarcted area of NS-injected mice as expected. Importantly, mice treated with N2 peptide have markedly less collagen in the LV that correlates with reduced cTnI release and leucocyte infiltration. This reduction of collagen in the hearts of N2-treated animals is directly proportional to the level of necrosis in the LV thereby resulting in less scar tissue formation. Additionally, because mature scar formation is known to lead to cardiac dysfunction, echocardiography was also performed on both NS- and N2-treated mice at 3-week reperfusion when a mature scar is clearly delineated. Although N2-treated animals trended towards improved ejection fractions, the difference in means were not statistically different (data not shown). This is likely due to not only the experimental variability within the groups but also the relatively moderate infarct sizes targeted in this model (i.e. 20–25% of LV).
Finally, the success of recent trials of cyclosporine, which blocks mitochondrial-dependent cell death in patients with ST-elevation undergoing percutaneous coronary intervention,4 supports the importance of a continuing search for inhibitors of lethal reperfusion.45 Therefore, the novel pathway described in this study could potentially be targeted with alternative inhibitors of inflammation [i.e. N2 mimetope or anti-N2 F(ab′)2] as their apparent mechanisms differ from those previously described.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was partly funded by grants from the National Institutes of Health (GM52585 for M.C.C. and F.D.M.; HL084821 for E.M.A.) as well as support from DecImmune Therapeutics, Inc.
Supplementary Material
Acknowledgements
We thank Drs Mark Entman and Lloyd Michaels and members of their lab at the Methodist Hospital, Houston, TX, USA, for their help and advice with the murine LAD model. We thank Dr Ronglih Liao and Soeun Ngoy at the Brigham and Women's Hospital Boston for their critical discussions and data analysis. We like to thank Harry Leung for his excellent technical assistance with all of the image quantitation. We would also like to thank Yi Zheng for her technical assistance with the confocal image processing and helpful scientific discussions.
Conflict of interest: M.C.C., F.D.M., E.M.A., and M.S.H. have financial interests in DecImmune Therapeutics, Inc.
References
- 1.Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Contran Pathologic Basis of Disease. Professional Edition. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010. [Google Scholar]
- 2.Wu B, Ootani A, Iwakiri R, Fujise T, Tsunada S, Toda S, et al. Ischemic preconditioning attenuates ischemia-reperfusion-induced mucosal apoptosis by inhibiting the mitochondria-dependent pathway in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G580–G587. doi: 10.1152/ajpgi.00335.2003. doi:10.1152/ajpgi.00335.2003. [DOI] [PubMed] [Google Scholar]
- 3.Thiemermann C, Bowes J, Myint FP, Vane JR. Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc Natl Acad Sci USA. 1997;94:679–683. doi: 10.1073/pnas.94.2.679. doi:10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. doi:10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. doi:10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 6.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. doi:10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Jackson R. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. doi:10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, et al. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 10.Busche MN, Pavlov V, Takahashi K, Stahl GL. Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose-binding lectin. Am J Physiol Heart Circ Physiol. 2009;297:H1853–H1859. doi: 10.1152/ajpheart.00049.2009. doi:10.1152/ajpheart.00049.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177:4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 12.Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. doi:10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 13.Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. doi:10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisman HF, Bartow T, Leppo MK, Marsh HC, Carson GR, Concino MF, et al. Soluble human complement receptor type I: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. doi:10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Kim L, Mealey R, Marsh H, Zhang Y, Tenner A, et al. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285:595–599. doi: 10.1126/science.285.5427.595. doi:10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay TF, Hill J, Ortiz F, Rudolph A, Valeri CR, Hechtman HB, et al. Blockade of complement activation prevents local and pulmonary albumin leak after lower torso ischemia-reperfusion. Ann Surg. 1992;216:677–683. doi: 10.1097/00000658-199212000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD., Jr Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149:1723–1728. [PubMed] [Google Scholar]
- 18.Reid RR, Woodcock S, Shimabukuro-Vornhagen A, Austen WG, Jr, Kobzik L, Zhang M, et al. Functional activity of natural antibody is altered in Cr2-deficient mice. J Immunol. 2002;169:5433–5440. doi: 10.4049/jimmunol.169.10.5433. [DOI] [PubMed] [Google Scholar]
- 19.Weiser M, Williams J, Moore F, Kobzik L, Ma M, Hechtman H, et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JP, Moore FD, Kobzik L, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. doi:10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, et al. Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2006;139:236–243. doi: 10.1016/j.surg.2005.05.028. doi:10.1016/j.surg.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, et al. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995;269:H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 24.Leung H. Using photoshop for measurement and analysis. Bull Micro Soc Canada. 2003;31:19–22. [Google Scholar]
- 25.Arnold JN, Wormald MR, Suter DM, Radcliffe CM, Harvey DJ, Dwek RA, et al. Human serum IgM glycosylation: identification of glycoforms that can bind to mannan-binding lectin. J Biol Chem. 2005;280:29080–29087. doi: 10.1074/jbc.M504528200. doi:10.1074/jbc.M504528200. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Michael L, Grosjean S, Kelly R, Carroll M, Entman M. The role of natural IgM in myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2006;41:62–67. doi: 10.1016/j.yjmcc.2006.02.006. doi:10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. doi:10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Alicot EM, Carroll MC. Human natural IgM can induce ischemia/reperfusion injury in a murine intestinal model. Mol Immunol. 2008;45:4036–4039. doi: 10.1016/j.molimm.2008.06.013. doi:10.1016/j.molimm.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd-Jones D, Adams RJ, Todd M, Ford E, Furie K, Gillespie C, et al. Heart disease and stroke statistics 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 30.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. doi:10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. doi:10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 32.Chan RK, Ibrahim SI, Takahashi K, Kwon E, McCormack M, Ezekowitz A, et al. The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J Immunol. 2006;177:8080–8085. doi: 10.4049/jimmunol.177.11.8080. [DOI] [PubMed] [Google Scholar]
- 33.Johnson AR, Hugli TE, Muller-Eberhard HJ. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975;28:1067–1080. [PMC free article] [PubMed] [Google Scholar]
- 34.el-Lati SG, Dahinden CA, Church MK. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J Invest Dermatol. 1994;102:803–806. doi: 10.1111/1523-1747.ep12378589. doi:10.1111/1523-1747.ep12378589. [DOI] [PubMed] [Google Scholar]
- 35.Lim HW, He D, Esquenazi-Behar S, Yancey KB, Soter NA. C5a, cutaneous mast cells, and inflammation: in vitro and in vivo studies in a murine model. J Invest Dermatol. 1991;97:305–311. doi: 10.1111/1523-1747.ep12480568. doi:10.1111/1523-1747.ep12480568. [DOI] [PubMed] [Google Scholar]
- 36.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. doi:10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 37.De Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75:375–382. doi: 10.1097/01.TP.0000044455.05584.2A. doi:10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- 38.Lefer DJ, Shandelya SM, Serrano CV, Jr, Becker LC, Kuppusamy P, Zweier JL. Cardioprotective actions of a monoclonal antibody against CD-18 in myocardial ischemia-reperfusion injury. Circulation. 1993;88:1779–1787. doi: 10.1161/01.cir.88.4.1779. [DOI] [PubMed] [Google Scholar]
- 39.Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91:1872–1885. doi: 10.1161/01.cir.91.6.1872. [DOI] [PubMed] [Google Scholar]
- 40.Testa L, Van Gaal WJ, Bhindi R, Biondi-Zoccai GG, Abbate A, Agostoni P, et al. Pexelizumab in ischemic heart disease: a systematic review and meta-analysis on 15,196 patients. J Thorac Cardiovasc Surg. 2008;136:884–893. doi: 10.1016/j.jtcvs.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 41.Faxon DP, Gibbons RJ, Chronos NA, Gurbel PA, Sheehan F. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40:1199–1204. doi: 10.1016/s0735-1097(02)02136-8. doi:10.1016/S0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 42.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. doi:10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. doi:10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Sign. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. doi:10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 45.Hausenloy DJ, Yellon DM. Time to take myocardial reperfusion injury seriously. N Engl J Med. 2008;359:518. doi: 10.1056/NEJMe0803746. doi:10.1056/NEJMe0803746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.