Abstract
Aims
Cardiomyocyte apoptosis contributes to the development of diabetic cardiomyopathy. How the elevated glucose levels associated with diabetes cause cell death is unknown. Here we report that high glucose-induced cardiomyocyte death is mediated via monocyte chemotactic protein-1 (MCP-1) production and induction of a novel zinc-finger protein.
Methods and results
H9c2 cardiomyoblasts treated with 28 mmol/L glucose were evaluated for MCP-1 production and induction of the zinc-finger protein, MCP-1-induced protein (MCPIP). Disruptors of MCP-1 interaction with its receptor, CCR2, and knockdown of MCPIP with siRNA were used to determine if MCP-1 and MCPIP mediate glucose-induced cell death. The molecular mechanisms were evaluated by assessing reactive oxygen species (ROS) production, endoplasmic reticulum (ER) stress, and autophagy. Key findings were confirmed in isolated neonatal rat cardiomyocytes. Glucose treatment of H9c2 cardiomyoblasts and isolated cardiomyocytes caused MCP-1 production, MCPIP induction, ROS production, ER stress, autophagy, and cell death. Treatment with CCR2 antagonists and knockdown of MCPIP attenuated glucose-induced ROS production, ER stress, autophagy, and cell death. Inhibition of ROS with 1400 W, tiron, and cerium oxide (CeO2) nanoparticles attenuated ER stress, autophagy, and cell death. Specific inhibitors of ER stress and knockdown of IRE-1 attenuated glucose-induced autophagy and cell death. Inhibitors of autophagy and knockdown of beclin-1 attenuated glucose-induced death.
Conclusion
Glucose-induced cardiomyocyte death is mediated via MCP-1 production and MCPIP induction, which causes sequential events—ROS production, ER stress, autophagy, and cell death.
Keywords: MCP-1, MCPIP, Hyperglycaemia, Diabetic cardiomyopathy, Cerium oxide nanoparticles
1. Introduction
The number of individuals with diabetes is expected to reach 300 million by the year 2025.1 Individuals with diabetes have an increased risk for developing heart failure.2 Moreover, cardiovascular complications are considered to be the leading cause of mortality in diabetic patients.3 Diabetes is known to alter cardiac structure and function independent of coronary artery disease and hypertension, a condition known as diabetic cardiomyopathy.4
Inflammation plays a critical role in the pathophysiological progression of cardiovascular diseases and heart failure.5,6 A role for inflammation in diabetic cardiomyopathy has also been implicated.7 Experimental evidence obtained with CCR2 or monocyte chemotactic protein-1 (MCP-1) deficient mice8,9 and mice with cardiac-targeted expression of MCP-110,11 demonstrate a key role for MCP-1 in the development of cardiovascular disease and heart failure. Increased serum levels of MCP-1 found in humans correlate with markers of the metabolic syndrome, including obesity, insulin resistance, type 2 diabetes, hypertension, and increased serum TG concentration.12 Furthermore, endothelial cells and monocytes exposed to high glucose levels are known to produce MCP-1.13 We recently reported that MCP-1 induces a novel zinc-finger protein, termed MCP-1-induced protein (MCPIP).14 The presence of MCPIP was found to be associated with apoptotic cardiomyocytes in a murine model of heart failure. Association of MCPIP with human ischaemic heart disease was also found.14 If and how glucose-induced MCP-1 and MCPIP might play a role in the pathophysiological progression of diabetic cardiomyopathy remains unknown.
Recent studies suggest that cardiomyocyte loss plays a key role in diabetic cardiac damage in both animals and humans.15–17 Hyperglycaemia is known to cause apoptosis16,17 that leads to diabetic cardiomyopathy. Attenuation of hyperglycaemia-induced cardiomyocyte cell death has been shown to prevent the progression of cardiac complications associated with diabetes.18 However, the molecular mechanisms by which hyperglycaemia contributes to development of diabetic cardiomyopathy remain unclear.
In this report, we provide evidence that exposure of H9c2 cardiomyoblasts to high glucose causes production of MCP-1 and expression of MCPIP. We show that glucose-induced cardiomyoblast death is mediated via induction of MCPIP caused by MCP-1 production. Moreover, H9c2 cells exposed to high glucose levels display increased reactive oxygen species (ROS) production that results in an endoplasmic reticulum (ER) stress response that leads to autophagy and H9c2 cardiomyoblast death. We found that MCP-1 induction of MCPIP was responsible for the mediation of this series of events that led to cell death. The key findings observed in H9c2 cardiomyoblasts were further validated in isolated neonatal rat cardiomyocytes. Thus, our results strongly suggest that hyperglycaemia-induced MCP-1 production and the consequent induction of MCPIP would lead to death of cardiomyocytes and thus contribute to the pathophysiological progression of diabetic cardiomyopathy.
2. Methods
The experimental procedures in mice and protocols used in this study were approved by Animal Care and Use Committee of the University of Central Florida, animal protocol number 08-42, conforming with the Guide for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.1. Cell culture
Isolated neonatal rat ventricular myocytes (Supplementary materials) and H9c2 cardiomyoblasts (ATCC) were grown in modified Dulbecco's Modified Eagles Medium (Supplementary materials). Cells were treated with/without 28 mmol/L D-glucose for 0, 12, 24, or 48 h. Cells were treated with/without appropriate amounts of inhibitors 1–3 h prior to glucose treatment (Supplementary materials).
2.2. Neonatal rat cardiomyocyte isolation
Neonatal rat ventricular myocytes were isolated from hearts of 2–3-day-old Sprague–Dawley rats by Trypsin digestions as previously described.19,20
In brief, hearts were removed surgically and ventricular cardiomyocytes were prepared by 0.12% Trypsin (Invitrogen) in Calcium-free phosphate-buffered saline (PBS: 137 mM NaCl, 2.68 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4). Cardiomyocytes were pre-plated for 2 h in DMEM supplemented with 15% FBS containing appropriate antibiotics to reduce non-myocyte contamination and then plated (2.0 × 106 cells) in culture flasks and incubated at 37°C and 5% CO2 in humidified atmosphere.
2.3. Cell death assays
Cell viability and death were measured by (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)(Promega), trypan blue (Gibco), and TMR TUNEL21 assays using manufacturer's instructions. For MTT assay, cells were plated on 96-well plates prior to treatment with/without glucose and was evaluated using a spectrophotometer measuring absorbance at 570 nm. For trypan blue, 1 × 105 cells were collected and treated with a 1:4 dilution of trypan blue. After 2 min, cells were counted on a haemacytometer. TMR TUNEL experiments were performed in four-well chamber slides, and cells were seeded at 0.5 × 105 cells per well prior to experimentation.
2.4. ROS measurements
ROS production was evaluated flurometrically using Dihydrorhodamine 123 (DHR123). Briefly, 24 h after treatment with 28 mM glucose, cells were treated with 1 µmol/L DHR123 for 30 min at 37°C and 5% CO2. Cells were then washed 3× with 1x PBS. 5 × 105 cells were plated on a 96-well plate and were subjected to fluorometric analysis (excitation: 550 nm; emission: 590 nm).
2.5. RT–PCR
Total RNA was isolated with the RNAeasy kit (Invitrogen) and first-strand cDNA was synthesized using 1 µg total RNA (DNase-treated) using I script cDNA synthesis kit (Bio-Rad); β-actin served as an internal control. Primers designed for real-time PCR are listed in Supplementary materials.
2.6. Immunoblot analysis
Cells were treated with cell lyses buffer and protein samples were collected and subjected to immunoblot using the appropriate polyclonal antibodies (Supplementary material).
2.7. siRNA treatment
Cells were treated with 100 nmol/L of a chemically synthesized siRNA targeted for MCPIP, BECN1, or IRE1 (Ambion) or with 100 nmol/L non-specific siRNA (Ambion) using Dharmafect transfection reagent 12 h prior to treatment with/without 28 mM glucose.
2.8. Statistical analysis
The experimental data were analysed by using SPSS statistical software (SPSS Inc.) under Windows XP. All values are presented as mean ± SEM. Results were compared between groups by ANOVA analysis followed by t-tests. Differences were considered significant at a P-value of <0.05.
3. Results
3.1. High glucose-induced death of H9c2 cardiomyoblasts is mediated via MCP-1 production and induction of MCPIP
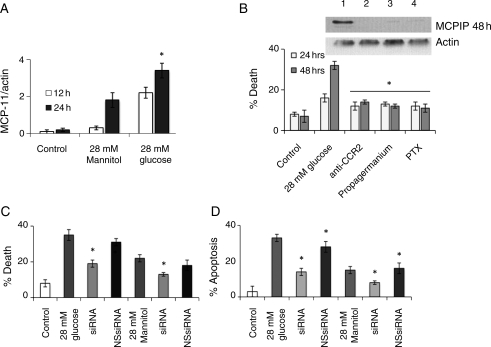
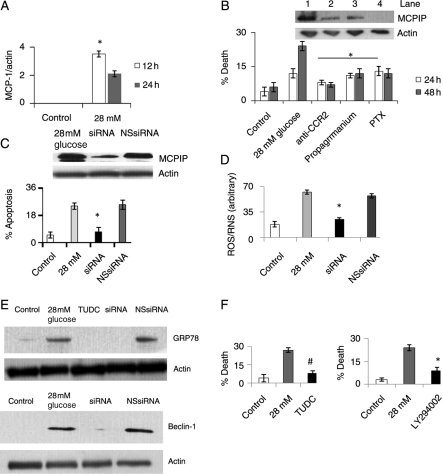
Since it is known that hyperglycaemic conditions can induce the expression of MCP-1 in cultured monocytes,12 we tested whether H9c2 cardiomyoblasts exposed to high glucose (28 mmol/L glucose) concentration could also cause production of MCP-1. We found that MCP-1 protein levels significantly increased in H9c2 cardiomyoblast 12 h after treatment with 28 mmol/L glucose and reached maximal levels at 24 h after treatment (Figure 1A, see Supplementary material online, Figure S1). H9c2 cells treated with 28 mM mannitol, to control for osmolarity, resulted in increased MCP-1 protein levels, but the induction was not as significant as cells treated with 28 mM glucose (Figure 1A, see Supplementary material online, Figure S1). H9c2 cardiomyoblasts treated with high glucose and 28 mM mannitol were found to induce cell death in a concentration-dependent manner as determined by trypan blue. Although both high glucose and mannitol treatment were found to induce cell death (Supplementary material online, Figure S1), the percentage of dead cells was greater in H9c2 cardiomyoblasts treated with high glucose than in cardiomyoblasts treated with 28 mM mannitol. To determine whether the MCP-1 produced by H9c2 cardiomyoblasts is involved in cell death induced by high glucose exposure, we tested whether inhibition of MCP-1 binding to its CCR2 receptor would attenuate high glucose-induced cell death in H9c2 cardiomyoblasts. Antibody specific to CCR2 significantly attenuated glucose-induced cell death as measured by trypan blue (Figure 1B). Inhibitors of G-protein coupled receptor, propagermanium and pertussis toxin, attenuated high glucose-induced cell death (Figure 1B). These results indicate that MCP-1 binding to its receptor mediates high glucose-induced H9c2 death.
Figure 1.
H9c2 cardiomyoblasts treated with 28 mmol/L glucose resulted in MCP-1 production and MCPIP induction that caused cell death. H9c2 cardiomyoblasts were treated with/without 28 mmol/L glucose. (A) At 12 and 24 h, cell lysate was collected and analysed using immunoblot with MCP-1 antibody. Results were quantified against β-actin (*P < 0.03). (B) Cardiomyoblasts treated with 28 mmol/L glucose were treated with/without 25 µg/mL CCR2 (lane 2) antibody, 2 µg/mL propagermanium (lane 3), or 250 ng/mL pertussis toxin (PTX) (lane 4) for 24 and 48 h. (Top) Cell lysate was collected at 48 h and analysed using immunoblot with MCPIP antibody. No treatment, lane 1. β-Actin served as a control. (Bottom) Cells were evaluated for death using trypan blue (*P < 0.03). (C and D) Cardiomyoblasts were treated with 28 mmol/L glucose or mannitol with/without siRNA specific for MCPIP or with non-specific siRNA (NS siRNA). At 24 and 48 h, cell death was analysed using trypan blue (C) or TUNEL assay (D; *P < 0.03).
We tested whether high glucose treatment of H9c2 cardiomyoblasts could induce MCPIP. High glucose and mannitol treatment induced MCPIP production with glucose treatment resulting in a more profound effect (Supplementary material online Figure S1). That the MCPIP induction was mediated via MCP-1 interaction with CCR2 was shown by the fact that treatment with CCR2 antibody, and the G-protein coupled receptor inhibitors propagermanium and pertussis toxin attenuated induction of MCPIP by high glucose treatment (Figure 1B, top). To determine if MCPIP is involved in glucose-induced cardiomyoblast death, we tested whether knockdown of MCPIP could attenuate glucose-induced cell death. Knockdown of MCPIP with MCPIP-specific siRNA (Supplementary material online, Figure S1) resulted in the attenuation of glucose-induced cardiomyoblast death as measured by trypan blue or TUNEL assay while treatment with non-specific siRNA had little effect (Figure 1C and D). Knockdown of MCPIP also resulted in the attenuation of mannitol-induced death (Figure 1C and D). These results demonstrate that glucose-induced cardiomyoblast death is mediated via MCP-1 induction of MCPIP.
3.2. Exposure of H9c2 cardiomyoblasts to high glucose levels induces ROS production mediated via MCPIP
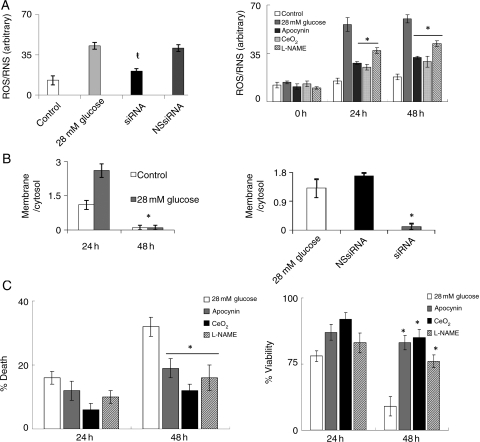
Since high glucose concentration was reported to elevate ROS levels,15–17 we tested whether MCPIP plays a role in this induction of ROS in H9c2 cardiomyoblasts. Exposure of H9c2 to 28 mmol/L glucose caused an increase in fluorescence of the free radical dye DHR123 (Figure 2A, left). Knockdown of MCPIP with specific siRNA significantly attenuated ROS production when compared with untreated and non-specific siRNA controls (Figure 2A, left). We tested whether this MCPIP-mediated ROS induction was caused by an increase in NADPH oxidase activity. Treatment of cardiomyoblasts with 28 mmol/L glucose resulted in the migration of the NADPH oxidase subunit phox47 to the plasma membrane (Figure 2B, left; see Supplementary material online, Figure S2), indicating activation of phox47, while knockdown of MCPIP with specific siRNA abolished this migration to the membrane (Figure 2B, right; see Supplementary material online, Figure S2). Thus, MCPIP mediates the activation of NADPH oxidase resulting in the increased production of ROS by glucose treatment. This notion is further supported by our observation that the treatment of H9c2 cells with the ROS/RNS inhibitors apocynin, CeO2 nanoparticles, and L-NAME prior to treatment with 28 mmol/L glucose resulted in the attenuation of ROS production as detected by DHR123 (Figure 2A, right). Treatment with the ROS/RNS inhibitors apocynin, CeO2 nanoparticles, and L-NAME resulted in the attenuation of high glucose-induced cardiomyoblast death as detected by trypan blue (Figure 2C, left) and caused increased viability in H9c2 cardiomyoblasts as determined by MTT assay (Figure 2C, right). Thus, our results indicate that high glucose-induced H9c2 cardiomyoblast death occurs via MCPIP-induced oxidative stress.
Figure 2.
H9c2 cardiomyoblasts treated with 28 mmol/L glucose resulted in cell death that was mediated via MCPIP-induced ROS production. (A, left) H9c2 cardiomyoblasts were treated with 28 mmol/L glucose with/without siRNA specific for MCPIP or with non-specific siRNA. After 24 h, ROS was measured using DHR 123 (excitation: 550 nm; emission 590 nm, ŧP < 0.05). (A, right) Cardiomyoblasts treated with 28 mmol/L glucose were treated with/without 20 µM Apocynin, 300 nM CeO2 nanoparticles, or 50 µM L-NAME. At 0, 24, and 28 h, ROS was measured using DHR 123 (excitation: 550 nm; emission: 590 nm (*P < 0.03). (B, left) Cardiomyoblasts were treated with/without 28 mmol/L glucose. Cell lysate was collected and the membrane fractions were isolated. Both cytosol and membrane fractions were evaluated using immunoblot with phox47 antibody. Results were quantified by taking the membrane/cytosol fraction (*P < 0.03). (B, right) Cardiomyoblasts were treated with 28 mmol/L glucose with/without siRNA specific for MCPIP or with non-specific siRNA. Cell lysate was collected and the membrane fractions were isolated. Cytosol and membrane fractions were evaluated using immunoblot with phox47 antibody. Results were quantified by taking the membrane/cytosol fraction (*P < 0.03). (C) Cardiomyoblasts treated with 28 mmol/L glucose were treated with/without 20 µM Apocynin, 300 nM CeO2 nanoparticles, or 50 µM L-NAME. At 24 and 48 h, cells were evaluated for cell death using trypan blue (left) or for cell viability using MTT assay (right) (*P < 0.03).
3.3. Exposure of H9c2 cardiomyoblasts to high glucose-induces ER stress that is mediated via MCPIP-induced ROS production
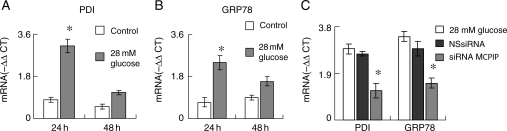
Since cardiac-targeted expression of MCP-1 is known to induce ER stress in murine cardiomyocytes,22 we tested whether exposure of H9c2 to high glucose concentrations would result in an increased expression of the ER stress chaperones PDI and GRP78. Treatment of H9c2 with 28 mmol/L glucose resulted in an increase in both transcript and protein levels of PDI and GRP78 (Figure 3A and B, see Supplementary material online, Figure S3). Knockdown of MCPIP with specific siRNA resulted in significant attenuation of both transcript and proteins levels of GRP78 and PDI when compared with H9c2 cardiomyoblasts treated with non-specific siRNA (Figure 3C, see Supplementary material online, Figure S3). Since ROS is known to induce ER stress,23 we tested whether MCPIP-induced ROS production plays a role in inducing ER stress in H9c2 cardiomyoblasts exposed to a high glucose concentration. Treatment of cardiomyoblasts with the ROS/RNS inhibitors apocynin and CeO2 nanoparticles prior to treatment with 28 mmol/L glucose resulted in drastically reduced induction of PDI and GRP78 protein levels (Supplementary material online, Figure S3). This result suggests that MCPIP-induced ROS production does cause ER stress in H9c2 treated with 28 mmol/L glucose.
Figure 3.
H9c2 cardiomyoblasts treated with 28 mmol/L glucose resulted in MCPIP-induced ER stress. H9c2 cardiomyoblasts were treated with/without 28 mmol/L glucose. RNA was isolated at 24 and 48 h. Transcript levels of PDI (A) and GRP78 (B) were evaluated using real-time RT–PCR. The results are expressed as the −ΔΔCT of treated/untreated. Results normalized to β-actin (*P < 0.03). (C) Cardiomyoblasts were treated with 28 mmol/L glucose with/without siRNA specific for MCPIP or with non-specific siRNA. RNA was isolated at 24 h. Transcript levels of MCPIP were evaluated using real-time RT–PCR. The results are expressed as the −ΔΔCT of treated/untreated. Results normalized to β-actin (*P < 0.03).
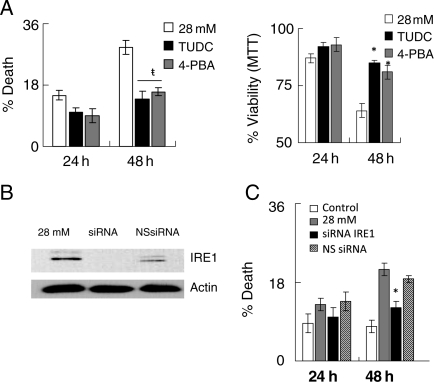
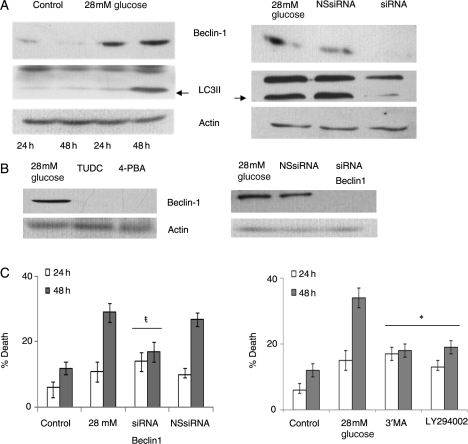
ER stress is known to lead to cell death.24–26 We tested whether chemical chaperones, known to specifically inhibit ER stress,27 could prevent high glucose-induced H9c2 death. Inhibition of ER stress with tauroursodeoxycholate (TUDC) and 4′ phenylbutyric acid (4-PBA) resulted in the attenuation of cardiomyoblast death as determined by trypan blue (Figure 4A, left) and resulted in increased cell viability as detected by MTT (Figure 4A, right). Knockdown of IRE1 an ER stress protein that is known to signal cell death24–28 (Figure 4B), attenuated high glucose-induced cardiomyoblast death (Figure 4C). Thus, high glucose treatment of H9c2 cardiomyoblasts results in the induction of MCPIP that leads to ER stress that causes cell death.
Figure 4.
H9c2 cardiomyoblasts treated with 28 mmol/L glucose resulted in cell death that was mediated via MCPIP-induced ER stress. (A) H9c2 cardiomyoblasts were treated with 28 mmol/L glucose and with/without 100 µM TUDC or 50 µM 4-PBA. Cells were then evaluated for cell death using trypan blue (left; ŧP < 0.05) or cell viability evaluated using MTT assay (right; *P < 0.03). (B and C) Cardiomyoblasts were treated with 28 mmol/L glucose with/without siRNA specific for IRE1 or with non-specific siRNA. Cell death was evaluated using trypan blue (*P < 0.03).
3.4. Exposure of H9c2 cardiomyoblasts to high glucose induces autophagy via MCPIP-induced ER stress
Since ER stress is known to induce autophagy that is associated with cardiomyocyte death involved with heart failure,29,30 we tested whether high glucose induction of MCPIP results in autophagy that is mediated via ER stress. We found that treatment of H9c2 with 28 mmol/L glucose resulted in an increase in the autophagy marker beclin-1 (Figure 5A, left). Knockdown of MCPIP with specific siRNA resulted in the attenuation of beclin-1 expression caused by glucose treatment (Figure 5A, right). Since LC3 cleavage is a key event that occurs during autopahgy,29,30 we tested whether high glucose treatment of H9c2 cardiomyoblasts could mediate this event. The observation that cardiomyoblasts treated with 28 mmol/L glucose resulted in cleavage of LC3 is indicative of autophagy (Figure 5A, left). Knockdown of MCPIP with specific siRNA prevented the cleavage of LC3I (Figure 5A, right). That ER stress induced by MCPIP was responsible for the observed autophagy caused by treatment with 28 mmol/L glucose was suggested by the finding that inhibition of ER stress with the specific chemical chaperones TUDC and 4-PBA27 attenuated induction of beclin-1 (Figure 5B). These results indicate that high glucose-induced autophagy occurs via MCPIP induction of ER stress.
Figure 5.
MCPIP-induced ER stress resulted in autophagy that mediated high glucose-induced H9c2 cardiomyoblast death. H9c2 cardiomyoblasts were treated with/without 28 mmol/L glucose. (A, left) At 24 and 48 h, cell lysate was collected and analysed using immunoblot with beclin-1 or LC3 antibody. β-Actin served as a control. (A, right) Cardiomyoblasts were treated with 28 mmol/L glucose with/without siRNA specific for MCPIP or with non-specific siRNA. At 48 h, cell lysate was collected and analysed using immunoblot with beclin-1 or LC3 antibody. β-Actin served as a control. (B, left) Cardiomyoblasts were treated with 28 mmol/L glucose and with 100 µM TUDC or with 50 µm 4-PBA. At 48 h, cell lysate was collected and analysed using immunoblot with antibody specific for beclin-1. β-Actin served as a control. (B, right) Cardiomyoblasts treated with 28mM glucose were treated with or without siRNA for Beclin-1 or non-specific siRNA (NS siRNA). At 48 h, cell lysate was collected and analysed using immunoblot with beclin-1 antibody. (C) Cardiomyoblasts were treated with 28 mmol/L glucose with/without siRNA specific for beclin-1 or with non-specific siRNA. At 24 and 48 h after treatment, cell death was analysed using trypan blue (left; *P < 0.05). (C, right) Cardiomyoblasts were treated with 28 mmol/L glucose and with/without 50 µM 3′MA or 1 µM LY294002. At 24 and 48 h after treatment, cell death was analysed using trypan blue (#P < 0.03).
Since autophagy is known to cause cardiomyocyte death associated with heart failure,29–31 we tested whether high glucose-induced cell death is mediated via MCPIP induction of autophagy. Knockdown of beclin-1 and treatment with the autophagy inhibitors 3′MA and LY294002 resulted in the attenuation of glucose-induced cell death as detected by trypan blue (Figure 5C). These results show that the treatment of H9c2 cardiomyoblasts with high glucose concentration results in the induction of MCPIP that leads to ROS production that results in ER stress that causes autophagy and the induction of cell death.
3.5. The molecular mechanisms underlying the high glucose-induced death of isolated neonatal cardiomyocytes are similar to those observed in high glucose-treated H9c2 cardiomyoblasts
To test whether the molecular events that lead to glucose-induced death of H9c2 cardiomyoblasts also occur in real cardiomyocytes, we examined the glucose-induced events in isolated neonatal rat ventricular cardiomyocytes. We found that cardiomyocytes treated with 28 mmol/L glucose resulted in increased MCP-1 protein expression (Figure 6A, see Supplementary material online, Figure S4). Furthermore, inhibition of the interaction of MCP-1 with its receptor, CCR2, with antibody for CCR2, and the G-coupled protein receptor inhibitors, propagermanium and pertussis toxin, resulted in the attenuation of glucose-induced cardiomyocyte death as measured by trypan blue (Figure 6B). High glucose treatment of cardiomyocytes resulted in induction of MCPIP while treatment of cardiomyocytes with CCR2 antibody, propagermanium, and pertussis toxin attenuated MCPIP induction (Figure 6B, see Supplementary material online, Figure S4). The involvement of MCPIP in glucose-induced cell death was confirmed as knockdown of MCPIP with specific siRNA resulted in the attenuation of glucose-induced cardiomyocyte death as measured by TUNEL assay (Figure 6C).
Figure 6.
High glucose treatment of cardiomyocytes resulted in MCP-1 production and induction of MCPIP that caused ROS production, ER stress, autophagy, and cell death. Isolated cardiomyocytes were treated with/without 28 mmol/L glucose. (A) At 12 and 24 h, cell lysate was collected and analysed using immunoblot with MCP-1 antibody. Results normalized to β-actin (*P < 0.03). (B) Cardiomyocytes treated with 28 mmol/L glucose were treated with or without 25 µg/mL CCR2 antibody, 2 µg/mL propagermanium, or 250 ng/mL PTX for 24 and 48 h. Cells were then evaluated for death using trypan blue (*P < 0.03). (B, top) Cardiomyocytes treated with 28 mmol/L glucose were treated with/without 25 µg/mL CCR2 antibody (lane 2), 2 µg/mL propagermanium (lane 3), or 250 ng/mL PTX (lane 4) for 24 h. Lane 1, control. Cell lysate was collected and analysed using immunoblot with MCPIP antibody. β-Actin served as control. (C) Cardiomyocytes were treated with 28 mmol/L glucose with/without siRNA specific for MCPIP or with non-specific siRNA. At 48 h, cell death was analysed using TUNEL assay (*P < 0.03). (D) Cardiomyocytes were treated with 28 mmol/L glucose with/without siRNA specific for MCPIP or with non-specific siRNA. ROS was then measured using DHR 123 (excitation: 550 nm; emission: 590 nm; *P < 0.03). (E) Cardiomyoyctes were treated with 28 mmol/L glucose with/without siRNA specific for MCPIP and non-specific siRNA or with/without 100 µM TUDC. At 48 h, cell lysate was collected and analysed using immunoblot with GRP78 and Beclin-1. β-Actin served as control. (F) Cardiomyocytes were treated with 28 mmol/L glucose and with/without 100 µM TUDC. (Left) At 48 h, cells were evaluated for cell death using trypan blue (#P < 0.02). (Right) Cardiomyocytes were treated with 28 mmol/L glucose with/without LY294002. At 48 h after treatment, cell death was analysed using trypan blue (*P < 0.03).
Glucose-induced ROS/RNS production in isolated cardiomyocytes as measured with DHR 123 was attenuated by knockdown of MCPIP with specific siRNA (Figure 6D). Glucose induction of ER stress was mediated via MCPIP in cardiomyocytes as knockdown of MCPIP with siRNA resulted in virtual elimination of the induction of GRP78 (Figure 6E, top). Treatment with the ER stress-specific inhibitor, TUDC, resulted in the attenuation of glucose-induced cardiomyoycte death as determined by trypan blue (Figure 6F, left). High glucose-induced cardiomyocyte death was found to be mediated via MCPIP induction of autophagy. Cardiomyocytes treated with high glucose resulted in increased levels of the autophagy marker protein, beclin-1, while knockdown of MCPIP with siRNA prevented its expression (Figure 6E, bottom). Moreover, treatment of cardiomyocytes with 28 mmol/L glucose and the autophagy inhibitor LY294002 resulted in the attenuation of glucose-induced cardiomyocyte death as determined by trypan blue (Figure 6F, right). These results strongly suggest that high glucose-induced cardiomyocyte death occurs via production of MCP-1 and induction of MCPIP that results in ROS production that leads to ER stress that causes autophagy and eventual cell death.
4. Discussion
Hyperglycaemia is known to cause cardiomyocyte apoptosis that plays a key role in the pathophysiological development of diabetic cardiomyopathy.15–17 However, the molecular mechanism by which hyperglycaemia induces cardiomyocyte apoptosis is poorly understood. Here we report that hyperglycaemia induces MCP-1 production in H9c2 cardiomyoblasts and neonatal cardiomyocytes that lead to the induction of the zinc-finger protein MCPIP that mediates high glucose-induced cell death. Furthermore, our study revealed that glucose-induced H9c2 cardiomyoblast and isolated cardiomyocyte death are mediated via MCPIP induction of ROS that leads to ER stress that causes autophagy and eventual cell death.
Diabetic cardiomyopathy is a major complication associated with diabetes mellitus.1–3 Inhibition of hyperglycaemic conditions has been shown to attenuate the cardiovascular complications associated with diabetes mellitus.18 Furthermore, cardiomyocytes exposed to hyperglycaemic conditions are known to undergo apoptosis.15–17 Our results are consistent with these observations and provide a molecular explanation into how hyperglycaemia can result in cardiomyocyte cell death involved with diabetic cardiomyopathy.
Diabetes is associated with elevated serum levels of MCP-1.32 MCP-1 is well known to be a critical player in the pathophysiological progression of heart failure attributed to coronary artery disease.8–11–33,34 Our results demonstrate that MCP-1 probably plays a critical role in the development of diabetic cardiomyopathy. Increased MCP-1 expression has been found in STZ-induced diabetic animal hearts.35 Serum levels of MCP-1 are elevated in diabetic individuals and expression of CCR2 on monocytes is known to be elevated in diabetic patients.32 Glucose treatment of monocytes has been reported to result in MCP-1 production.13 Our results show that hyperglycaemia causes MCP-1 production by H9c2 cardiomyoblasts and isolated cardiomyocytes. The glucose-induced production of MCP-1 observed was, to a large extent, due to the hypertonicity created by elevated glucose levels as mannitol treatment also resulted in MCP-1 production. How hypertonicity may contribute to MCP-1 production remains unclear and is any area for further exploration. Furthermore, our results suggested that there may be other possible mechanisms whereby hyperglycaemia can induce MCP-1 production as glucose-induced MCP-1 production was greater than mannitol-induced MCP-1 production. Hyperglycaemia is known to lead to the increased production and activation of Angiotensin II (Ang II) signalling pathways in cardiomyoyctes.36 Although activation of Ang II signalling has been shown to result in MCP-1 production in cardiac fibroblasts, it was previously shown that Ang II treatment of cardiomyocytes does not induce MCP-1 expression.37 However, such as MCP-1, glucose-induced Ang II can induce NADPH oxidase-mediated ROS production.38 Thus, a possible interaction between the Ang II and MCP-1 signalling pathways might exist. Our results suggest MCP-1 as a critical player in glucose-induced death of cardiomyocytes as inhibition of the interaction of MCP-1 with CCR2-attenuated high glucose-induced death of H9c2 cardiomyoblasts and isolated cardiomyocytes. Our finding that knockdown of MCPIP in H9c2 cardiomyoblasts and isolated cardiomyocytes attenuated high glucose-induced death supports a role for MCPIP as a key mediator of cardiomyocyte loss that leads to diabetic cardiomyopathy.
Increased ROS production is observed in type 1 and type 2 diabetes and is considered to be a major contributing factor in the development of diabetic cardiomyopathy.39–41 Several reports demonstrate a role for ROS in glucose-induced cardiomyocyte cell death.15–17 Consistent with these findings, we showed that high glucose treatment of H9c2 and isolated cardiomyocytes-induced ROS production mediated via MCP-1 production and consequent MCPIP induction. NADPH oxidase has been shown to be a major source of ROS production in cardiomyocytes treated with high glucose levels.16,17 Our results demonstrate that MCPIP mediates glucose-induced NADPH oxidase activation. CeO2 nanoparticles act as free radical scavengers due to their dual oxidative states, Ce4+ to Ce3+, that creates an oxygen vacancy.42 CeO2 nanoparticles were reported to protect HT22 cells from oxidative stress-induced cell death.43 Here we show that CeO2 nanoparticles attenuate glucose-induced ROS production and cardiomyocyte death. That inhibition of MCPIP-induced ROS production was found to attenuate high glucose-induced cardiomyoycte death places MCPIP as a key regulator of hyperglycaemia-induced ROS production that leads to cardiomyocyte death.
Oxidative stress is known to lead to an ER stress response.23–44 Major chaperone proteins involved in ER stress include GRP78 and PDI.23–44–45 Elevated levels of ER stress-associated chaperone proteins have been observed in glucose and glucosamine-treated hepatocytes, monocytes, and smooth muscle cells.46 Our results show that exposure of H9c2 cardiomyoblasts and isolated cardiomyocytes to high glucose levels caused induction of both GRP78 and PDI, mediated via MCPIP, as knockdown of MCPIP attenuated the expression levels of GRP78 and PDI. CeO2 nanoparticles have been previously shown to provide a cardioprotective effect through the attenuation of ER stress in a MCP-1 transgenic model of ischaemic heart disease.11 Here we show that CeO2 nanoparticles attenuated MCPIP-mediated ER stress induced by high glucose. These results demonstrate a role for ER stress in high glucose-induced cardiomyocyte death that contributes to the pathophysiological progression of diabetic cardiomyopathy.
Prolonged ER stress is known to result in signalling events known as the unfolded protein response that eventually leads to cell death.24–28 ER stress-induced cardiomyoycte apoptosis has been reported to occur in the hearts of diabetic animals.47 IRE1 is a major signalling protein involved in ER stress-induced apoptosis.24–28 IRE1alpha is known to induce JNK activation that in turn can result in an increase in pro-apoptotic proteins such as p53 and PUMA.24 We have previously found that MCPIP expression in H9c2 cells causes JNK activation and induction of p53 and PUMA.48 The present results show that MCPIP-induced ER stress is involved in hyperglycaemia-induced cardiomyocyte death. Thus, the chemical chaperones TUDC and 4′PBA27 caused attenuation of glucose-induced cell death. Furthermore, knockdown of IRE1 attenuated high glucose-induced H9c2 cardiomyoblast death demonstrating the involvement of ER stress signalling events in high glucose-induced cell death. Thus, our results demonstrate that MCPIP-induced ER stress plays an important role in hyperglycaemia-induced cardiomyocyte death.
Prolonged ER stress is known to cause the induction of autophagy that is known to be involved in cardiovascular diseases.29,30 Although autophagy is thought at first to protect the cell from increased stress,21 it is known that prolonged autophagy can lead to cell death.49 Autophagy depends on several key proteins including beclin-1 and LC3. Beclin-1 interacts with PI3K II resulting in the eventual cleavage of LC3 that is required for assembly of the autophagosome.26–50 Our results show that hyperglycaemia caused an increase in beclin-1 levels and a significant increase in LC3 cleavage product demonstrating that autophagy was involved in H9c2 cardiomyoblast and cardiomyoycte death caused by hyperglycaemia. Moreover, our finding that inhibition of autophagy with specific inhibitors and via knockdown of beclin-1 attenuated high glucose-induced H9c2 and cardiomyoycte death strongly suggests the involvement of MCPIP-induced autophagy in glucose-induced cell death.
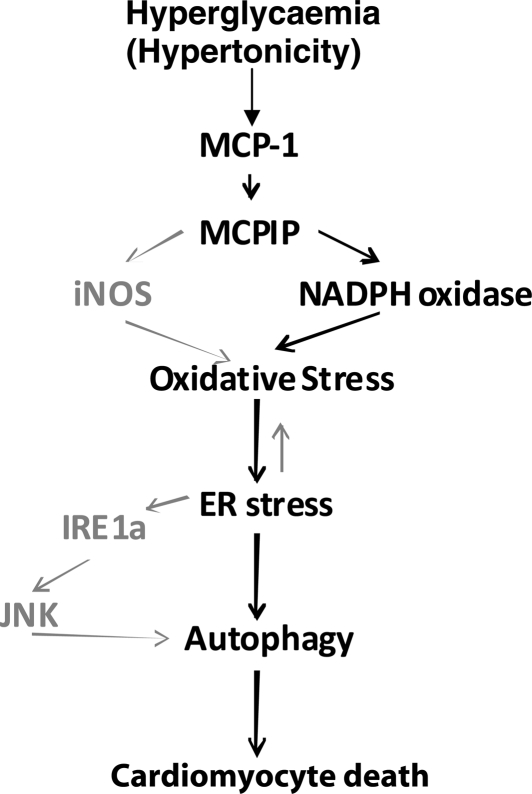
Our results link hyperglycaemia to MCP-1 production and MCPIP induction and thus provide a molecular linkage between hyperglycaemia, inflammation, and diabetic cardiomyopathy (Scheme 1). Thus MCPIP might be a potential therapeutic target in the prevention and treatment of heart failure that occurs in diabetic individuals.
Scheme 1.
Hyperglycaemia causes MCP-1 production and MCPIP induction that results in oxidative stress that leads to ER stress that causes autophagy and eventual cardiomyocyte death. Hyperglycaemia results in MCP-1 production and the induction of MCPIP. MCPIP is known to induce oxidative stress via iNOS or NADPH oxidase. In turn, oxidative stress results in increased ER stress and ultimately activation of the unfolded protein response (UPR). MCPIP-induced UPR activation can occur through IRE1 that results in JNK activation and eventually autophagy. Prolonged autophagy finally results in cardiomyocyte death. Signalling events demonstrated in this study are outlined in bold black. Signalling events induced by MCPIP that were previously shown but not included here are outlined in grey.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (grant HL-69458).
Supplementary Material
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. doi:10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Asghar O, Al-Sunni A, Khavandi K, Khavandi A, Withers S, Greenstein A, et al. Diabetic cardiomyopathy. Clin Sci (Lond) 2009;116:741–760. doi: 10.1042/CS20080500. doi:10.1042/CS20080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spector KS. Diabetic cardiomyopathy. Clin Cardiol. 1998;21:885–887. doi: 10.1002/clc.4960211205. doi:10.1002/clc.4960211205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–2951. doi: 10.2337/diacare.26.10.2949. doi:10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- 5.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. doi:10.1161/01.RES.0000043825.01705.1B. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. doi:10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 7.Tschope C, Walther T, Escher F, Spillmann F, Du J, Altmann C, et al. Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. FASEB J. 2005;19:2057–2059. doi: 10.1096/fj.05-4095fje. [DOI] [PubMed] [Google Scholar]
- 8.Peters W, Charo IF. Involvement of chemokine receptor 2 and its ligand, monocyte chemoattractant protein-1, in the development of atherosclerosis: lessons from knockout mice. Curr Opin Lipidol. 2001;12:175–180. doi: 10.1097/00041433-200104000-00011. doi:10.1097/00041433-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, et al. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. doi:10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 10.Kolattukudy PE, Quach T, Bergese S, Breckenridge S, Hensley J, Altschuld R, et al. Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am J Pathol. 1998;152:101–111. [PMC free article] [PubMed] [Google Scholar]
- 11.Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. doi:10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simeoni E, Hoffmann MM, Winkelmann BR, Ruiz J, Fleury S, Boehm BO, et al. Association between the A-2518G polymorphism in the monocyte chemoattractant protein-1 gene and insulin resistance and Type 2 diabetes mellitus. Diabetologia. 2004;47:1574–1580. doi: 10.1007/s00125-004-1494-4. doi:10.1007/s00125-004-1494-4. [DOI] [PubMed] [Google Scholar]
- 13.Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009;11:241–249. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98:1177–1185. doi: 10.1161/01.RES.0000220106.64661.71. doi:10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiordaliso F, Bianchi R, Staszewsky L, Cuccovillo I, Doni M, Laragione T, et al. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol. 2004;37:959–968. doi: 10.1016/j.yjmcc.2004.07.008. doi:10.1016/j.yjmcc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Feng Q, Arnold M, Peng T. Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc Res. 2009;84:100–110. doi: 10.1093/cvr/cvp189. doi:10.1093/cvr/cvp189. [DOI] [PubMed] [Google Scholar]
- 17.Shen E, Li Y, Shan L, Zhu H, Feng Q, Arnold JM, et al. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes. 2009;58:2386–2395. doi: 10.2337/db08-0617. doi:10.2337/db08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–1697. doi: 10.1016/j.jacc.2006.07.022. doi:10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Bonci D, Latronico MV, Condorelli G. Cardiomyocytes. Methods Mol Biol. 2003;229:169–179. doi: 10.1385/1-59259-393-3:169. [DOI] [PubMed] [Google Scholar]
- 20.Iwaki K, Chi SH, Dillmann WH, Mestril R. Induction of HSP70 in cultured rat neonatal cardiomyocytes by hypoxia and metabolic stress. Circulation. 1993;87:2023–2032. doi: 10.1161/01.cir.87.6.2023. [DOI] [PubMed] [Google Scholar]
- 21.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. doi:10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol. 2006;291:H1411–H1420. doi: 10.1152/ajpheart.01378.2005. doi:10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. doi:10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 24.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. doi:10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, et al. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. doi:10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 26.Moretti L, Cha YI, Niermann KJ, Lu B. Switch between apoptosis and autophagy: radiation-induced endoplasmic reticulum stress? Cell Cycle. 2007;6:793–798. doi: 10.4161/cc.6.7.4036. [DOI] [PubMed] [Google Scholar]
- 27.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. doi:10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder M, Kaufman RJ. Divergent roles of IRE1alpha and PERK in the unfolded protein response. Curr Mol Med. 2006;6:5–36. doi: 10.2174/156652406775574569. doi:10.2174/156652406775574569. [DOI] [PubMed] [Google Scholar]
- 29.Rothermel BA, Hill JA. Myocyte autophagy in heart disease: friend or foe? Autophagy. 2007;3:632–634. doi: 10.4161/auto.4913. [DOI] [PubMed] [Google Scholar]
- 30.Takemura G, Miyata S, Kawase Y, Okada H, Maruyama R, Fujiwara H. Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy. 2006;2:212–214. doi: 10.4161/auto.2608. [DOI] [PubMed] [Google Scholar]
- 31.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. doi:10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mine S, Okada Y, Tanikawa T, Kawahara C, Tabata T, Tanaka Y. Increased expression levels of monocyte CCR2 and monocyte chemoattractant protein-1 in patients with diabetes mellitus. Biochem Biophys Res Commun. 2006;344:780–785. doi: 10.1016/j.bbrc.2006.03.197. doi:10.1016/j.bbrc.2006.03.197. [DOI] [PubMed] [Google Scholar]
- 33.Kim WJ, Chereshnev I, Gazdoiu M, Fallon JT, Rollins BJ, Taubman MB. MCP-1 deficiency is associated with reduced intimal hyperplasia after arterial injury. Biochem Biophys Res Commun. 2003;310:936–942. doi: 10.1016/j.bbrc.2003.09.088. doi:10.1016/j.bbrc.2003.09.088. [DOI] [PubMed] [Google Scholar]
- 34.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. doi:10.1016/S1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 35.Drimal J, Knezl V, Navarova J, Nedelcevova J, Paulovicova E, Sotnikova R, et al. Role of inflammatory cytokines and chemoattractants in the rat model of streptozotocin-induced diabetic heart failure. Endocr Regul. 2008;42:129–135. [PubMed] [Google Scholar]
- 36.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H939–H948. doi: 10.1152/ajpheart.00391.2007. doi:10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 37.Omura T, Yoshiyama M, Kim S, Matsumoto R, Nakamura Y, Izumi Y, et al. Involvement of apoptosis signal-regulating kinase-1 on angiotensin II-induced monocyte chemoattractant protein-1 expression. Arterioscler Thromb Vasc Biol. 2004;24:270–275. doi: 10.1161/01.ATV.0000112930.40564.89. doi:10.1161/01.ATV.0000112930.40564.89. [DOI] [PubMed] [Google Scholar]
- 38.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, et al. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. doi:10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 39.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. doi:10.1097/01.ASN.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 40.Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. doi:10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 41.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–H2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 42.Chung D. Nanoparticles have health benefits too. New Scientist. 2003;179:2410–2416. [Google Scholar]
- 43.Schubert D, Dargusch R, Raitano J, Chan SW. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun. 2006;342:86–91. doi: 10.1016/j.bbrc.2006.01.129. doi:10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 44.Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhotak S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. doi:10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh Y-H, Su I-J, Lei H-Y, Lai M-D, Chang W-W, Huang W. Differential endoplasmic reticulum stress signaling pathways mediated by iNOS. Biochem Biophys Res Commun. 2007;359:643–648. doi: 10.1016/j.bbrc.2007.05.154. doi:10.1016/j.bbrc.2007.05.154. [DOI] [PubMed] [Google Scholar]
- 46.Werstuck GH, Khan MI, Femia G, Kim AJ, Tedesco V, Trigatti B, et al. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 2006;55:93–101. doi:10.2337/diabetes.55.01.06.db05-0633. [PubMed] [Google Scholar]
- 47.Li Z, Zhauy T, Dai H, Liu G, Wang H, Sun Y, et al. Endoplasmic reticulum stress is involved in myocardial apoptosis of streptozocin-induced diabetic rats. J Endocrinol. 2008;196:565–572. doi: 10.1677/JOE-07-0230. doi:10.1677/JOE-07-0230. [DOI] [PubMed] [Google Scholar]
- 48.Younce CW, Kolattukudy PE. MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel zinc-finger protein, MCPIP. Biochem J. 426:43–53. doi: 10.1042/BJ20090976. doi:10.1042/BJ20090976. [DOI] [PubMed] [Google Scholar]
- 49.Martin DN, Baehrecke EH. Caspases function in autophagic programmed cell death in Drosophila. Development. 2004;131:275–284. doi: 10.1242/dev.00933. doi:10.1242/dev.00933. [DOI] [PubMed] [Google Scholar]
- 50.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. doi:10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.