Abstract
Aims
Toll-like receptor (TLR)-mediated signalling pathways have been implicated in myocardial ischaemia/reperfusion (I/R) injury. Activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway protects the myocardium from ischaemic injury. We hypothesized that the modulation of TLR2 would induce cardioprotection against I/R injury via activation of the PI3K/Akt signalling.
Methods and results
Mice were treated with TLR2 ligands, peptidoglycan (PGN) or Pam3CSK4, respectively, 1 h before the hearts were subjected to ischaemia (1 h), followed by reperfusion (4 h). Infarct size was determined by triphenyltetrazolium chloride staining. Cardiac function and haemodynamic performance were evaluated. Infarct size was significantly reduced in PGN- or Pam3CSK4-treated mice compared with untreated I/R mice. Administration of TLR2 ligands improved cardiac function following I/R. PGN treatment increased the levels of phospho-Akt and phospho-GSK-3β (glycogen synthase kinase-3β), compared with untreated I/R hearts. PGN stimulation increased TLR2 tyrosine phosphorylation and association of the p85 subunit of PI3K with TLR2. To investigate the role of PI3K/Akt signalling in PGN-induced cardioprotection, we administered the PI3K inhibitor, Wortmannin, to the mice 15 min before PGN treatment. We also administered PGN to kinase-deficient Akt (kdAkt) transgenic mice 1 h before myocardial I/R. Both PI3K inhibition and kdAkt mice abolished the cardioprotection induced by PGN. To examine the role of TLR2 in PGN-induced cardioprotection, we administrated PGN to TLR2 knockout mice 1 h before the hearts were subjected to I/R. PGN-induced cardioprotection was lost in TLR2-deficient mice.
Conclusion
These results demonstrate that TLR2 ligands induced cardioprotection, which is mediated through a TLR2/PI3K/Akt-dependent mechanism.
Keywords: Toll-like receptors, Myocardial ischaemia/reperfusion, Signalling pathways, PI3K/Akt signalling
1. Introduction
The innate immune system plays a central role in the pathophysiology of myocardial ischaemia/reperfusion (I/R) injury and heart failure1; however, the mechanisms are unknown. Furthermore, the physiological mechanisms that attempt to limit the inflammatory response, to promote survival of the myocardium, and to maintain homeostasis following cardiac I/R remain unclear.
Toll-like receptors (TLRs) are pattern recognition receptors that play an important role in the induction of innate immune and inflammatory responses.2,3 Recent evidence suggests that the TLR4-mediated NFκB activation pathway plays an important role in myocardial I/R injury.4–9 We and others have reported that modulation of the TLR4-mediated signalling pathway or TLR4 deficiency results in protection against myocardial I/R.6–9 TLR2 also plays a critical role in the induction of innate and inflammatory responses.2,3 However, the role of TLR2 in myocardial ischaemic injury is unclear. We have previously reported that glucan phosphate treatment protects the myocardium from I/R injury6 and attenuates cardiac dysfunction in polymicrobial sepsis.10 Glucan phosphate activates intracellular signalling through a Dectin-1/TLR2-dependent pathway.11 We have also reported that treatment of mice with Pam3CSK4, a specific TLR2 ligand, induces protection against cerebral ischaemic injury12 and attenuates cardiac dysfunction in septic mice.13 These observations suggest that the administration of TLR2 ligands may induce cardioprotection against I/R injury.
Phosphoinositide 3-kinases (PI3Ks) and their downstream target serine/threonine kinase Akt (also known as protein kinase B) are a conserved family of signal transduction enzymes, which are involved in regulating cellular activation, inflammatory responses, and apoptosis.14 Recent studies have identified cross-talk between TLR signalling and the PI3K/Akt pathway.15 We6,16 and others17,18 have reported that the PI3K/Akt signalling pathway may be an endogenous negative feedback regulator and/or compensatory mechanism that serves to limit proinflammatory and apoptotic events in response to injurious stimuli. We have shown that activation of the PI3K/Akt signalling pathway is associated with decreased myocardial ischaemic injury.6 Activation of PI3K/Akt-dependent signalling has been shown to prevent cardiac myocyte apoptosis and to protect the myocardium from I/R injury.19,20 Recent evidence suggested that Mal, an adaptor protein in the TLR-mediated signalling pathway, connects TLR2 to PI3K activation.21 Therefore, it is possible that the modulation of TLR2 will activate the PI3K/Akt signalling pathway, which will be responsible for cardioprotection.
In the present study, we examined the effect of TLR2 ligands, peptidoglycan (PGN) and Pam3CSK4, on myocardial I/R injury. We observed that administration of TLR2 ligands protects the myocardium against I/R injury and improves cardiac function and haemodynamic performance following I/R. However, the protection is lost in TLR2-deficient mice. Importantly, we observed that blocking the PI3K/Akt signalling pathway abolished the TLR2 ligand, PGN-induced cardioprotection. Our data suggest that modulation of TLR2-induced cardioprotection is mediated through a PI3K/Akt-dependent mechanism.
2. Methods
2.1. Experimental animals
TLR2 knockout (KO) mice (B6.129-TLR2tm1kir/J) and wild-type C57B6/L mice were purchased from Jackson Laboratory. Transgenic mice with cardiac-specific expression of kinase-defective Akt (kdAkt) with an FVB background have been described previously22 by Shioi and McMullen in the laboratory of Izumo, Beth Israel Deaconess Medical Center. Wild-type mice (FVB) were bred and maintained in the Division of Laboratory Animal Resources at East Tennessee State University (ETSU). All experiments were performed in accordance with the guidelines for the ‘Principles of Laboratory Animal Care’ and the ‘Guide for the Care and Use of Laboratory Animals’ published by NIH (NIH Publication No. 85-23, revised 1996). All aspects of the animal care and experimental protocols were approved by the ETSU Committee on Animal Care.
2.2. Induction of myocardial I/R injury
Myocardial I/R injury was induced as described previously.6,7,23 Briefly, male mice were anaesthetized by isoflurane inhalation before the left anterior descending coronary artery (LAD) was ligated with a 7-0 silk ligature over a 1 mm polyethylene tube (PE-10). After completion of 60 min of occlusion, the coronary artery was reperfused by pulling on the exteriorized suture to release the knot. After 4 h of reperfusion, the mice were sacrificed and the hearts were harvested.
2.3. Experimental protocols
To investigate the effects of TLR2 ligands on myocardial infarction, mice (n = 8 per group) were treated with or without PGN (50 µg/25 g body weight, Sigma-Aldrich, St Louis, MO, USA) or Pam3CSK4 (n = 7 per group, 50 µg/25 g body weight, InvivoGen, San Diego, CA, USA) by intraperitoneal (i.p.) injection 1 h before the hearts were subjected to I/R. The dose of PGN and PamsCSK4 was chosen according to our previous studies,12,13 in which a range of doses were tested initially.
To examine the effect of TLR2 ligand on cardiac function following myocardial I/R, Pam3CSK4 (50 µg/25 g body weight) was administered to the mice (5 per group) 1 h before myocardial I/R was induced. Untreated mice served as I/R control. Cardiac function was measured by echocardiography13 before myocardial I/R, 1 day, and 7 days after myocardial I/R.
We also evaluated haemodynamic performance using a Millar conductance catheter system (Millar Instruments Inc., Houston, TX, USA), as described previously.10,13 Mice (n = 4) were treated with or without PGN (50 µg/25 g body weight) 1 h before the hearts were subjected to ischaemia (60 min), followed by reperfusion for 3 days. Sham-operated mice that were treated with or without PGN (n = 4 per group) served as sham controls. Indices of systolic and diastolic cardiac performances were derived from left ventricular (LV) pressure–volume data obtained at the steady state.10,13
To examine whether TLR2 is essential for TLR2 ligand-induced cardioprotection, TLR2 KO mice (n = 6 per group) were treated with or without PGN (50 µg/25 g body weight) 1 h before hearts were subjected to LAD ligation.
To evaluate the effect of a TLR2 ligand on the activation of the PI3K/Akt signalling pathway, mice were treated with PGN (50 µg/25 g body weight) for 0, 5, 15, 30, and 60 min by i.v. injection, respectively. The hearts were harvested for the examination of Akt phosphorylation by Western blot. There were four mice at each time point.
To determine the role of the PI3K/Akt signalling pathway in TLR2 ligand-induced cardioprotection, mice (n = 6 per group) were injected with the PI3K inhibitor Wortmannin (i.p., 25 µg/25 g body weight, Sigma-Aldrich) 15 min before PGN administration. The dose of Wortmannin was chosen according to our previous studies.7,16 One hour after PGN treatment, mice were subjected to I/R. In a separate experiment, kdAkt transgenic mice (n = 6 per group) were treated with or without PGN 1 h before the hearts were subjected to I/R. Wild-type mice (FVB) served as a control. The hearts were harvested, and the infarct size was determined by triphenyltetrazolium chloride (TTC) staining, as described below.6,7,23
To examine the effect of PGN administration on activation of the PI3K/Akt signalling pathway, mice were treated with or without PGN (50 µg/25 g body weight) by i.p. injection 1 h prior to myocardial I/R. Sham-operated mice that were treated with or without PGN served as sham controls. The PI3K inhibitor Wortmannin (25 µg/25 g body weight) was administered for 15 min before the administration of PGN.7,16 There were eight groups: sham, sham + PGN, I/R, I/R + PGN, sham + Wort, I/R + Wort, sham + Wort + PGN, and I/R + Wort + PGN. There were six mice in each group.
2.4. Determination of myocardial infarct size
Infarct size was established by TTC (Sigma-Aldrich) staining, as described previously.6,7,23 Briefly, the hearts were removed and perfused with saline on a Langendorff system to wash blood from the coronary vasculature before staining with 1% Evans Blue. Each heart was then sliced horizontally to yield five slices. The slices were incubated in 1% TTC for 15 min at 37°C. Ratios of risk area (RA) vs. LV and infarct area (IA) vs. RA were calculated and expressed as a percentage.
2.5. In vitro experiments
The H9C2 rat cardiomyoblasts were obtained from the American Type Culture Collection (Rockville, MD, USA) and were maintained in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum under 5% CO2 at 37°C. When the cells reached 70–80% confluence, they were treated with PGN at a final concentration of 1 µg/mL for 0, 5, 15, 30, and 60 min with four replicates at each time point. The cells were harvested, and cellular proteins were isolated for the examination of TLR2 tyrosine phosphorylation and association with the PI3K p85 subunit by immunoprecipitation (IP) followed by immunoblots.6
2.6. Immunoprecipitation
Approximately 800 µg of cellular proteins was immunoprecipitated with 2 µg of antibodies to TLR2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at 4°C followed by the addition of 15 µL of protein A/G-agarose beads (Santa Cruz Biotechnology), as described previously.6 The precipitates were washed four times with lysis buffer and subjected to immunoblotting (IB) with the appropriate antibodies.
2.7. Western blot
Western blot was performed as described previously.6,7,23 Briefly, the cellular proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred onto Hybond ECL membranes (Amersham Pharmacia, Piscataway, NJ, USA). The ECL membranes were incubated with the appropriate primary antibody [anti-phospho-Akt (Ser473), anti-phospho-GSK3β (Ser9) (Cell Signaling Technology, Inc., Beverly, MA, USA), anti-GSK-3β, anti-Akt, and anti-PTyr20 (Santa Cruz Biotechnology, Inc.)], respectively, followed by incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Inc.). The signals were detected with the ECL system (Amersham Pharmacia).
2.8. Electrophoretic mobility shift assay
Nuclear proteins were isolated from heart samples as described previously.6,7,23 NFκB binding activity was examined by electrophoretic mobility shift assay (EMSA) in a 15 µL binding reaction mixture containing 15 µg of nuclear proteins and 35 fmol of [γ-32P] labelled double-stranded NFκB consensus oligonucleotide.
2.9. Immunohistochemistry
Immunohistochemistry was performed to examine NFκB nuclear translocation in the heart section using a specific antibody against the p50 (Santa Cruz Biotechnology, Inc.) subunit of NFκB, as described previously.7,10 Three slides from each block were evaluated.
2.10. Myeloperoxidase activity measurement
Myeloperoxidase (MPO) activity in the heart tissues was measured using a commercially available kit (Invitrogen). The measurement was performed according to the instructions of the manufacturer.
2.11. Statistical analysis
Data are expressed as mean ± SE. Comparisons of data between groups were made using one-way analysis of variance, and Tukey's procedure for multiple range tests was performed. P < 0.05 was considered significant.
3. Results
3.1. TLR2 ligands treatment attenuated myocardial injury following I/R
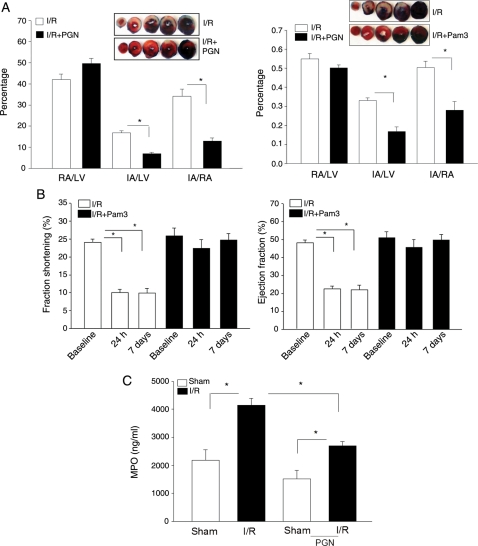
We have previously reported that glucan phosphate significantly reduced myocardial infarction following I/R.6 Glucan phosphate activates cellular signalling through Dectin-1/TLR2-dependent pathways11; therefore, we examined the role of the TLR2 ligands, PGN, and Pam3Sck4 on myocardial infarction. As shown in Figure 1A, treatment of mice with the TLR2 ligand significantly attenuated I/R-induced myocardial infarction. I/R induces a substantial (34.1 ± 3.42%) infarct size/area at risk (IA/RA, index for myocardial injury). However, infarct size in both PGN-treated mice (12.9 ± 1.45 vs. 34.1 ± 3.42%) and Pam3Sck4-treated mice (16.7 ± 2.53 vs. 33.0 ± 1.48%) was significantly smaller compared with the untreated I/R group. There is no significant difference in the RA/LV, which reflects the position of coronary artery ligation, between untreated and PGN-treated mice.
Figure 1.
(A) Administration of TLR2 ligands decreased myocardial infarct size following I/R injury. Mice were treated with and without PGN (n = 8 per group) or Pam3CSK4 (n = 7 per group) 1 h before the hearts were subjected to myocardial ischaemia (60 min) followed by reperfusion for 4 h. The hearts were harvested, and the infarct size was determined by TTC staining. The IA (white) and the area at risk (red + white) from each section were measured using an image analyser. Ratios of RA vs. LV and IA vs. RA were calculated and are presented in the graphs. Photographs of representative heart sections are shown above. *P < 0.01 compared with the untreated WT I/R group. RA, risk area; LV, left ventricular area; IA, infarct area; PGN, peptidoglycan; Pam3, Pam3CSK4. (B) Pam3CSK4 treatment improved cardiac function following myocardial I/R. Mice were treated with Pam3CSK4 (n = 4 per group) 1 h before the hearts were subjected to myocardial ischaemia (60 min) followed by reperfusion for 4 h. Cardiac function was examined by echocardiography before I/R (baseline), 1 day, and 7 days after I/R. *P < 0.01 compared with the baseline (before I/R). (C) PGN administration reduced MPO activity in the heart. Mice were treated with (n = 4) or without PGN (n = 4) 1 h before the hearts were subjected to ischaemia (60 min), followed by reperfusion for 3 days. Sham-operated mice served as sham controls (n = 3 per group). Hearts were harvested for the measurement of MPO activity. *P < 0.01 compared with indicated group.
3.2. TLR2 ligands improved cardiac function and haemodynamic performance following I/R
To examine the effect of a TLR2-specific ligand on cardiac function following I/R, we treated mice with Pam3CSK4 (50 µg/25 g body weight) 1 h before the mice were subjected to I/R. Cardiac function was examined by echocardiography before I/R and 1 and 7 days after I/R. As shown in Figure 1B, ejection fraction (EF) and fractional shortening (FS) were significantly reduced on day 1 (53.5 and 58.1%) and day 7 (54.4 and 58.8%) after I/R. Pam3CSK4 administration significantly attenuated I/R-induced cardiac dysfunction in untreated mice. EF and FS values in Pam3CSK4-treated I/R mice were maintained at or near control levels.
In addition, we evaluated haemodynamic performance in mice treated with or without the TLR2 ligand, PGN. Hearts were subjected to ischaemia (60 min) followed by reperfusion for 3 days. Haemodynamic measurements were performed using a Millar conductance catheter system.10,13 As shown in Table 1, I/R resulted in decreased haemodynamic performance as indicated by a reduction in dp/dtmax, stroke volume, EF, and cardiac output compared with the sham group. In PGN-treated mice, the haemodynamic parameters were improved significantly. The data suggest that the administration of TLR2 ligands significantly improved cardiac function following myocardial I/R.
Table 1.
PGN administration improved haemodynamic performance in the myocardium following I/R
| Groups | HR | SV | EF | CO | SW | dp/dtmax |
|---|---|---|---|---|---|---|
| Sham | 537 ± 25 | 24.1 ± 0.38 | 74.4 ± 0.51 | 12.9 ± 0.43 | 1792.7 ± 109.96 | 10 596 ± 715 |
| Sham + PGN | 555 ± 16 | 26.9 ± 6.42 | 75.5 ± 2.22 | 14.8 ± 3.14 | 1930.7 ± 364.58 | 10 181 ± 909 |
| I/R | 483 ± 14 | 18.3 ± 4.53a | 53.7 ± 4.22a | 8.7 ± 0.76a | 1271.8 ± 111.74a | 7477 ± 715a |
| I/R + PGN | 499 ± 13 | 32.5 ± 3.00b | 74.7 ± 3.10b | 15.8 ± 1.36b | 1741.3 ± 187.21 | 9691 ± 507b |
HR, heart rate; SV, stroke volume; EF, ejection fraction; CO, cardiac output; SW, stroke work.
aP < 0.05 compared with sham control.
bP < 0.05 compared with the I/R group.
3.3. PGN administration decreased neutrophil infiltration into the myocardium following I/R
Neutrophil infiltration during myocardial I/R plays an important role in causing cardiac dysfunction. MPO activity is an established marker of neutrophil infiltration into tissue. To examine the effect of PGN on neutrophil infiltration in the myocardium during I/R, we examined MPO activity in the heart tissues after 60 min of ischaemia, followed by 3 days of reperfusion. Figure 1C shows that I/R significantly increased MPO activity by 89% compared with the sham control. In the PGN-treated group, MPO activity was reduced significantly compared with untreated I/R hearts. MPO activity in PGN-treated I/R hearts was still higher than that of the PGN-treated sham control.
3.4. TLR2 deficiency abolished PGN-induced cardioprotection against I/R injury
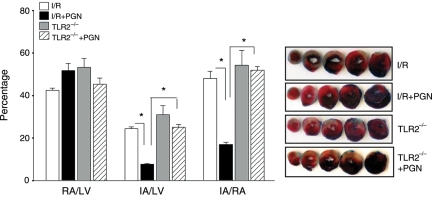
We examined the role of TLR2 in PGN-induced cardioprotection. TLR2-deficient (TLR2−/−) mice were treated with or without PGN 1 h before the hearts were subjected to ischaemia (60 min) followed by reperfusion (4 h). Myocardial infarction was determined by TTC staining. Figure 2 shows that IA/RA in TLR2−/− mice was similar to that of age-matched WT mice (54.2 ± 6.89 vs. 47.9 ± 3.41%). There was no significant difference in the myocardial infarct size between PGN-treated TLR2−/− mice and untreated TLR2−/− mice following I/R (51.9±1.74 vs. 54.2 ± 6.89%). There was also no significant difference in the myocardial infarct size between PGN-treated TLR2−/− mice and untreated WT mice that were subjected to I/R. However, the infarct size in PGN-treated TLR2−/− mice was significantly greater than that in PGN-treated WT mice (51.9 ± 1.74 vs. 16.9 ± 1.01%, P < 0.01, Figure 2). There was no significant difference in the RA/LV between TLR2−/− and WT mice.
Figure 2.
TLR2 deficiency abolished PGN-induced cardioprotection following myocardial I/R injury. TLR2-deficient (TLR2−/−) mice and age-matched WT mice were treated with and without PGN (n = 6 per group) 1 h before the hearts were subjected to myocardial ischaemia (60 min) followed by reperfusion for 4 h. Hearts were harvested, and the infarct size was determined by TTC staining. Ratios of RA vs. LV and IA vs. RA were calculated. Photographs of representative heart sections are shown to the right. *P < 0.01 compared with the untreated WT I/R group. RA, risk area; LV, left ventricular area; IA, infarct area.
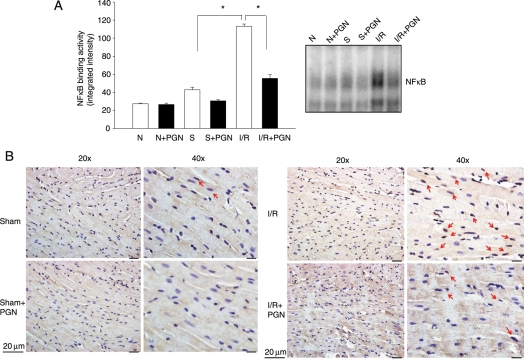
3.5. PGN administration decreased NFκB binding activity in the myocardium following I/R
NFκB activation plays an important role in myocardial I/R injury.4–6 We examined NFκB binding activity in the myocardium following I/R in the presence and absence of PGN. Figure 3A shows that NFκB binding activity is low in the hearts from normal and sham control mice. I/R significantly increased NFκB binding activity by 163.4% compared with sham control (113.2 ± 2.5 vs. 42.9 ± 2.7%). PGN administration did not alter NFκB binding activity in both normal and sham control hearts. However, PGN treatment significantly blunted the I/R-stimulated NFκB binding activity by 50.8% (55.7 ± 3.9 vs. 113.2 ± 2.5%). Figure 3B shows that I/R-induced NFκB nuclear translocation occurred in cardiac myocytes and endothelial cells. PGN administration reduced the NFκB nuclear translocation. These data are consistent with EMSA results.
Figure 3.
(A) PGN treatment attenuated I/R-increased NFκB activation in the myocardium following I/R. Mice were pre-treated with or without PGN 1 h before the hearts were subjected to ischaemia (60 min) and reperfusion (4 h) (n = 6 per group). Sham-operated mice served as sham controls (n = 6 per group). The hearts were harvested and cellular proteins were isolated. NFκB binding activity was determined by EMSA. *P < 0.05 compared with indicated groups. N, normal; S, sham surgery; I/R, ischaemia/reperfusion. (B) PGN treatment reduced NFκB nuclear translocation in the myocardium following I/R. Immunohistochemistry was performed with a specific antibody to the p50 subunit of NFκB. Red arrows indicate darker brown colour in the nucleus, which suggests NFκB nuclear translocation.
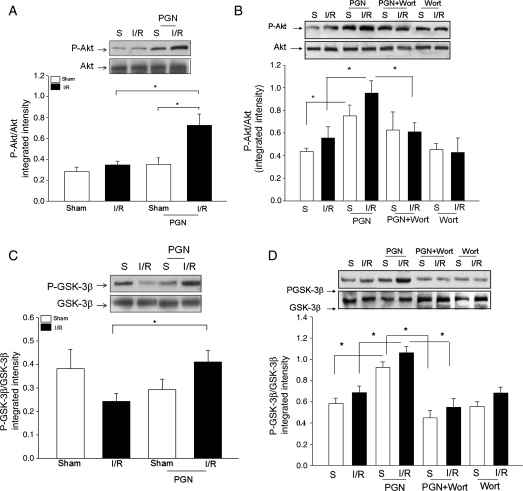
3.6. PGN treatment increased the levels of phospho-Akt and phospho-GSK3β in the myocardium following I/R
Activation of the PI3K/Akt signalling pathway has been shown to protect the myocardium from ischaemic injury6,20,23; therefore, we examined whether PGN treatment will induce the activation of PI3K/Akt signalling in the myocardium following I/R. First, we examined the effect of PGN treatment on Akt phosphorylation in hearts subjected to ischaemia (60 min), followed by reperfusion for 10 min. As shown in Figure 4A, PGN treatment significantly increased the levels of phospho-Akt/Akt in the myocardium compared with untreated I/R hearts. Next, we examined the effect of PGN on myocardial Akt phosphorylation after 4 h of reperfusion, following 60 min of ischaemia. Figure 4B shows that the levels of phospho-Akt in PGN-treated I/R hearts were significantly higher than those in untreated I/R hearts (0.95 ± 0.11 vs. 0.56 ± 0.10). In addition, PGN administration also increased significantly the levels of phospho-Akt in the myocardium in sham mice (0.75 ± 0.10), compared with untreated sham mice (0.44 ± 0.03). Akt is an important downstream target of PI3K.6,20,23 We examined the effect of blocking PI3K activity with Wortmannin on PGN-induced Akt phosphorylation in the myocardium. As shown in Figure 4B, administration of Wortmannin to mice did not affect the levels of phospho-Akt in the myocardium, which were subjected to I/R in the absence of PGN treatment. However, blocking PI3K activity with Wortmannin significantly attenuated PGN-induced Akt phosphorylation both in sham and in I/R hearts.
Figure 4.
PGN treatment increased the levels of phosphorylated Akt and phosphorylated GSK-3β in the myocardium. The increased phosphorylation of Akt and GSK3β was attenuated by PI3K inhibition. Mice were pre-treated with or without PGN 1 h before the hearts were subjected to ischaemia (60 min) and reperfusion for 10 min (A, n = 4 per group) and 4 h (n = 6 per group). Sham-operated mice served as sham controls (n = 4 per group for 10 min of reperfusion and n = 6 per group for 4 h of reperfusion). A PI3K inhibitor, Wortmannin, was also administered to the mice (n = 6 per group) treated with and without PGN. The hearts were harvested and cellular proteins were isolated. The levels of phospho-Akt (A and B) and phospho-GSK-3β (C and D) were examined by Western blot with specific antibodies. *P < 0.05 compared with indicated groups. S, sham; I/R, ischaemia/reperfusion; PGN, peptidoglycan; Wort, Wortmannin.
We also examined the levels of phospho-GSK-3β (Ser-9), which is an important kinase downstream of Akt24 in hearts, which were subjected to shorter (10 min) and longer (4 h) time of reperfusion following ischaemia (60 min). As shown in Figure 4C, PGN administration maintained phospho-GSK-3β/GSK-3β at the sham control level in the hearts subjected to 10 min of reperfusion, following 60 min of ischaemia. After 4 h of reperfusion following 60 min of ischaemia, the levels of phospho-GSK-3β/GSK-3β in the myocardium were significantly higher in the PGN-treated mice than those in the untreated I/R hearts (1.06 ± 0.06 vs. 0.68 ± 0.06) (Figure 4D). The levels of phospho-GSK-3β/GSK-3β in PGN-treated sham mice were also significantly higher than those of untreated sham control (0.93 ± 0.05 vs. 0.58 ± 0.05). Wortmannin administration significantly reduced PGN-increased levels of phospho-GSK-3β in the myocardium both in the sham (0.45 ± 0.07) and in the I/R (0.55 ± 0.08) mice.
3.7. PGN administration increased cardiac TLR2 tyrosine phosphorylation and TLR2 association with the p85 subunit of PI3K
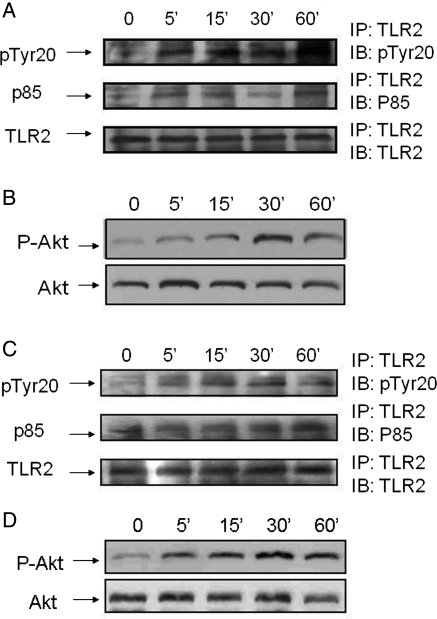
To examine the mechanisms by which PGN administration activates PI3K/Akt signalling, we examined the effect of PGN treatment on TLR2 tyrosine phosphorylation. We also determined whether the p85 regulatory subunit of PI3K will associate with TLR2 following PGN administration. Mice were treated with PGN for 0, 5, 15, 30, and 60 min, respectively, and the hearts were harvested. Cellular proteins were isolated from heart samples and subjected to IP with anti-TLR2 antibody followed by IB with the indicated antibodies. As shown in Figure 5A, PGN treatment rapidly increased TLR2 tyrosine phosphorylation. In addition, the levels of PI3K/p85 found in the TLR2 immunoprecipitates increased (Figure 5A). Figure 5B shows that PGN treatment increased the levels of cardiac phospho-Akt/Akt compared with untreated controls.
Figure 5.
PGN administration increased TLR2 phosphotyrosine levels and association of the p85 subunit of PI3K with TLR2. (A) Mice were treated with PGN for 0, 5, 15, 30, and 60 min, respectively. Hearts were harvested and cellular proteins were isolated for IP with a specific anti-TLR2 antibody and immunoblots using antibodies to p85 and PTyr20 (A) and for phospho-Akt and Akt (B). The immunoblot is representative of four hearts. (C and D)H9C2 cells were treated with PGN for 0, 5, 15, 30, and 60 min, respectively, and cellular proteins were isolated and examined for IP with a specific anti-TLR2 antibody followed by immunoblots using antibodies to p85 and PTyr20 (C) and for phospho-Akt/Akt (D). Each time point was repeated four times.
3.8. In vitro PGN administration induced the association between TLR2 and p85α of PI3K and increased Akt phosphorylation in cardiomyoblasts
We examined the effect of PGN treatment on TLR2 tyrosine phosphorylation and association with the p85 subunit of PI3K in cultured cardiomyocytes (H9C2). Cardiomyocytes were treated with PGN (1 µg/mL) for 0, 5, 15, 30, and 60 min, respectively. The cells were harvested, and cellular proteins were isolated. We performed IP with an anti-TLR2 antibody and immunoblot, which indicated antibodies on H9C2 cellular proteins. Figure 5C shows that PGN stimulation increased TLR2 tyrosine phosphorylation and enhanced TLR2 association with the p85 subunit of PI3K. We also examined the levels of phospho-Akt in the cells treated with and without PGN. As shown in Figure 5D, PGN administration significantly increased the levels of phospho-Akt in the cells. The data suggest that stimulation of TLR2 activated the PI3K/Akt signalling pathway through TLR2 tyrosine phosphorylation and association with the p85 subunit of PI3K.
3.9. Pharmacological inhibition of PI3K abrogated PGN-induced myocardial protection against I/R injury
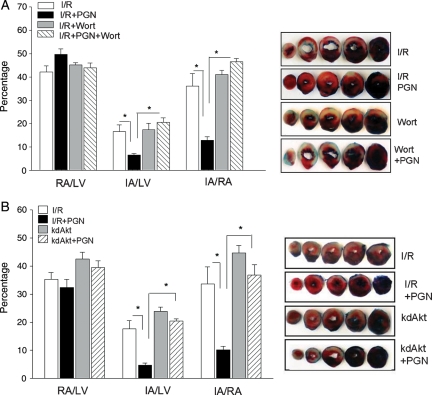
To examine whether increased activation of the PI3K/Akt pathway by PGN administration was responsible for the protection of the myocardium against I/R injury, we administered the PI3K inhibitor, Wortmannin, to PGN-treated mice. As shown in Figure 6A, pharmacological inhibition of PI3K with Wortmannin abrogated the cardioprotection observed in PGN-treated mice following I/R injury. The IA/RA was significantly greater in PGN + Wortmannin (46.5 ± 1.34%, P < 0.05) compared with PGN-treated mice that did not receive the inhibitor (12.9 ± 1.45%). Administration of Wortmannin alone did not significantly affect I/R-induced myocardial infarct size. The data suggest that PI3K inhibition abolished PGN-induced cardioprotection against I/R injury.
Figure 6.
Pharmacological inhibition of PI3K (A) and genetic deficiency of Akt (B) abrogate the cardioprotection induced by PGN administration. (A) Mice were pre-treated with and without PGN (n = 8 per group) 1 h before the hearts were subjected to myocardial ischaemia (60 min) followed by reperfusion for 4 h. In the pharmacological inhibition group, mice (n = 8 per group) were treated with PGN for 1 h and were injected i.p. with Wortmannin (25 µg/25 g body weight) 1 h prior to ischaemia (1 h) followed by reperfusion for 4 h. (B) kdAkt transgenic mice (n = 8 per group) were pre-treated with or without PGN for 1 h before the hearts were subjected to ischaemia (1 h) followed by reperfusion (4 h). The hearts were harvested, and the infarct size was determined by TTC staining. Ratios of RA/LV and IA vs. RA were calculated and are presented in the graph. *P < 0.01 compared with the indicated group. RA, risk area; LV, left ventricle; IA, infarct area; Wort, Wortmannin.
3.10. Genetic deficiency of Akt abrogated PGN-induced myocardial protection against I/R injury
Akt is an important downstream kinase that is phosphorylated and activated by PI3K. We examined the effect of Akt deficiency on PGN-induced cardioprotection against I/R injury using mice that express a kinase-deficient form of Akt (kdAkt). PGN was administered to kdAkt transgenic mice22 1 h before the hearts were subjected I/R. Age-matched WT mice served as a control. Figure 6B shows that PGN treatment induced cardioprotection in WT mice as we expected. However, PGN-induced cardioprotection was abolished in kdAkt mice. IA/RA in PGN-treated kdAkt mice was similar to that in WT I/R mice (Figure 6B). The data suggest that PGN-induced cardioprotection is mediated through Akt activation.
4. Discussion
A significant finding in the present study is that administration of the TLR2 ligands, PGN and Pam3CSK4, induced cardioprotection against I/R injury. Cardioprotection was lost in TLR2 deficiency, suggesting that the presence of TLR2 is required for the TLR2 ligand-induced cardioprotection. PGN administration significantly increased TLR2 tyrosine phosphorylation and the association of the p85 regulatory subunit of PI3K with TLR2, resulting in the activation of the PI3K/Akt pathway. Of greater significance, PI3K/Akt inhibition abolished TLR2 ligand-induced cardioprotection. Our results suggest that TLR2 ligands induce cardioprotection against I/R injury through a PI3K/Akt-dependent mechanism.
We have previously reported that glucan phosphate protected the myocardium from I/R injury6 and attenuated cardiac dysfunction in septic mice.10 Glucan phosphate acts through Dectin-1/TLR2-dependent signalling.11 Recently, we observed that administration of the TLR2 ligand, Pam3CSK4, significantly attenuated cardiac dysfunction in septic mice13 and cerebral ischaemic injury.12 These observations indicated that the modulation of TLR2-mediated signalling by its ligands may induce protection against deleterious challenges. In the present study, we observed that administration of TLR2 ligands, i.e. PGN and Pam3CSK4, resulted in significant protection against myocardial I/R injury and improved cardiac function following I/R. The protective effect induced by PGN was abolished in TLR2-deficient mice, suggesting that TLR2 ligand-induced cardioprotection is mediated through a TLR2-dependent mechanism.
The role of TLR2 in myocardial I/R injury is still controversial. Recent studies have shown that a TLR2 ligand, Pam3CSK4, can induce preconditioning to reduce myocardial infarct size25 and improve cardiac function following myocardial I/R.26 Sakata et al.27 reported that following in vitro I/R, the recovery of LV-developed pressure in wild-type mice was relatively lower than that observed in TLR2-deficient mice. However, the creatinine kinase levels were similar in both wild-type and TLR2-deficient mice, suggesting that zero-flow ischaemia resulted in similar infarct size in both groups.27 Favre et al.28 reported that knockout of TLR2 can induce protection against myocardial ischaemic injury. The observed differences may be caused by use of different models and different ischaemia and reperfusion time periods. We measured myocardial infarction after 60 min of ischaemia followed by reperfusion for 4 h, whereas Favre et al.28 used 30 min of ischaemia and 60 min of reperfusion.28
It has been well documented that innate immune and inflammatory responses are involved in the pathophysiological processes of myocardial ischaemic injury.1 The TLR-mediated NFκB signalling pathway plays a critical role in the induction of innate and immune responses2 and contributes to myocardial I/R injury.4–9 We observed in the present study that I/R significantly increased myocardial NFκB binding activity and nuclear translocation. Administration of the TLR2 ligand attenuated I/R-stimulated NFκB activation. Although the TLR4-mediated NFκB pathway contributes to myocardial ischaemic injury,4–9 recent evidence suggests that activation of the PI3K/Akt signalling pathway could negatively regulate TLR/NFκB-mediated innate and inflammatory responses.17,18,29 We and others have previously shown that activation of the PI3K/Akt pathway may be a negative feedback mechanism that prevents excessive innate immune and/or inflammatory responses during myocardial I/R injury and polymicrobial sepsis.6,18,23,29,30 Activation of PI3K/Akt-dependent signalling has been demonstrated to protect cardiac myocytes from I/R injury and to inhibit I/R-induced cardiac myocyte apoptosis.6,19,20,23 In the present study, we observed that PGN administration significantly increased the levels of myocardial phosphorylated Akt and reduced I/R-increased NFκB binding activity. It is possible, therefore, that activation of the myocardial PI3K/Akt signalling pathway in PGN-treated mice may be responsible for the cardioprotection against I/R injury. To evaluate this hypothesis, we administered the PI3K inhibitor, Wortmannin, to the mice prior to PGN administration. We observed that pharmacological inhibition of PI3K with Wortmannin abrogated PGN-induced cardioprotection against I/R injury. Akt is an important kinase downstream of PI3K.24 We also observed that cardioprotection induced by PGN administration was abolished in kdAkt transgenic mice. Thus, using both pharmacological and genetic approaches that inhibit PI3K and Akt, we demonstrated that PGN-induced cardioprotection is mediated through activation of the PI3K/Akt-dependent signalling pathway.
The cytosolic domain of TLR2 contains a PI3K binding motif (YXXM), which binds the phosphorylated form of the p85 subunit of PI3K.31 We observed that PGN administration significantly increased the levels of phosphorylated Akt in the myocardium, suggesting that the PI3K/Akt signalling pathway was activated. Recent evidence suggests that stimulation of TLRs leads to activation of the PI3K/Akt signalling pathway.6,15,31 For example, stimulation of TLR2 results in the recruitment of active Rac1 and PI3K to the TLR2 cytosolic domain, resulting in the activation of the PI3K/Akt pathway.31 Mal, an adaptor in TLR-mediated signalling, has been shown to connect TLR2 to PI3K activation.21 We observed in the present study that PGN administration significantly induced TLR2 tyrosine phosphorylation and increased the association of the p85 subunit of PI3K with TLR2. When considered together, these data indicate that PGN administration increases phosphorylation of TLR2 with subsequent recruitment of the p85 subunit of PI3K, which results in the activation of PI3K/Akt-dependent signalling.
In summary, our data indicate that TLR2 ligand administration induced cardioprotection. PGN-induced cardioprotection is mediated through activation of the PI3K/Akt signalling pathway. These data are significant because they demonstrate that TLR2 is essential for the induction of protection against myocardial I/R injury and that activation of the PI3K/Akt signalling pathway plays an important role in protecting the myocardium from I/R injury.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (HL071837 to C.L., GM083016 to C.L. and D.L.W., GM53552 to D.L.W.) and by AHA grant (09GRNT2020111 to R.L.K.).
References
- 1.Linde A, Mosier D, Blecha F, Melgarejo T. Innate immunity and inflammation—new frontiers in comparative cardiovascular pathology. Cardiovasc Res. 2007;73:26–36. doi: 10.1016/j.cardiores.2006.08.009. doi:10.1016/j.cardiores.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 3.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. doi:10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Browder W, Kao RL. Early activation of transcription factor NF-κB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–H552. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- 5.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element ‘decoy’ against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. doi:10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, et al. Modulating Toll-like receptor mediated signaling by (1–>3)-β-d-glucan rapidly induces cardioprotection. Cardiovasc Res. 2003;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. doi:10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, et al. Protection against myocardial ischemia/reperfusion injury in TLR4 deficient mice is mediated through a phosphoinositide 3-kinase dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 8.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, et al. Reduced myocardial ischemia–reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. doi:10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 9.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, et al. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004;128:170–179. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Ha T, Hua F, Grant D, Xia Y, Ma J, Gao X, et al. Glucan phosphate attenuates cardiac dysfunction and inhibits cardiac MIF expression and apoptosis in septic mice. Am J Physiol Heart Circ Physiol. 2006;291:H1910–H1918. doi: 10.1152/ajpheart.01264.2005. doi:10.1152/ajpheart.01264.2005. [DOI] [PubMed] [Google Scholar]
- 11.Brown GD, Herre J, Williams DL, Willment JA, Marshall ASJ, Gordon S. Dectin-1 mediates the biological effects of β-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. doi:10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, et al. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. doi:10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha T, Lu C, Liu L, Hua F, Hu Y, Kelley J, et al. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide-3-kinase dependent mechanism. Am J Physiol Heart Circ Physiol. 2010;298:H984–H991. doi: 10.1152/ajpheart.01109.2009. doi:10.1152/ajpheart.01109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. doi:10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 15.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. doi:10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 16.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, et al. Modulation of the phosphoinositide 3-Kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 17.Guha M, Mackman N. The PI3K-Akt pathway limits LPS activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. doi:10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 18.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. doi:10.1016/S1471-4906(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 19.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia–reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui T, Ling L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, et al. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 21.Sierra SS, Deshmukh SD, Kalnitski J, Kuenzi P, Wymann MP, Golenbock DT, et al. Mal connects TLR2 to PI3 kinase activation and phagocyte polarization. EMBO J. 2009;28:2018–2027. doi: 10.1038/emboj.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. doi:10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, et al. Lipopolysaccharide-induced myocardial protection against ischemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78:546–553. doi: 10.1093/cvr/cvn037. doi:10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 24.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. doi:10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mersmann J, Berkels R, Zacharowski P, Tran N, Koch A, Lekushi K, et al. Preconditioning by toll-like receptor 2 agonist Pam3CSK4 reduces CXCL1-dependent leukocyte recruitment in murine myocardial ischemia/reperfusion injury. Crit Care Med. 2010;38:903–909. doi: 10.1097/CCM.0b013e3181ce50e6. doi:10.1097/CCM.0b013e3181ce50e6. [DOI] [PubMed] [Google Scholar]
- 26.Dong JW, Vallejo JG, Tzeng HP, Thomas JA, Mann DL. Innate immunity mediates myocardial preconditioning through Toll-like receptor 2 and TIRAP-dependent signaling pathways. Am J Physiol Heart Circ Physiol. 2010;298:H1079–H1087. doi: 10.1152/ajpheart.00306.2009. doi:10.1152/ajpheart.00306.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakata Y, Dong J-W, Vallejo JG, Huang C-H, Baker JS, Tracey KJ, et al. Toll-like receptor 2 modulates left ventricular function following ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H503–H509. doi: 10.1152/ajpheart.00642.2006. doi:10.1152/ajpheart.00642.2006. [DOI] [PubMed] [Google Scholar]
- 28.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, et al. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–1071. doi: 10.1161/ATVBAHA.107.140723. doi:10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 29.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. doi:10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 30.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide-3-kinase signaling pathway alters host resistance to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. doi:10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- 31.Arbibe L, Mira J-P, Teusch N, Kline L, Guha M, Mackman N, et al. Toll-like receptor 2-mediated NF-κB activation requires a RAC I-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. doi:10.1038/82797. [DOI] [PubMed] [Google Scholar]