Abstract
Aims
Numerous lines of evidence suggest a role of oxidative stress in initiation and progression of heart failure. We identify novel pathways of oxidative stress in cardiomyocytes using proteomic technology.
Methods and results
Cardiomyocytes and cardiac fibroblasts isolated from rat hearts were treated with sublethal doses of H2O2 for detection of secreted protein factors in the conditioned media by mass spectrometry-based proteomics. Comparison between the two cell types leads to the finding that H2O2 caused an elevated cystatin C protein in the conditioned medium from cardiomyocytes. When cardiomyopathy was induced in mice by chronic administration of doxorubicin, elevated cystatin C protein was detected in the plasma. Myocardial ischaemia by left anterior descending coronary artery occlusion causes an increase in the level of cystatin C protein in the plasma. In myocardial tissue from the ischaemic area, an increase in cystatin C correlates with the inhibition of cathepsin B activity and accumulation of fibronectin and collagen I/III. Overexpressing cystatin C gene or exposing fibroblasts to cystatin C protein results in an inhibition of cathepsin B and accumulation of fibronectin and collagen I/III.
Conclusion
Oxidants induce elevated cystatin C production from CMCs. Cystatin C plays a role in cardiac extracellular matrix remodelling.
Keywords: Oxidative stress, Cardiomyocytes, Proteomics, Cysteine protease inhibitor, Heart failure
1. Introduction
Clinical, epidaemiologic, and biomedical studies have demonstrated an association of oxidative stress with the process of heart failure. Imperfect coupling of mitochondrial respiration produces reactive oxygen species (ROS) as a byproduct. Cardiomyocytes (CMCs), the main cell type of the myocardium, have the highest content of mitochondria among all cell types.1 About 40% cell volume is occupied by mitochondria, making CMCs most vulnerable to ROS generation.1 Oxidation and lipid peroxidation products have been detected in failing human hearts and in experimental models of heart failure.2–5 However, despite of well-documented association of oxidative stress and heart failure, how oxidative stress contributes to heart failure remains unclear.
Recent development in the proteomic technology provides an opportunity for the discovery of novel molecular pathways of oxidative stress. A shotgun approach of proteomics takes advantage of the separation capacity of liquid chromatography instrumentation. This approach can be quick and productive given a well-defined subproteome. Comparing to the whole proteome of a specific cell type, the subproteome of secreted proteins is less complex, allowing meaningful detection using the shotgun LC-MS/MS proteomics.6 Once a protein candidate is identified from in vitro cell culture systems, the study can be extended to in vivo models of heart failure. While the discovery of novel pathways expands our knowledge regarding the molecular mechanisms underlying the process of heart failure, those encoding the proteins ultimately being secreted by CMCs and circulated into the bloodstream may serve as non-invasive biomarkers of CMC injury.
The myocardium mainly contains two cell types: CMCs and fibroblasts. During the process of heart failure, a small proportion of CMCs undergo apoptosis, while the remaining CMCs develop hypertrophy and eventually deteriorate in contractile function. In contrast, cardiac fibroblasts (CFs) produce extracellular matrix (ECM) proteins and play a critical role in the alignment of CMCs and the texture of the myocardium. Myocardial fibrosis often occurs in association with heart failure and results from changes in ECM proteins secreted by fibroblasts. Since CMCs are responsible for the contractile function of the heart, proteins produced due to CMC injury become important in predicting the disease state and for studying the mechanism of heart failure.
2. Methods
2.1. Tissue culture
CMCs and CFs were isolated from 1–2-days-old Sprague–Dawley rats.7 At day 5 after seeding (2 × 106 cells per 100 mm dish), cells were treated with 300 µM H2O2 for 2 h and cultured in fresh DMEM containing 10% FBS for three additional days. Cells were placed in serum-free DMEM for 3 days for collecting conditioned medium.
2.2. Protein carbonyl assay
Protein carbonyls were measured using 4-dinitrophenylhydrazine in trichloroacetic acid-precipitated protein pellets according to manufacturer's instruction (Cayman Chemical Co).
2.3. ESI-LC-MS/MS analysis
Concentrated conditioned media was digested by trypsin for analyses using a quadrupole ion trap ThermoFinnigan LCQ DECA XP PLUS equipped with a Michrom Paradigm MS4 HPLC and a nanospray source. Peptides (7 µg) were loaded and eluted from a Vydac C18 capillary column using a gradient of 0–65% solvent (98% methanol/2% water/0.5% formic acid/0.01% triflouroacetic acid) over 60 mins at 350 nL/min. Dependent data scanning was performed with Xcalibur v1.3 software and Turbo SEQUEST™ v3.1 scores the matches using the criteria of Xcorr >2.5 for +2, 3.5 for +3, or 1.8 for +1 peptide ions, ΔXcorr >0.08, and the ratio of ions >50%.6 The spectra were searched against the non-redundant protein database downloaded from NCBI on 14 July 2005, when it contained 2 662 317 entries.
2.4. Western blot analysis
Proteins from conditioned media (20 µg), cell lysates (20 µg), animal plasma (100 µg), or heart tissue (40 µg) were separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE, 15%) for western blots using antibody against cystatin C, cathepsin (Upstate Biotechnology), atrial natriuretic peptide (ANP), GAPDH (Abcam), fibronectin, vinculin (Sigma-Aldrich), matrix metalloproteinase 2 (MMP-2, Chemicon), collagen I, or collagen III (Santa Cruz Biotechnology). Horseradish peroxidase-conjugated secondary antibodies (Zymed) were used for enhanced chemiluminescence reaction.6,7
2.5. DNA constructs and cell transfections
The full-length cDNA of cystatin C was cloned by RT–PCR using RNA extracted from rat CFs with the primer pair of 5′-TATCTCTCACAATTGGGTAAAAGC-3′ and 5′-GGATAAAACACTACAGGGTAGG-3′. The PCR product (460 kb fragment) was inserted between KpnI and XbaI sites in pcDNA4/HisMax© vector (Invitrogen). CFs were transfected using Fugene 6 (Roche) in serum-free DMEM medium. Cells were then incubated in the presence of pcDNA4-cystatin C construct for 24 h at 37°C in DMEM with 10% FBS followed by incubation in fresh 0.5% FBS/DMEM overnight before measurements. The transfection efficiency was about 10%.
2.6. RNA isolation and semiquantitative RT–PCR
Semiquantitative RT–PCR was performed using 2 µg of total RNA extracted with TriZol (Invitrogen) using primer sets designed by Primer 3 Input Program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3.cgi, Table 1) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control.
Table 1.
Sequence, expected fragment size, and annealing temperature of primers used in the semi-quantitative RT–PCR analysis of mRNA levels
| Gene | Sequence | Expected fragment size (bp) | Temp. (°C) | GenBank accession number |
|---|---|---|---|---|
| Cystatin C (rat) | Sense:tggtgagagctcgtaagcag | 208 | 62 | Rn.106351 |
| Antisense:gctggattttgtcagggtgt | ||||
| Cystatin C (mouse) | Sense:aaaggcacacactccctgac | 249 | 58 | Mm.4263 |
| Antisense:cctgcagcagctcctttact | ||||
| ANP (mouse) | Sense:gtgtacagtgcggtgtccaa | 153 | 62 | Mm.19961 |
| Antisense:acctcatcttctaccggcatc | ||||
| CollagenI | Sense:tgctgccttttctgttcctt | 179 | 56 | Rn.2953 |
| Antisense:aaggtgctgggtagggaagt | ||||
| CollagenIII | Sense:gtccacgaggtgacaaaggt | 189 | 56 | Rn.3247 |
| Antisense:catcttttccaggaggtcca | ||||
| Fibronectin | Sense:gaaaggcaaccagcagagtc | 230 | 56 | Rn.1604 |
| Antisense:ctggagtcaagccagacaca | ||||
| GAPDH (rat) | Sense:tgaaggtcggtgtcaacggatttggc | 223 | 62 | Rn.64496 |
| Antisense:catgtaggccatgaggtccaccac | ||||
| GAPDH (mouse) | Sense:cctgcaccaccaactgctta | 222 | 62 | Mm.379644 |
| Antisense:tcatgagcccttccacaatg |
2.7. Animal experiments
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Experimental protocols were approved by University of Arizona Institutional Animal Care and Use Committee. Male BL6/129SF1 mice (5–7 weeks old, 18–22 g, the Jackson's Laboratory) were treated with doxorubicin (Dox) (i.p.) twice a week for a total of 10 injections at 4 mg/kg per injection.6 After the first four injections, animals were not injected for 2 weeks to allow recovery of bone marrow depression.8 Cardiac haemodynamic measurements show cardiomyopathy 2 weeks after the final Dox injection.8 The blood was collected via the abdominal vena cava.6
The surgical procedure of left anterior descending (LAD) coronary artery occlusion was performed using male BL6/129SF1 mice aged 7–8 weeks.9,10 An anterior thoracotomy was performed after induction of anaesthesia with 2.5% Avertin. Upon exposure of the heart, an 8-0 silk suture was tightened rapidly around the proximal LAD (1–3 mm from the tip of the left atrium). At 24 h after occlusion, the hearts were perfused with 2% Evans Blue for 5 min via the ascending aorta for separation of non-ischaemic vs. ischaemic area.
2.8. Cathepsin B (CTB) activity assay
Cathepsin B (CTB) activity was measured using Z-Arg-Arg-AMC as a substrate per instruction of the manufacturer (EMD Biosciences).
2.9. Zymography
Cell lysates (50 µg protein) or conditioned medium (2 µg protein) was loaded onto a 10% acrylamide gel contained 0.5 mg/mL gelatin. Following electrophoresis for 3 h at 25 mA at 4°C, the gels were soaked in 2.5% Triton X-100 before incubation in reaction buffer (50 mM Tris–HCl, pH 7.6, 0.2 M NaCl, 5 mM CaCl2, 0.02% Brij-35) for 15 h at 37°C. The gel was stained with 0.05% Coomassie Blue for the detection of negative staining due to enzymatic digestion of gelatin.
2.10. Statistics
The student's t-test was used for comparisons of means from control vs. treated group. One-way analysis of variance was used to compare groups of means followed by the Bonferroni Correction for multiple samples using Stata 8.2 software.
3. Results
3.1. Identification of cystatin C in conditioned media of CMCs
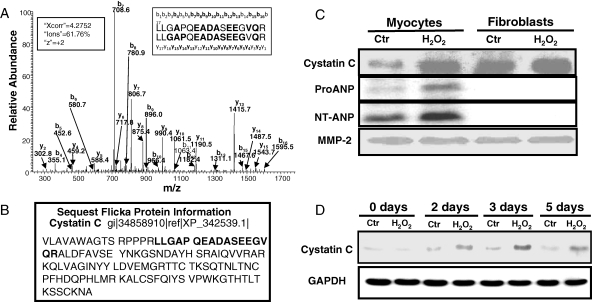
H2O2 causes oxidative stress in CMCs or CFs within 2 h time frame of treatment as measured by increases in protein carbonyls (Table 2). Cells were able to recover from protein oxidation after 2 days (Table 2). A fraction of cells die off within 24–72 h, whereas the majority of CMCs survive and develop hypertrophy.11 Conditioned media were collected after 3 days, when protein carbonyl levels returned to the basal level and when only surviving cells remained as measured by morphology and trypan blue exclusion, for identifying protein factors secreted by CMCs or CFs due to H2O2 treatment. Seven proteins were found among all groups: Biglycan, Collagen alpha 1 (III), Collagen alpha 2 (V), MMP2, Fibronectin 1, Osteonectin, and Procollagen, type I alpha 2. A +2 peptide ion LLGAPOEADASEEGVQR of cystatin C was found in H2O2 treated CMCs (Xcorr = 4.28) but not control CMCs (Figure 1A and B). For CFs, cystatin C was present in both control (Xcorr = 4.81, +2 ion, LLGAPQEADASEEGVQR) and H2O2 treated group (Xcorr = 2.23, +1 ion, ALDFAVSEYNK, and Xcorr = 4.67, +2 ion, LLGAPQEADASEEGVQR).
Table 2.
Time course of protein oxidation
| Day | Cardiomyocytes |
Fibroblasts |
||
|---|---|---|---|---|
| Ctr | Hp | Ctr | Hp | |
| 0 | 1.65 ± 0.39 | 2.90 ± 0.37* | 1.64 ± 0.27 | 2.55 ± 0.19* |
| 1 | 1.67 ± 0.43 | 2.15 ± 0.51 | 1.71 ± 0.03 | 1.88 ± 0.08 |
| 2 | 1.46 ± 0.13 | 1.58 ± 0.46 | 1.73 ± 0.004 | 1.60 ± 0.11 |
| 3 | 1.45 ± 0.11 | 1.00 ± 0.12 | 1.59 ± 0.25 | 1.42 ± 0.36 |
CMCs and CFs in 100 mm dishes were treated with 300 µM H2O2 for 2 h. Cells were harvested immediately (day 0) or were cultured in fresh DMEM containing 10% FBS for measurements of protein carbonyl. The data represent means ± standard deviation from one experiment representative of three.
*P < 0.05 when means from H2O2 treated samples are compared with that of control using Student's t-test.
Figure 1.
H2O2 treatment of cardiomyocytes causes elevation of cystatin C in conditioned medium. CMCs and CFs in 100 mm dishes were treated with 300 µM H2O2 for 2 h. Cells were placed in fresh DMEM containing 10% FBS for 3 days before being placed in DMEM with 0% FBS for additional 3 days to collect the conditioned medium for ESI-LC-MS/MS (A and B) or for western blot analyses (C) to detect cystatin C (20 µg protein/lane), or ProANP and NT-ANP (100 µg protein/lane) with the loading control MMP-2. In the MS/MS spectrum of cystatin C peptide detected from the conditioned media of H2O2-treated CMCs, bolded letters indicate the detected b and y ions matching the predicted ion mass in sequence database (A). SEQUEST Flicka information page shows the fragment of cystatin C detected by LC-MS/MS (B). Cell lysates were harvested from CMCs in 6-well plates (0.5 × 106 cells/well) following 300 µM of H2O2 treatment for 2 h and were then cultured in fresh DMEM containing 10% FBS for indicated time for detection of cystatin C (20 µg/lane) with GAPDH as a loading control (D).
To verify cystatin C induction by H2O2 treatment in CMCs, conditioned media were collected for western blot analyses. MMP-2 was used as a loading control.6 Consistent with the finding from LC-MS/MS analyses, CFs contain a higher basal level of cystatin C compared with CMCs, but the level of cystatin C did not change with H2O2 treatment (Figure 1C). In contrast, elevated cystatin C protein was detected in the conditioned medium of H2O2 treated CMCs (Figure 1C). With cell lysates, an increased cystatin C protein was detected 2 days after H2O2 treatment (Figure 1D).
ANP has been used as a biomarker of heart failure in many experimental models. ANP, a 28-amino acid peptide, is derived from a 126-amino acid pro hormone (ProANP) due to the cleavage by a serine protease.12 The protease also generates a 98-amino acid N-terminal peptide (NT-ANP). Levels of ProANP and NT-ANP were measured for the purpose of comparison with the cystatin C data. With 20 µg of proteins from the conditioned medium, we could detect elevation of cystatin C but not ANP in its Pro- or NT-form (data not shown). With 100 µg proteins of conditioned medium, there is a significant elevation of ProANP and NT-ANP in the conditioned medium of H2O2 treated CMCs (Figure 1C).
3.2. Serum cystatin C elevation following cardiac injury
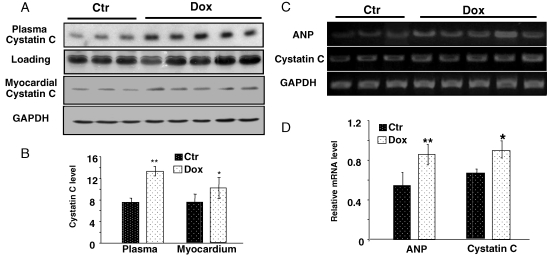
Two animal models were used to address whether cystatin C elevates in association with cardiac injury: Dox-induced cardiomyopathy and LAD coronary artery occlusion-induced myocardial ischaemia, both models involve oxidative stress. The protocol of chronic treatment of Dox induces cardiomyopathy.8 Measurements of alanine transaminase (ALT), aspartate aminotransferase (AST) and bilirubin for the liver function, or blood urea nitrogen (BUN) and creatinine for kidney function confirmed no liver or kidney injury (Table 3). However, we cannot exclude the possibility that Dox causes toxicity in other organs or systems. Regardless, western blot analyses indicate a clear elevation of cystatin C in the plasma of Dox-treated mice (Figure 2A and B). RT–PCR and western blot analyses using heart tissue samples show elevated levels of cystatin C mRNA and protein in animals treated with Dox (Figure 2A–D), although the changes were less dramatic than that of the plasma samples. Elevated levels of ANP mRNA were detected in parallel with cystatin C mRNA in the cardiac tissue from Dox-treated mice (Figure 2C and D). ProANP or NT-ANP protein was not detected in the myocardium or the plasma by western blot, likely due to the low abundance or short half-life of the protein. To demonstrate the specificity of cystatin C induction in the heart, the brain, liver, and kidney tissues were collected for western blot analyses. The assays did not detect elevation of cystatin C protein in these tissues from Dox-treated mice (data not shown).
Table 3.
Laboratory values after doxorubicin treatment in mice
| Ctr (n = 3) | Dox (n = 5) | |
|---|---|---|
| ALT (17–77 U/L) | 9.87 ± 12.69 | 2.83 ± 2.67 |
| AST (54–298 U/L) | 34.03 ± 16.51 | 32.43 ± 7.13 |
| Bilirubin (0–0.9 mg/dL) | 0.23 ± 0.15 | 0.17 ± 0.21 |
| BUN (8–33 mg/dL) | 15.7 ± 2.12 | 10.63 ± 1.37 |
| Creatinine (0.2–0.9 mg/dL) | 0.15 ± 0.07 | 0.08 ± 0.05 |
The blood was collected at the end of 10 weeks Dox treatment for measurements of liver and kidney function biomarkers by the Pathological Service Core at University of Arizona animal care facility. The data are presented as means ± SD.
Figure 2.
Dox induced elevation of cystatin C in the plasma and the myocardium. The plasma of male BL6/129SF1J mice was collected 2 weeks after final Dox injection for measurements of cystatin C protein levels (A and B). An area of Coomassie blue-stained gel corresponding to the molecular weight (Mw) of cystatin C was included to show equal plasma protein loading (A). The hearts were collected to detect levels of cystatin C protein by western blot (C and D), or cystatin C or ANP mRNA by RT–PCR (E and F) with GAPDH as a loading control (C and E). The intensities of the bands were quantified using NIH imaging J software and are presented as means ± SE (B, D, F, G). An asterisk (*) indicates P < 0.05 while double asterisks (**) indicate P < 0.01 when means from Dox treated samples are compared with that of control using Student's t-test.
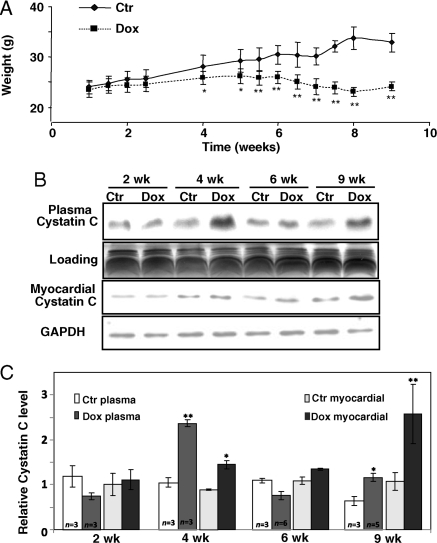
Since cardiomyopathy typically develops within 10 weeks after the first injection of Dox, this well-defined time frame allows us to address whether elevated cystatin C can be detected earlier in the time course of cardiomyopathy development. The plasma was collected at 2, 4, 6, and 9 weeks from vehicle and Dox-treated mice for western blot analyses to detect cystatin C. Dox treatment causes decreases in animal body weight starting at the fourth week, when the animals show no visible change in locomotion activity. The body weight decreased further in a time-dependent manner as Dox-treated animals progressing towards cardiomyopathy (Figure 3A). Western blot analyses indicate a clear elevation of cystatin C in the plasma of mice at 4 and 9 weeks (Figure 3B and C). An increase of cystatin C was observed starting from fourth week and was profound at ninth week in the homogenates of heart tissue (Figure 3B and C). These results indicate that cystatin C elevation can be detected early in the time course of heart failure.
Figure 3.
Time-dependent induction of cystatin C in the plasma of Dox-treated mice. Male BL6/129SF1J mice treated with Dox were weighted at indicated time points (A) with the plasma (B) and whole heart tissues (C) collected at second, fourth, sixth, and ninth weeks for western blot analyses of cystatin C protein levels (100 µg protein/lane). An area of Coomassie blue staining corresponding to cystatin C Mw was shown to indicate equal plasma protein loading (B). GAPDH was used as an internal loading control for the myocardial tissues (C). The intensities of the bands were quantified using NIH imaging J software and are presented as means ± SD from indicated number (C). An asterisk (*) indicates P < 0.05 while double asterisks (**) indicates P<0.01 when means from Dox-treated samples are compared with that of control using Student's t-test.
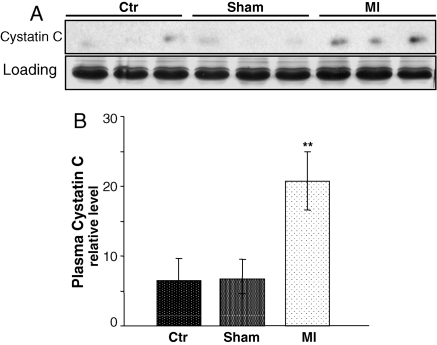
With LAD coronary artery ligation surgery, the plasma was collected from control, sham operated, and myocardial infracted mice at 7 days after the surgery for western blot analyses. Compared with the control or sham-operated mice, there is a significant elevation of cystatin C protein in the plasma of MI mice (Figure 4). An increase of cystatin C protein was also observed in the ischaemic area compared with normal area of the myocardium (Figure 5A).
Figure 4.
Elevation of cystatin C in the plasma of mice with regional ischaemia. Male BL6/129SF1J mice were used for LAD coronary artery occlusion surgery. The plasma collected seventh day after the surgery was used for western blot analyses to detect cystatin C (100 µg protein/lane, A). An area of Coomassie blue staining corresponding to cystatin C Mw was included to show equal plasma protein loading (A). The intensities of the bands quantified by NIH imaging J software are presented as means ± SE (B). Double asterisks (**) indicate P < 0.01 when the means of mice with myocardial ischaemia were compared with that of sham-operated or control animals by Student's t-test.
Figure 5.
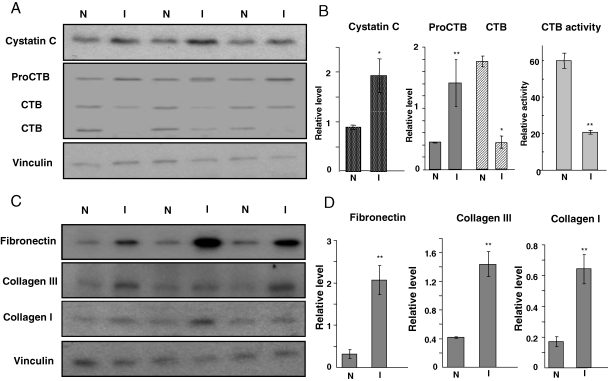
Cystatin C, cathepsin B, and ECM proteins in the ischaemic area of the myocardium. At 24 h after LAD coronary artery occlusion surgery, the non-ischaemic (N) and ischaemic area (I) were separated for western blot analyses (60 µg protein/lane) to detect cystatin C, cathepsin B (CTB, A and B), collagen III, collagen I, and fibronectin (C and D) with vinculin as a loading control. The intensities of the bands quantified by NIH imaging J software were normalized to that of vinculin and presented as means ± SE (B and D). Cathepsin B activity was measured using the substrate of Z-Arg-Arg-AMC (B). Double asterisks (**) indicate P < 0.01, and an asterisk (*) indicates P < 0.05 when the means from the ischaemic area was compared with that of non-ischaemic area by Student's t-test.
3.3. Cystatin C, cathepsin B, and ECM proteins
Cystatin C, an inhibitor of cysteine protease, preferentially binds and inhibits CTB, which can be secreted in the extracellular space in addition to its intracellular lysosomal location.13,14 The pro-CTB is a 45 kDa protein that can be processed to active forms of 36 and 25 kDa. CTB has been shown to participate in ECM remodelling and cleavage of ECM.13,14 In the myocardium, the major forms of ECM proteins include collagen I, collagen III, and fibronectin, which are produced mainly by fibroblasts.15 With LAD coronary artery induced regional ischaemia in the myocardium, corresponding to an accumulation of cystatin C protein are decreases of active forms of CTB, increases in the proenzyme, and suppression of CTB activity (Figure 5A and B). Measurements of collagen I, collagen III, and fibronectin indicate increases in the ischaemic area (Figure 5C and D).
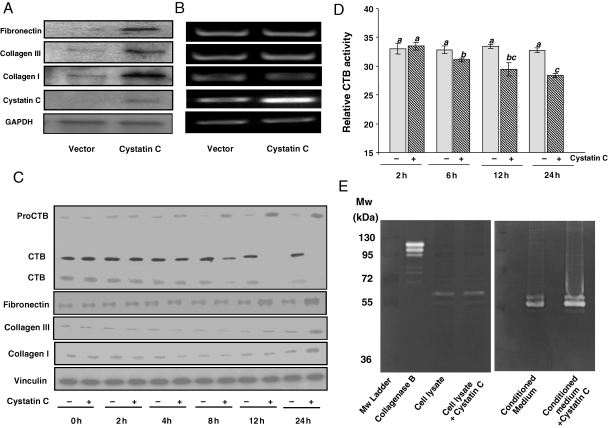
To verify the effect of cystatin C on cardiac ECM remodelling, we overexpressed cystatin C gene in CFs. Cell number counting indicates that cystatin C overexpression does not affect cell growth. Western blot analyses indicate that cystatin C overexpression caused accumulation of collagen I, collagen III, and fibronectin (Figure 6A). RT–PCR analyses were carried out to eliminate such accumulation resulting from transcriptional activation of these genes (Figure 6B).
Figure 6.
Cystatin C modulates ECM proteins. CFs were seeded in 6-well plates (Falcon) at a density of 0.3 × 106 cells/well for 24 h transfection with 0.5 µg/well pcDNA4-cystatin C construct. At 24 h after transfection, the cells were harvested for western blot analyses (20 µg protein/lane) with vinculin as a loading control (A) or RT–PCR with GAPDH as an internal control (B). Without transfection, CFs were starved 3 days after seeding overnight in 0.5% FBS/DMEM before treatment with 5 nM cystatin C for indicated time (C and D) or 24 h (E). Cells (C–E) or conditioned medium (E) were harvested for western blot analyses with vinculin as a loading control (C), for cathepsin B activity assay (D) or zymography (E). The means that is not significantly different from another is labelled with a common letter symbol. The means in the ‘a’ group are significantly different from means in the ‘b’ or ‘c’ group.
To confirm that cystatin C indeed plays a role in ECM remodelling, we treated CFs with purified cystatin C protein. The western blot results indicate that cystatin C treatment caused inhibition of CTB activity and prevented the conversion of the proenzyme to active form (Figure 6C). CTB activity measurements confirmed time-dependent inhibition by cystatin C (Figure 6D). Cystatin C treatment caused elevation of collagen I, collagen III, and fibronection proteins (Figure 6C). Since collagens and fibronectin can be cleaved by MMPs, we excluded the possibility that cystatin C inhibited the activity of MMPs by zymography. CF cell lysates show MMP activity around molecular weight (Mw) of 63 kDa, corresponding to MMP-2. The conditioned medium showed MMP activity corresponding to the Mw for MMP-2, MMP-3, MMP-9, or MMP-13, which is 63, 55, 92, or 43 kDa, respectively. The MMP activities from cell lysate or conditioned medium were not affected by cystatin C treatment (Figure 6E). These data suggest that cystatin C regulates CTB activity and subsequent accumulation of ECM proteins.
4. Discussion
LC-MS/MS-based shotgun proteomics has lead to the discovery of cystatin C in the conditioned media of CMCs treated with H2O2. With in vivo models of heart failure, such as Dox treatment-induced cardiomyopathy or coronary artery ligation induced myocardial ischaemia, elevated levels of cystatin C was detected in the plasma. Elevation of cystatin C correlates with an inhibition of CTB, accumulation of collagen I, collagen III, and fibronectin in the ischaemic area of the myocardium. Overexpression of cystatin C gene or treating fibroblasts with purified cystatin C protein leads to inhibition of CTB activity and accumulation of the ECM proteins. These data suggest that while circulating cystatin C may serve as a possible biomarker of oxidative injury in association of heart failure, functionally elevated cystatin C results in inhibition of CTB. Since CTB can cleave ECM proteins, the net outcome of cystatin C elevation is accumulation of ECM proteins in the myocardium.
Cystatin C is a low molecular weight alkaline protein (Mw 13 359 Da) belonging to a family of cysteine protease inhibitors. As a highly abundant inhibitor of cysteine proteases, cystatin C has been found in most cell types, including cardiac muscle fibres.16,17 This protein is typically secreted by cells and ends up in various biological fluids including urine, blood, saliva, seminal fluid, and cerebrospinal fluid.18 At the cellular level, cystatin C regulates the activity of lysosomal cysteine proteases, such as cathepsins B, H, and L.19,20 Like cystatin C, CTB can be secreted into the extracellular space. CTB can degrade ECM protein directly, or indirectly by activating urokinase plasminogen activator (uPA) or MMPs such as collagenase I.14 Our data with zymography have excluded the possibility of cystatin C affects MMPs, leaving the possibility that CTB either directly degrades collagen I, collagen III, and fibronectin or indirectly through activation of uPA. Regardless, cystatin C can bind tightly to CTB to inhibit its activity. An imbalance between cysteine proteases and cystatin C has been shown in human atherosclerotic and aneurismal aortic lesions.21,22 Decreased cystatin C expression in general is associated with an increased incidence of atherosclerosis and the severity of cardiovascular disease.21,23,24 The observed increase of cystatin C due to regional myocardial ischaemia indicates a role in protection of ECM from being cleaved by CTB and perhaps other cysteine proteases. Fibrosis and associated increases of collagen I/III is a common phenomenon of failing hearts. Our finding suggests that cystatin C can be a molecular target for blocking the detrimental effect ECM remodelling.
Our data suggest that cystatin C produced by CMCs may serve as a possible biomarker of heart failure. ANP or BNP currently is used as a diagnostic biomarker of heart failure. Like ANP, cystatin C in the blood is usually filtered through the glomeruli, being absorbed in the proximal tubule of the kidney. Dysfunction of the proximal tubule results in increases in the level of cystatin C in the circulating system. While several recent studies indicate that serum cystatin C is superior to creatinine as an indicator of kidney failure involving defective glomerular filtration,25 elevated levels of serum cystatin C have been found in heart failure patients independent of renal dysfunction.26–28 A clinical study from Japan has shown that heart failure patients have significantly higher levels of cystatin C in the circulating system.29 Sarnak et al.30 reported that high cystatin C concentration in the plasma imposes a risk factor of heart failure in individuals older than 65 years of age. In the USA, a recent clinical study with 139 subjects indicates the correlation of cystatin C elevation with advanced left ventricular diastolic dysfunction and right ventricular systolic dysfunction.28 Three additional recent reports have demonstrated the association of elevated serum cystatin C with the risk of heart failure.26,27,31 These lines of evidence support our postulation that cystatin C can be used as a biomarker of heart failure. Practically, a simple solution for using cystatin C as a diagnostic tool for heart failure is to include a renal failure specific marker, such as creatinine, to differentiate heart failure from renal failure.
At present, the natriuretic peptides are the only FDA-approved biomarkers for biochemical diagnosis of heart failure. ANP and BNP are secreted from ventricular CMCs when the heart progresses into failure with various types of inducers, e.g. hypertension and aortic stenosis. However, due to rapid degradation by proteases in the plasma, the half life of ANP or BNP is about 3 or 20 min, respectively.32,33 In addition to a short half life, the serum level of BNP is ≤100 pg/mL for normal individuals. In contrast, the concentration of serum cystatin C for a normal person is in the range of 800–1200 pg/mL,19 about 10 times higher than that of BNP. More importantly, cystatin C is stable for at least 6 months when serum samples are kept at −80°C.19,34 Finney et al.34 found that the blood can be left untreated up to 24 h without affecting the detection of cystatin C. In our experiments, detection of ProANP or NT-ANP by SDS–PAGE and western blot requires five times more proteins from conditioned medium (100 µg protein for Pro or NT-ANP) than detection of cystatin C (15–20 µg protein) by the same method. With the serum samples that show elevated cystatin C from Dox treated or myocardial infracted animals, ProANP or NT-ANP could not be detected by western blot, indicating a low level or rapid degradation of these proteins in the plasma. These lines of evidence can be translated to the speculation of using cystatin C as a quick, non-invasive, and minimal blood requiring biochemical measurement of heart failure.
Funding
Work from our laboratory has been supported by US National Institute of Health R01s ES 10826, HL 076530, HL 089958, Arizona Biomedical Research Commission, and Colgate Palmolive Award for Alternative Research (Q.M.C.). G.T. and the Proteomics Facility Core are supported by Southwest Environmental Health Sciences Center P30 ES06694.
Acknowledgements
We thank Thai Nho Dinh for performing Zymography experiments. B.X. is a recipient of Mark and Mary Anne Fay Investigator Award from the University of Arizona Sarver Heart Center.
Conflict of interest: none declared.
References
- 1.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. doi:10.1016/0022-2828(92)93381-S. [DOI] [PubMed] [Google Scholar]
- 2.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. doi:10.1016/S0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Dhalla AK, Seneviratne C, Singal PK. Oxidative stress and heart failure. Mol Cell Biochem. 1995;147:77–81. doi: 10.1007/BF00944786. doi:10.1007/BF00944786. [DOI] [PubMed] [Google Scholar]
- 4.Sawyer DB, Colucci WS. Mitochondrial oxidative stress in heart failure: ‘oxygen wastage’ revisited. Circ Res. 2000;86:119–120. doi: 10.1161/01.res.86.2.119. [DOI] [PubMed] [Google Scholar]
- 5.Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid Redox Signal. 2006;8:2111–2124. doi: 10.1089/ars.2006.8.2111. doi:10.1089/ars.2006.8.2111. [DOI] [PubMed] [Google Scholar]
- 6.Xie L, Tsaprailis G, Chen QM. Proteomic identification of insulin-like growth factor binding protein-6 induced by sublethal H2O2 stress from human diploid fibroblasts. Mol Cell Proteomics. 2005;4:1861–1873. doi: 10.1074/mcp.M500032-MCP200. [DOI] [PubMed] [Google Scholar]
- 7.Purdom S, Chen QM. Epidermal growth factor receptor-dependent and -independent pathways in hydrogen peroxide-induced mitogen-activated protein kinase activation in cardiomyocytes and heart fibroblasts. J Pharmacol Exp Ther. 2005;312:1179–1186. doi: 10.1124/jpet.104.077057. doi:10.1124/jpet.104.077057. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, Zhou Z, Kang YJ. Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Res. 2001;61:3382–3387. [PubMed] [Google Scholar]
- 9.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, et al. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995;269:H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 10.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 2004;16:349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Tu V, Wu Y, Bahl J. Hydrogen peroxide dose dependent induction of cell death or hypertrophy in cardiomyocytes. Arch Biochem Biophys. 2000;373:242–248. doi: 10.1006/abbi.1999.1558. doi:10.1006/abbi.1999.1558. [DOI] [PubMed] [Google Scholar]
- 12.Rosenzweig A, Seidman CE. Atrial natriuretic factor and related peptide hormones. Ann Rev Biochem. 1991;60:229–255. doi: 10.1146/annurev.bi.60.070191.001305. doi:10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- 13.Frlan R, Gobec S. Inhibitors of cathepsin B. Curr Med Chem. 2006;13:2309–2327. doi: 10.2174/092986706777935122. doi:10.2174/092986706777935122. [DOI] [PubMed] [Google Scholar]
- 14.Yan S, Sloane BF. Molecular regulation of human cathepsin B: implication in pathologies. Biol Chem. 2003;384:845–854. doi: 10.1515/BC.2003.095. doi:10.1515/BC.2003.095. [DOI] [PubMed] [Google Scholar]
- 15.Jane-Lise S, Corda S, Chassagne C, Rappaport L. The extracellular matrix and the cytoskeleton in heart hypertrophy and failure. Heart Fail Rev. 2000;5:239–250. doi: 10.1023/A:1009857403356. [DOI] [PubMed] [Google Scholar]
- 16.Abrahamson M, Olafsson I, Palsdottir A, Ulvsback M, Lundwall A, Jensson O, et al. A. Structure and expression of the human cystatin C gene. Biochem J. 1990;268:287–294. doi: 10.1042/bj2680287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barka T, van der Noen H. Expression of the cysteine proteinase inhibitor cystatin C gene in rat heart: use of digoxigenin-labeled probes generated by polymerase chain reaction directly for in situ and northern blot hybridizations. J Histochem Cytochem. 1993;41:1863–1867. doi: 10.1177/41.12.8245434. [DOI] [PubMed] [Google Scholar]
- 18.Tavera C, Prevot D, Girolami JP, Leung-Tack J, Colle A. Tissue and biological fluid distribution of cysteine proteinases inhibitor: rat cystatin C. Biol Chem Hoppe Seyler. 1990;371:187–192. [PubMed] [Google Scholar]
- 19.Mussap M, Plebani M. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci. 2004;41:467–550. doi: 10.1080/10408360490504934. doi:10.1080/10408360490504934. [DOI] [PubMed] [Google Scholar]
- 20.Mason RW, Sol-Church K, Abrahamson M. Amino acid substitutions in the N-terminal segment of cystatin C create selective protein inhibitors of lysosomal cysteine proteinases. Biochem J. 1998;330:833–838. doi: 10.1042/bj3300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukhova GK, Wang B, Libby P, Pan JH, Zhang Y, Grubb A, et al. Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E-null mice. Circ Res. 2005;96:368–375. doi: 10.1161/01.RES.0000155964.34150.F7. doi:10.1161/01.RES.0000155964.34150.F7. [DOI] [PubMed] [Google Scholar]
- 22.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. doi:10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bengtsson E, To F, Hakansson K, Grubb A, Branen L, Nilsson J, et al. Lack of the cysteine protease inhibitor cystatin C promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscl Thromb Vasc Biol. 2005;25:2151–2156. doi: 10.1161/01.ATV.0000179600.34086.7d. doi:10.1161/01.ATV.0000179600.34086.7d. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson P, Deguchi H, Samnegard A, Lundman P, Boquist S, Tornvall P, et al. Human evidence that the cystatin C gene is implicated in focal progression of coronary artery disease. Arterioscl Thromb Vasc Biol. 2004;24:551–557. doi: 10.1161/01.ATV.0000117180.57731.36. doi:10.1161/01.ATV.0000117180.57731.36. [DOI] [PubMed] [Google Scholar]
- 25.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. doi:10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 26.Djousse L, Kurth T, Gaziano JM. Cystatin C and risk of heart failure in the Physicians' Health Study (PHS) Am Heart J. 2008;155:82–86. doi: 10.1016/j.ahj.2007.08.023. doi:10.1016/j.ahj.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muntner P, Mann D, Winston J, Bansilal S, Farkouh ME. Serum cystatin C and increased coronary heart disease prevalence in US adults without chronic kidney disease. Am J Cardiol. 2008;102:54–57. doi: 10.1016/j.amjcard.2008.02.098. doi:10.1016/j.amjcard.2008.02.098. [DOI] [PubMed] [Google Scholar]
- 28.Tang WH, Van Lente F, Shrestha K, Troughton RW, Francis GS, Tong W, et al. Impact of myocardial function on cystatin C measurements in chronic systolic heart failure. J Card Fail. 2008;14:394–399. doi: 10.1016/j.cardfail.2008.01.006. doi:10.1016/j.cardfail.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Arimoto TT, Yasuchika N, Takeshi T, Noriaki O, Hidenobu F, Akio T, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail. 2005;11:595–601. doi: 10.1016/j.cardfail.2005.06.001. doi:10.1016/j.cardfail.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 31.Moran A, Katz R, Smith NL, Fried LF, Sarnak MJ, Seliger SL, et al. Cystatin C concentration as a predictor of systolic and diastolic heart failure. J Card Fail. 2008;14:19–26. doi: 10.1016/j.cardfail.2007.09.002. doi:10.1016/j.cardfail.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munagala VK, Burnett JC, Jr, Redfield MM. The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol. 2004;29:707–769. doi: 10.1016/j.cpcardiol.2004.07.002. doi:10.1016/j.cpcardiol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Lee DS, Vasan RS. Novel markers for heart failure diagnosis and prognosis. Curr Opin Cardiol. 2005;20:201–210. doi: 10.1097/01.hco.0000161832.04952.6a. doi:10.1097/01.hco.0000161832.04952.6a. [DOI] [PubMed] [Google Scholar]
- 34.Finney HN, Newman DJ, Gruber W, Merle P, Price CP. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II) Clin Chem. 1997;43:1016–1022. [PubMed] [Google Scholar]