Abstract
Spermatogonia- stem cells and progenitors of adult spermatogenesis- are killed through a p53-regulated apoptotic process after γ-irradiation but the death effectors are still poorly characterized. Our data demonstrate that both intrinsic and extrinsic apoptotic pathways are involved, and especially that spermatogonia can be split into two main populations, according to apoptotic effectors. Following irradiation both Dr5 and Puma genes are upregulated in the α6-integrin-positive Side Population (SP) fraction, which is highly enriched in spermatogonia. Flow cytometric analysis confirms an increased number of Dr5-expressing SP cells, and Puma-β isoform accumulates in α6-integrin positive cellular extracts, enriched in spermatogonia. Trail−/− or Puma−/− spermatogonia display a reduced sensitivity to radiation-induced apoptosis. The TUNEL kinetics strongly suggest that the extrinsic and intrinsic pathways, via Trail/Dr5 and Puma respectively, could be engaged in distinct subpopulations of spermatogonia. Indeed flow cytometric studies show that Dr5 receptor is constitutively present on more than half of the undifferentiated progenitors (Kit− α6 + SP) and half of the differentiated ones (Kit+ α6 + SP). In addition after irradiation, Puma is not detected in the Dr5-positive cellular fraction isolated by immunomagnetic purification, while Puma is present in the Dr5-negative cell extracts. In conclusion, adult testicular progenitors are divided into distinct sub-populations by apoptotic effectors, independently of progenitor types (immature Kit-negative versus mature Kit-positive), underscoring differential radiosensitivities characterizing the stem cell/progenitors compartment.
Introduction
Among the consequences of genotoxic stress, subfertility and transient sterility are an important issue for adult males. Injured germ cells, like in somatic self-renewing tissues, are located in the progenitor population, composed of mitotic spermatogonia that are the pre-meiotic cells in spermatogenesis. DNA damage results in apoptosis of part of the spermatogonia but resistant testicular stem cells allow afterwards the recovery of functional differentiation. As for somatic cells, apoptosis of damaged spermatogonia is controlled by the tumor suppressor p53, but its downstream apoptotic effector(s) remain far less characterized [1], [2].
Among the apoptotic factors, procaspases −2, −7, −8 and −9 are constitutively expressed in adult mouse spermatogonia [3]. After a genotoxic stress, the Fas/CD95/Tnfrsf6 death receptor gene had been identified as a p53 target in somatic cells [4] and the involvement of the extrinsic death receptor pathway has been further evoked in germ cells. Nevertheless, the requirement for Fas/Fas-Ligand in radiation-induced apoptosis of testicular germ cells remains controversial [5], [6]. Trail/Dr5 pathway could represent a better candidate. In the mouse Dr5/Trail-R2/Tnfrsf10b is the only receptor of the ligand Trail (TNF-related apoptosis inducing ligand) and activation of this signaling pathway can trigger apoptosis of infectious and cancer cells [7]. Dr5 is a p53-inducible gene, and Dr5−/− mice are viable but present impaired apoptotic response to irradiation [8], [9]. Trail induces in vitro apoptosis of normal testicular cells, via expression of Dr5 on spermatocytes [10], but the involvement of Trail/Dr5 pathway in stress-induced death of spermatogonia has not been assayed yet.
The role of the Bcl2 family, and therefore of the intrinsic/mitochondrial death pathway, in the control of germ cell development is known. The pro-apoptotic Bax and the anti-apoptotic Bcl-xL are necessary as well as pro-apoptotic BH3-only proteins [11]–[13]. Nevertheless, the role of Bcl2 proteins in radiation-induced apoptosis of adult male germ cell is far less demonstrated, while some are known to be widely involved in genotoxic damage tissular response (i.e, Bax, Puma).
One reason may be the difficulty to access spermatogonia. Testicular stem cells and progenitors represent less than 10% of the adult germ cells and are located along the basal membrane of the seminiferous tubule, which also includes meiotic and haploid cells. According to histological criteria, undifferentiated spermatogonia include stem cells (Asingle) and less committed progenitors (Apaired and Aaligned), whereas spermatogonia from A1 to B constitute the more differentiated sub-populations [14]. Immature spermatogonia can be identified on tissue sections by their expression of stem cell markers, like Plzf/Zbtb16 [15]. The improvement of their characterization allows their isolation by association of several stem cell markers. Thus, a α6-integrin-positive (α6 +) population enriched in spermatogonia can be isolated after immunomagnetic purification [16]. Testicular germ cells display the Side Population (SP) phenotype - based on the Hoechst 33342 (Ho42) efflux -that characterizes stem cells [17], [18]. In combination with anti α6 +-integrin pre-purification, SP criterion selects a fraction (α6 + SP) highly enriched in testicular stem cells. An additional screening of α6 + SP cells based on the expression of the c-Kit receptor allows the separation between immature (c-Kit negative) and differentiated spermatogonia (c-Kit positive) [19]–[21].
In order to identify the effectors responsible for genotoxic-induced apoptosis of spermatogonia, we demonstrate that different p53-regulated pathways are engaged: mitochondrial via Puma and extrinsic via Trail/Dr5. According to Dr5 expression, our results show that spermatogonia can be constitutively divided up into sub-populations that overlaps the traditional distribution -undifferentiated Kit− versus differentiated Kit+- and potently reflects different death- sensitivities.
Results
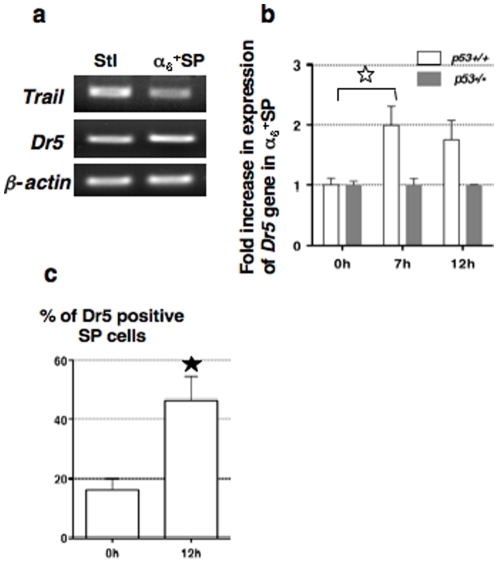
γ rays induce Dr5 expression in spermatogonia in a p53-dependent manner
As the p53-controlled Dr5 gene is involved in radiation-induced apoptosis of various somatic cells, we asked whether the Trail/Dr5 signaling pathway could be responsible for death of spermatogonia (Death marker evolution is presented in Figure S1). Trail and Dr5 genes were expressed in primary Sertoli cells and in the testicular α6 + SP fraction (Fig. 1a). Then we measured the variation in Dr5 gene expression levels in α6 + SP cells prior to and post-IR (post irradiation) (Fig. 1b): Dr5 expression was two-fold higher than in non-irradiated cells and remained stable at 12 h. Consistent with these results, the number of SP cells expressing the Dr5 receptor was increased a 2.8 fold, 12 h post-IR (Fig. 1c and analysis details in Figure S2). Conversely, in irradiated p53−/− cells, Dr5 expression level did not vary, demonstrating that the stress- induced upregulation of Dr5 in damaged spermatogonia was p53-dependent.
Figure 1. Dr5 receptor expression is induced in a p53-dependent manner on irradiated spermatogonia.
(a) Trail and Dr5 genes are expressed in Sertoli cells (Stl) and in α6 + SP cells, (negative for CD45 and highly enriched in spermatogonia); PCR amplifications (35 cycles) were carried out using 5 µL of first strand reaction for Trail and Dr5 genes and normalized to β-actin PCR product (30 cycles). (b) Comparison of Dr5 expression in WT or p53−/− sorted CD45− α6 +SP cells (highly enriched in spermatogonia), by semi-quantitative RT-PCR prior to, 7 and 12 hours after irradiation. Each expression level of Dr5 was normalized to its value in non-irradiated WT cells and is indicated as a fold increase (± SEM, three independent experiments using pooled populations that were independently purified from 5 WT and 3 p53−/− males). A significant difference (star, p = 0.0232) was observed in WT cells prior to and 7 h post-IR. (c) Wild-type SP cells (n = 5) were analyzed independently for Dr5 receptor expression by FACS prior to and 12 h after irradiation, according to their DNA content. Mean percentages of Dr5- positive SP cells are shown (± SEM) and the star indicates a significant variation (p = 0.01033). See Figure S2 for details on flow cytometric analysis.
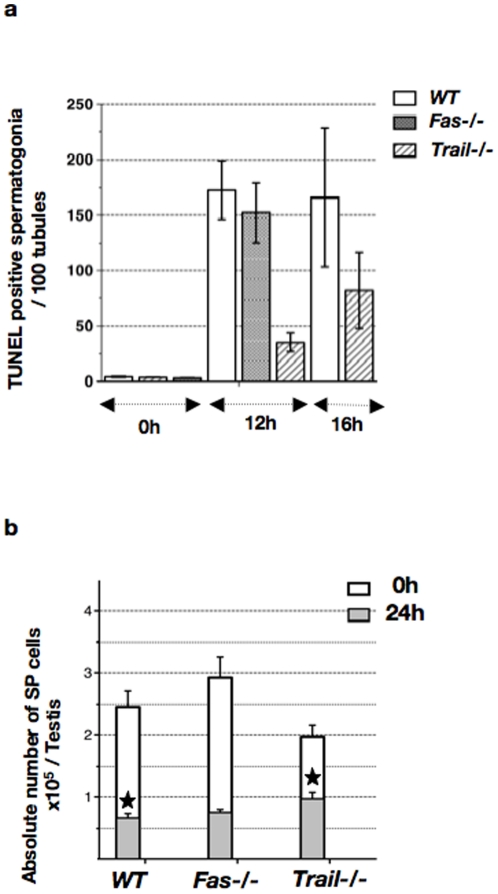
The Trail/Dr5 signaling cascade is the only Death Receptor pathway involved in radiation-induced apoptosis of spermatogonia
We further investigated the in vivo involvement of the Trail/Dr5 pathway by monitoring the death response of irradiated Trail−/− cells. Twelve hours post-IR, the number of TUNEL-positive Trail−/− spermatogonia was four-fold lower than that of WT cells (Fig. 2a). The number of positive Trail−/− cells was doubled at 16 h, while the number of TUNEL-positive WT spermatogonia remained constant. By contrast, the number of positive spermatogonia in irradiated Fas−/− males was comparable to that of WT counterparts. The lower sensitivity to radiation-induced apoptosis of Trail−/− germ cells was confirmed by in situ labeling for cleaved caspase-7 (data not shown).
Figure 2. Trail/Dr5 is involved in irradiated spermatogonia apoptosis.
(a) Comparison of TUNEL labeling between irradiated Trail−/−, Fas−/− and WT testes. The number of TUNEL-positive cells, located along the basal membrane, was determined prior to and post-IR, and indicated as the mean per 100 tubules (± SEM). Experiments were repeated 3 times and at least 200 tubules were counted in 2 males per genotype. (b) Viability of Trail−/−, Fas−/− and WT SP cells prior to and 24 h post-IR. Means of absolute number of SP cells per testis (± SEM) were determined after Ho42 and PI staining of testicular cell suspensions, analyzed in triplicates by FACS in the presence of fluorescent beads as an internal reference. The irradiated WT SP fraction was significantly lower than control (white star p<0.0001, n≥5). Irradiated Trail−/− SP significantly differs from WT SP (black star, p = 0.022, n≥5). Basal and irradiated Fas−/− and WT SP fractions did not differ (p≫0.05, n>5).
Moreover, by monitoring at 24 h post-IR the absolute number of SP cells (Fig. 2b), we detected a 2.7-fold reduction in the viability of WT SP cells, while viability of Trail−/− SP cells was only a 2-fold decreased. In other words, the irradiated Trail−/− SP fraction was significantly higher than that of irradiated WT cells (1.4 fold), while there was no significant difference in the number of non-irradiated WT and Trail−/− SP cells. By contrast, viability of Fas−/− and WT SP cells was comparable.
In conclusion, these data demonstrate that the Trail/Dr5 extrinsic pathway is involved in the radiation-induced death of spermatogonia.
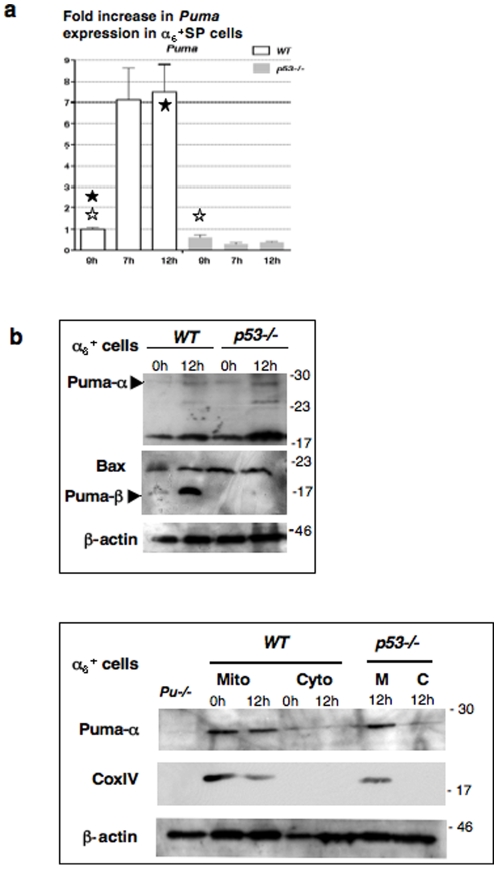
γ rays induce Puma expression in spermatogonia in a p53-dependent manner
The previous data suggest that Trail inactivation did not protect all spermatogonia from radiation-induced apoptosis, which is mainly triggered by the Bcl2 family in somatic cells, through the intrinsic pathway. The pro-apoptotic members (e.g. Bax) interact with the mitochondrial membrane inducing cytochrome c release and consecutive pro-caspase-9 activation. Upstream, the subgroup of BH3-only proteins controls activities of pro- and anti-apoptotic Bcl2 family members. In order to identify Bcl2-related factors involved in spermatogonia death following irradiation, we analyzed several candidate genes by Real-Time PCR in α6 + SP cells. Puma (p53 upregulated modulator of apoptosis) was the only “apoptotic” gene exhibiting a significant 7- fold increase in irradiated α6 + SP cells (Fig. 3a and Figure S3) [22], [23]. This upregulation was directly correlated to the p53 status as indicated by the absence of changes in RNA level in p53−/− α6 + SP cells.
Figure 3. Increased Puma gene expression in a p53-dependent manner in α6 +SP cells.
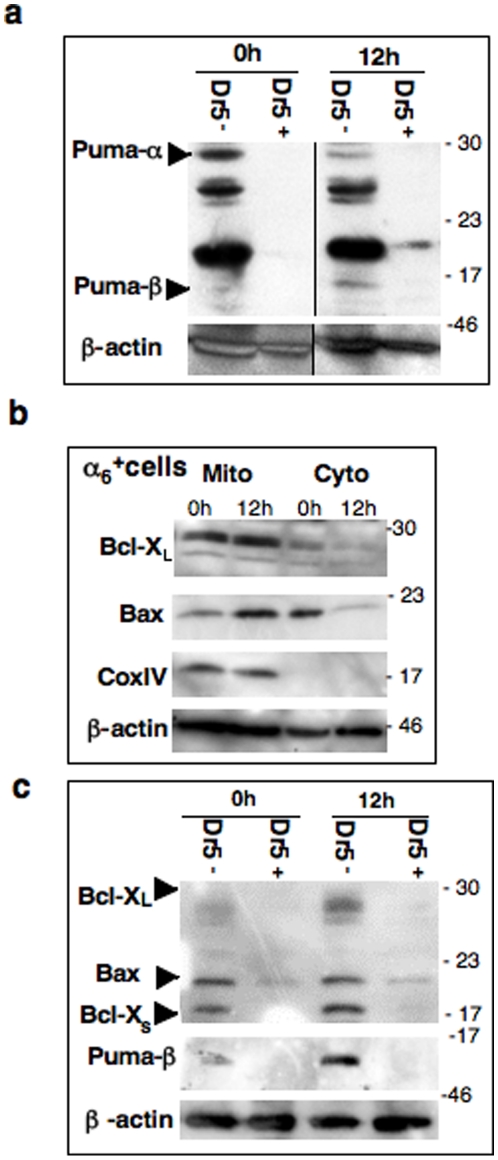
(a) Comparison in WT or p53−/− sorted CD45−α6 +SP cells (highly enriched in spermatogonia) by semi-quantitative RT-PCR prior to, 7 and 12 hours after irradiation. Each expression level was normalized to its value in non-irradiated WT cells and is indicated as a fold increase (± SEM, three separate experiments from pooled CD45− α6 +SP populations, which were independently purified from 5 WT and 3 p53−/− mice). A significant difference in Puma expression level was observed in WT α6 +SP cells before irradiation and 12 h post-IR (black star, p = 0.015), and the basal level was significantly reduced in p53−/− α6 +SP cells (white star, p = 0.00074). See Figure S3 for complete semi-quantitative RT-PCR analysis. (b) Puma expression in α6 + cells (testicular cell fraction enriched in spermatogonia after immunomagnetic purification) was determined by western-blot analysis. The α isoform was identified on the upper picture with the 9643 antibody, using Puma−/− cell lysate as a negative control and according to Callus and col. [41]. The Puma-β isoform was detected with the 4976 antibody and β actin was used as loading control. On the last picture, testicular α6 + cells were collected prior and 12 h post-IR, and separated into cytosolic and mitochondrial-enriched fractions for puma-α location and expression level; the sample purity was assayed using an anti-Cox IV antibody (mitochondrial marker) and β actin as loading control. Data presented are representative of 2 independent experiments and each α6 + population was purified from 3 males.
Since Puma gene codes for different alternative transcripts we wondered which isoforms were expressed in healthy and damaged cells. Puma-α was detected by western-blot in α6 + populations enriched in spermatogonia (Fig. 3b upper). Its expression level appeared constant whatever the p53 status as confirmed in the mitochondrial fractions of the α6+ cells (Fig. 3b lower). Conversely, Puma-β accumulated in irradiated α6 + WT cells, while it remained undetectable in p53−/− α6 + populations (Fig. 3b upper). In parallel, Bax amounts remained constant whatever the conditions.
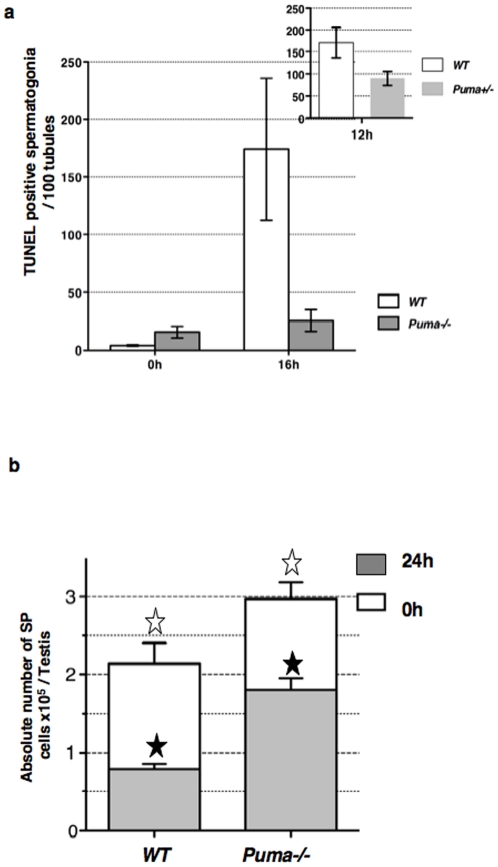
Puma is involved in radiation-induced apoptosis of spermatogonia
To confirm the involvement of the BH3-only Puma in apoptotic radiosensitivity, dead spermatogonia of irradiated Puma−/− mice were counted (Fig. 4a). The number of TUNEL-positive Puma−/− spermatogonia was 7-fold lower than that of WT cells at 16 h. The pro-apoptotic effect of Puma was correlated to allele number as shown by the 2-fold reduction in TUNEL-positive Puma+/− spermatogonia at 12 h post-IR (insert Fig. 4a). In addition 24 h post-IR (Fig. 4b), viability of Puma−/− SP cells was only 1.6 fold decreased in comparison to the 2.7 fold reduction observed for WT SP cells. The number of non-irradiated Puma−/− SP cells was 1.4 fold higher than that of WT control and this significant difference was further increased (2.3 fold) following irradiation.
Figure 4. Puma is involved in spermatogonia radiation-induced apoptosis.
(a) Comparison of TUNEL labeling between irradiated Puma−/− and WT testes. The number of TUNEL-positive cells, located along the basal membrane, was determined prior to and post-IR, and indicated as the mean per 100 tubules (± SEM). Experiments were repeated twice and at least 200 tubules were counted in 2 males per genotype. (b) Viability of Puma−/− and WT SP cells prior to and 24 h post-IR. Means of absolute number of SP cells per testis (± SEM) were determined after Ho42 and PI staining of testicular cell suspensions, which were analyzed by FACS in triplicates in the presence of fluorescent beads as an internal reference. The WT SP fraction significantly differs from the Puma−/− SP population prior (white star, p = 0.022; n = 5) and after irradiation (n = 5, black star p<0.0001).
In conclusion, Puma−/− spermatogonia are more resistant than WT cells to genotoxic stress demonstrating that Puma-controlled intrinsic apoptosis has a central role. Nevertheless, the overlay of the TUNEL assay kinetics (Fig. 2a and 4a) shows that the number of dead Trail−/− spermatogonia was 50% higher than that of Puma−/− cells at 16 h. This suggests that different populations of spermatogonia can be distinguished on the basis of their death-sensitivity to DNA damage: two subsets could be eliminated via Puma or via Trail/Dr5 exclusively, and a minor subpopulation by a cooperation of both pathways.
The extrinsic and intrinsic apoptotic pathways could be effective in different subsets of injured spermatogonia
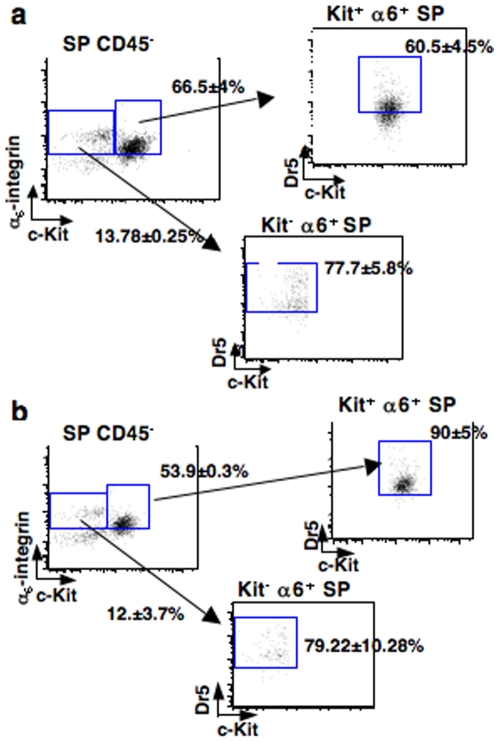
To go further into irradiated progenitors characterization, Dr5 receptor expression was analyzed by flow cytometry on immature (Kit– α6 + SP) and differentiated (Kit+ α6 + SP) spermatogonia, both populations expressing Puma RNA (data not shown). Dr5 was constitutively present on 75% of immature spermatogonia versus 60% of the differentiated progenitors (Fig. 5a). After irradiation, 90% of the differentiated spermatogonia were positive for Dr5 receptor, concomitant to an increase of the number of molecules per cell, as shown by fluorescence enhancement (Fig. 5b). By contrast, the number of irradiated immature cells expressing Dr5 was constant.
Figure 5. Dr5 is expressed in Kit− and Kit+ spermatogonia and is upregulated in committed progenitors post-IR.
Combined flow cytometric analysis of the expression of stem cell markers and Dr5 receptor in testicular cell suspensions. Testicular cell suspensions were stained with Ho42, and immunolabeled for the detection of α6-integrin, c-Kit and Dr5. Isotypic controls were performed in parallel. Hematopoietic CD45+ cells were excluded from the SP fraction and experiments were performed on cells (a) non-irradiated and (b) 12 h post-IR. FACS plots are gated on α6-integrin+ cells, c-Kit positive (mature progenitors) or negative (Stem cells and immature progenitors) cells and numbers indicate their percentage (±SEM).
We took advantage of the ability to separate testicular cell suspension into Dr5-positive (Dr5+) and Dr5-negative (Dr5−) fractions using Magnetic Activated Cell Sorting (MACS by Miltenyi Biotech). Spermatogonia were present in both Dr5+ and DR5− fractions, while the former was highly more enriched (Population characterization in Figures S4 and S5). On western-blots (Fig. 6a), Puma could not be detected in Dr5+ fractions. By contrast, Puma-α was constitutively present in Dr5− population, and appeared downregulated after irradiation. Puma-β isoform was also detected in irradiated Dr5− cells within it accumulated, as previously observed in the irradiated α6 + fractions (Fig. 3b). We then focused on the Puma “partners”. Indeed, BH3-only proteins maximize their effects through oligomeric interactions with pro- and/or anti- apoptotic members of the Bcl2 family: Bcl-XL and/or Bax for Puma [24]. In irradiated α6 + population (Fig. 6b), Bax was translocated to the mitochondrial-enriched extract, with a concomitant loss of its cytoplasmic form, suggesting Bax activation and apoptosis induction. By contrast, the anti-apoptotic Bcl-XL was strongly expressed in the mitochondrial-enriched lysates of both control and irradiated α6 + cells. However, only Bax was detected in both Dr5+ and Dr5− populations (Fig. 6c). Conversely, the anti-apoptotic Bcl-XL accumulated in irradiated Dr5− cells, while the pro-apoptotic Bcl-Xs appeared unaffected.
Figure 6. Puma is not detected in MACS Dr5-positive cells.
(a) Differential expression of Puma isoforms in testicular Dr5+ and Dr5− populations. Dr5 fractions were isolated by immunomagnetic purification prior and post-IR and analyzed by western-blot with β actin as loading control. Data presented are representative of 2 independent experiments and the Dr5 populations were purified from 3 males. See Figure S4, for characterization of MACS Dr5 populations. (b) Differential expression of Bcl-XL and Bax in cytosolic and mitochondrial fractions. Testicular α6 + cells, enriched in spermatogonia after selection by immunomagnetic purification, were collected prior and 12 h post-IR and separated into cytosolic and mitochondrial-enriched fractions for western blot analysis; the sample purity was assayed using an anti-Cox IV antibody (mitochondrial marker) and β actin as loading control. Data presented are representative of 2 independent experiments and the α6 + populations were purified from 3 males. See Figure S3 for corresponding semi-quantitative RT-PCR analysis. (c) Bax is the only Bcl2 family member detected in both Dr5+ and Dr5− populations.
In conclusion, these data show that most of spermatogonia can be divided up into a Dr5-positive subset potentially sensitive to Trail, and another Dr5-negative subpopulation deleted via intrinsic pathway after DNA damage.
Discussion
We demonstrate herein that two p53-dependent pathways are involved in radiation- induced apoptosis of spermatogonia via the transcriptional control of Puma and Dr5 genes. The inactivation of each pathway results in partial inhibition of apoptotic death of spermatogonia, and the overlay of TUNEL-kinetics suggested the existence of several spermatogonia populations according to death sensitivities. Unfortunately we were unable to obtain double mutated animals (Puma−/− Trail−/−), suggesting embryonic lethality.
The Puma-controlled intrinsic pathway is induced in one subset of progenitors, independently of the Trail/Dr5 system. Puma-regulated death is widely involved after genotoxic stress: in lymphocyte lineages, small intestine and central nervous system [25]–[27]. While Puma transcription levels were highly elevated in spermatogonia, Puma protein amounts remain limiting: (i) irradiated Puma+/− spermatogonia demonstrate an intermediate resistance to death, as previously reported for somatic cells [28], (ii) no obvious variation of Puma-α quantity is detected whatever the p53 status and this isoform is mainly located in the mitochondrial –enriched fraction of α6 + cells, treated or not. Our results suggest that the mitochondrial apoptosis could be induced by Puma-β that only accumulates in WT cells, illustrating the p53 dependence. Alternative splicing of Puma is conserved among species, but the preference for the isoform β could be male germ cell-specific. Indeed, in cisplatin-treated kidney cells, only isoform α is upregulated according to p53 status [29]. Puma-β markedly differs at the N-terminus from Puma-α [30], however except its mitochondrial location little is known about its activity (partners, apoptotic role…). Depending on the experimental systems, two different models for Puma action have been proposed: (i) a displacement of Bcl-XL to release constitutively active Bax, or (ii) a direct interaction with Bax to induce its activation [22], [30]. These potent partners are detected in α6 + cells: the pro-survival Bcl-XL was mainly located at mitochondria and the pro-apoptotic Bax translocates to mitochondrial membrane suggesting the conformational activation necessary to apoptosis induction. But they are differently distributed between Dr5-negative and Dr5-positive populations; the later undergoes extrinsic death via Trail/Dr5 without expression of Puma isoforms.
As for somatic cells, DNA damage upregulates Dr5 expression on spermatogonia in a p53-dependent manner, and injured adult testicular Trail−/− progenitors are resistant to apoptosis, like somatic Dr5−/− tissues [9]. The presence of Trail RNA in both Sertoli and α6 +SP cells suggests that spermatogonia can undergo apoptosis in an autocrine and/or paracrine manner. The Trail/Dr5 pathway can be associated with induction of procaspase-8 cleavage in α6 + cells (Figure S1). But the presence of Bax in Dr5+ population, as well as Bid truncation in irradiated α6 + cells (Data not shown), support that mitochondria might be recruited to amplify the process in normal testicular cells, as in cancer lines [31]. Nevertheless, our data do not allow the exclusion of a minor third sub-population exhibiting both death pathways.
These distinct populations of spermatogonia exist according to “death criteria” and appear to overlap with the classical distribution of spermatogonia. Indeed, among immature spermatogonia (Kit− α6 +SP), 75% express Dr5 receptor while Puma protein was not detected in the MACS-Dr5+ population. Irradiation does not modify Puma specific expression. We cannot exclude that part of the 25% Dr5-negative immature cells could be Puma-positive and/or Bcl-XL positive. It is important to note that undifferentiated spermatogonia include germinal stem cells (As) that resist to radiation-induced death and are responsible for differentiation recovery. Consequently, either they express Dr5 receptor and the signaling pathway might be inhibited after moderate stress, or they are negative for Dr5 and Bcl-XL accumulation could be a way to resist to death induction. The lack of suitable anti-Puma antibody for in situ studies did not allow co-labeling with stem cell marker (e.g. Plzf [15]) to refine characterization.
Conversely, the differentiated spermatogonia (Kit+ α6 +SP) contain about 60% of Dr5+ cells, with a 30% increase post IR. This enhancement in Dr5+ progenitors could be due to a real Dr5 upregulation, but also to the rapid death of Dr5− differentiated progenitors, potentially expressing Puma, thereby causing an apparent enrichment in more radio-resistant Dr5+ cells at 12 h post-IR.
These data raise few questions: (i) the constitutive presence of Dr5 on most of the immature spermatogonia suggests a role of Trail/Dr5 in differentiation entry; this is not surprising as this pathway has been reported to have regulatory functions in the immune and hematopoietic compartments, as well as in bone remodeling and muscle differentiation [32]–[35]. (ii) Spermatogonia death could be regulated according to a threshold mechanism: the intrinsic pathway would be activated rapidly and/or by low damage, and the extrinsic pathway could be recruited over a major stress level. It is important to note that the applied γ dose (1.6 Gy) induces a transient sterility. In conclusion, adult spermatogonia exhibit different sensitivities to DNA damage linked to specific metabolisms of reactive oxygen species, to DNA repair capacities and/or to cycling status. The less mature spermatogonia are more resistant to radiation-induced stress than committed progenitors, but over a critical damage threshold the extrinsic pathway could be activated, thereby limiting the mutational risk.
Materials and Methods
Mice
The Fas−/− [36], Trail−/− [37] and Puma−/− [38] mouse strains were raised on a C57BL/6 background in our animal facilities, as well as the p53−/− [39] strain on SV129 background. Whatever their genotypes, all the KO males are fertile. Since adult C57BL/6 germ cells are more sensitive to radiation-induced death than the SV129 cells, control and mutants of same genetic background were used in experiments. Adult males (2.5- to 3.5- months-old) were exposed to 1.5 or 2 Gy γ rays in a 60Co irradiator (dose rate 0.085Gy/mn). The Figure S1 describes the evolution of some death markers in irradiated SV129 testis. All animal procedures were carried out in accordance with French Government policies (Services Vétérinaires de la Santé et de la Production Animale, Ministère de l′Agriculture; N° A92-032-02).
Sertoli cell-enriched cultures
Primary cultures enriched in adult Sertoli cells were prepared by trypsin/collagenase I digestion of testes from 3- week-old males [40]. Isolated cells were seeded in DMEM-F12 medium supplemented with 15 mM Hepes pH 7.2, 1.2 g/L Na2CO3, 5 ng/mL EGF, 5 µg/mL transferin and 10 µg/mL bovine insulin, at a density of 5×105 cells/cm2 and maintained at 32°C for 48 h. A hypotonic shock in 20 mM Tris-HCl pH 7.4 (2.5 min at RT) allowed removal of most of germ cells. After one HBSS rinse, the adherent Sertoli cells were recovered for 48 h in the enriched DMEM-F12 medium and assayed by RT-PCR amplification for the expression of the germ cell marker c-Kit.
Testicular single-cell suspensions
Germ cells are prepared as previously described [18]. Briefly seminiferous tubules are dispersed in collagenase I at 32°C. Interstitial cells were further discarded by filtration, and the seminiferous tubules are digested in Cell dissociation buffer (Invitrogen). After washing and filtration, isolated cells are resuspended in (HBSS supplemented with 20 mM Hepes pH 7.2, 1.2 mM MgSO47H20, 1.3 mM CaCl22H20, 6.6 mM sodium pyruvate, 0.05% lactate, glutamine and 1% FCS) and incubated at 32°C.
Real-Time PCR amplification from sorted cells
The testicular cell suspension is enriched in α6-integrin positive cells using immunomagnetic purification (MACS by Miltenyi Biotech) [18]. The α6 + cellular fraction is incubated (50 min. at 32°C) with Ho42 and further labeled for CD45 (clone 30-F11, Becton Dickinson; 15 min at 4°C). The hematopoietic CD45-PE+ cells were discarded during FACS analysis and α6 +SP population was sorted with a FACStar Plus Flow Cytometer (Becton Dickinson). Populations were independently purified from 5 WT and 3 p53−/− mice and RNA further stoechiometrically pooled. RNA purification, and cDNA synthesis were performed as previously described [18], [21].
For cDNA synthesis the following primers were used:
Dr5 (CTgTgCATTCgTCTCTCTTgg/TgAgTCgTTTCCgTTTACCg); Trail (gTgTCTgTggCTgTgACTTACA/AATgCCCTTTCCgAgAggA); Puma (CACCCCATCgCCTCCTTTCT/ggAAggggCgCggACTgTCg); Noxa (TggAgTgCACCggACATAACT/CACTCgTCCTTCAAgTCTgCTg); Bax (TCAAggCCCTgTgCACTAA/TgAggACTCCAgCCACAAA); Bcl-XL (gATgCAggTATTggTgAgTCgg/ATCCACAAAAgTgTCCCAgCC); Bid (CTTTgCTCCgTgATgTCTTCC/TgCgggCTCCTCAgTCCATC); Gusβ (AgCCACAgCTgAATAgCCAgTT/ACTCCTCACTgAACATgCgAgg); βActin (TCgTgCgTgACATCAAAgAgA/gAACCgCTCgTTgCCAATAgT).
Real-time PCR amplifications were carried out with the Sybr Green® PCR kit (Applied Biosystems) and each reaction was performed at least twice in duplicate using a Abiprism 7000 apparatus. The β glucuronidase (Gusβ) gene was used as an internal control because Gusβ levels were constant in WT α6 +SP fractions whatever the p53 status. A second internal control, βactin (Mm00446953m1), was used in Taqman® assays (Applied Biosystems) to compare WT and p53−/− samples. After normalization, mean expressions were shown as fold increase (± SEM), after comparison with the mean expression levels of the WT control to which was ascribed an arbitrary value of 1.
Immuno-labeling and absolute SP cell count
For marker analysis one million testicular cells were firstly stained with Ho42 in 1 mL incubation buffer for 45 min. at 32°C, and then labeled with 1 µg of antibody (PE- anti-DR5 (MD5-1, eBioscience), or FITC-anti-human-CD49f, APC-anti-Kit and Pc5-anti-CD45 (BD Pharmingen)) for 15 min at 4°C. Isotype antibodies were used as negative controls and propidium iodide (PI) was added prior to analyses with a LSRII cytometer (Becton Dickinson). Mean percentages are shown (± SEM).
For population measurements, after Ho42 and PI staining of testicular cell suspensions, SP fractions were quantified by FACS using fluorescent TruCOUNT™ beads (BD Biosciences). A minimum of three different counts was performed per testis prior to and 24 h post-IR in the presence of the fluorescent beads as an internal reference. Elongated spermatids were excluded during the analysis to avoid any contamination of the SP.
TUNEL assay
TUNEL assay was performed as previously described [3] and labelings were repeated 3 times, with at least 200 tubules were counted per male. Cells located at the basal membrane were counted under an Olympus BX51 photomicroscope equipped with an Insight QE Spot cameram.
Protein purification and western blotting
The testicular cell suspensions are enriched in either in α6-integrin positive cells (anti-CD49- PE), or in Dr5 positive cells (anti-Dr5 PE) using immunomagnetic purification (MACS by Miltenyi Biotech). For total protein lysates, MACS purified fractions were lysed for 30 min at 4°C in 25 mM Tris pH 7.5, 2 mM EDTA, NP40 5% (v/v), 0.6 M NaCl, 15 mM NaF, Na3VO4 with protease inhibitor cocktail (Roche Diagnostics) then centrifuged for 30 min at 16 000 g, 4°C. In order to purify mitochondrial proteins from α6 + populations, the ProteoExtract® Cytosol/mitochondria kit (Calbiochem) was used with a 40 times dounce according to the manufacturer's instructions. Concentrations were measured with the Quant-it™ Protein assay kit `(Invitrogen). After migration of 30 µg of proteins on 12 or 14% SDS-Page gels, they were blotted on Immobilon™PSQ membranes using 35% methanol solution at the anode. Membranes were hybridized with anti-bactin (AC15, Sigma-Aldrich), anti-CoxIV (20E8, Molecular Probes), anti- Bcl-XL (S-18), anti-Bax (N-20) (Santa Cruz), anti-Puma (rabbit polyclonal 9643 (Abcam) or 4976 (Cell Signaling)) in TBS-milk. Proteins were visualized with peroxidase-coupled secondary antibodies (Pearce) using the ECL Plus System (GEHealth) on Kodak films. Puma-α isoform was identified with the 9643 antibody, using Puma−/− cell lysate as a negative control [41] and Puma-β isoform was detected with the 4976 antibody. β-actin was used as loading control and sample purity was assayed using an anti-Cox IV antibody (mitochondrial marker). Data presented are representative of 2 independent experiments and MACS-α6 + population and MACS-Dr5 populations were purified from 3 males. Characterization of MACS-Dr5 cell populations is presented in Figure S4.
Statistical analysis
Values were expressed as the mean ± standard error (±SEM). Unpaired Student's t test was used to compare data (Kaleidagraph, Synergy software). Taking into account the variance distribution, a p<0.05 was considered significant.
Supporting Information
Detection of apoptotic markers in irradiated testes. On the basis of TUNEL dose-response previously described by Hasegawa M, Wilson G, Russell LD, Meistrich ML (1997) Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiation research. 147: 457–467. Immunohistochemistry on SV129 testes, Immunostaining was performed on 5microm sections fixed in Bouin. After antigen retrieval by microwave irradiation in citrate buffer, sections were treated with 0.3% H2O2 in PBS and blocked with 3% BSA in PBS. They were then incubated with anti-cleaved caspase-9 (Asp353), -cleaved caspase-7 (Asp198) (Cell Signaling), -cytochrome c (6H2B4, Becton Dickinson) or -p53 (CM5, Novocastra) antibodies diluted in BSA overnight at 4°C. Labeling was revealed using the ABC Vectastain Kit (Vector Laboratories) and Sigma fast DAB (Sigma). Sections were counterstained with hematoxylin and analyzed under an Olympus BX51 photomicroscope equipped with an Insight QE Spot camera. (A) P53 is stabilized 4 h post-IR in spermatogonia, (B) Cytochrome c is released 7 h post-IR in some spermatogonia. Arrows indicate positive spermatogonia (bar = 20 microm). (C) Detection of dead spermatogonia 16h-post IR by TUNEL assay. (D) Cleavage of pro-caspase-9 and -7 in spermatogonia, with antibodies specifically recognizing the processed form. Arrows indicate positive spermatogonia (bar = 20 microm). (E) Processing of pro-caspase-2 and -8 followed by immunoblotting of whole lysates of MACS- alpha6+ fraction (enriched in spermatogonia) versus MACS - alpha6- fraction (including spermatocytes I, spermatocytes II and spermatids) prior to and post-IR; actin was used as a loading control. Representative results obtained from 3 male pooled testicular cells at each time point. Each experiment was performed at least twice. Membranes were hybridized with anti-caspase- 8 (1G12) and anti-caspase- 2 (11B4) (Alexis), anti-betaactin (AC15, Sigma-Aldrich) and proteins were visualized with peroxidase-coupled secondary antibodies (Pearce) using the ECL Plus System (GEHealth) on Kodak films.
(0.96 MB TIF)
Flow cytometric analysis of Dr5 labeling on testicular cell suspension. (a) Testicular suspension is analyzed according to Ho42 and PI staining; viable cells are gated in R1, and dead cells are excluded. (b) Elongated spermatids are excluded from the viable testicular cells (gate R2). (c) The SP cells are gated, and further analyzed for Dr5 labeling (d) according to the isotypic threshold (gate R4).
(0.14 MB TIF)
Analysis of the expression levels of Noxa, Bid, Bcl-XL and Bax genes. Comparison in p53+/+ or p53−/− sorted CD45- alpha6+ SP cells, by semi-quantitative RT-PCR prior to, 7 and 12 hours after irradiation. Each expression level was normalized to its value in non-irradiated cells and is indicated as a fold increase (± SEM, 3 separate experiments from pooled CD45- alpha6+ SP populations, independently purified from 5 males). On left panel, expression of the anti-apoptotic Bcl-XL gene did not vary significantly, like RNA levels of the pro-apoptotic BH3-only members Bid and Noxa, potently involved in DNA damage response. On right panel, expression of the pro-apoptotic Bax gene was significantly reduced in irradiated CD45- alpha6+ SP cells (black star p = 0.00031). The basal level in p53−/− cells was significantly increased (p = 0.0028).
(0.09 MB TIF)
Characterization of Dr5+ and Dr5- cell populations Total testicular sample was purified by MACS according to Dr5 expression. MACS cellular fractions were cytospined, fixed with 2% PFA-PBS and stained with anti-CD9, anti-Kit (Becton Dickinson) or anti-Plzf after Triton permeabilization (Santa Cruz). After incubation with a secondary antibody coupled to Alexa 488, cells were counterstained with Dapi. Kit is a marker of differentiated spermatogonia, and not of testicular stem cells. CD9 is expressed on spermatogonia and also on stem cells, which are specifically expressing plzf. Most of the Kit- spermatogonia are Plzf+.
(0.89 MB TIF)
(0.84 MB TIF)
Acknowledgments
We thank our Amgen collaborators for providing the Trail−/− mice, Dr Villunger and his laboratory for the Puma−/− mice and irradiated tissues, and Dr Soularue for help with the transcriptional studies. We also thank Dr Pflumio for cytospin assistance, Dr Racine for critical reading and E. Andermarcher for english editing of the manuscript. We also thank J.B. Lahaye for expertise in mice irradiation, C. Chauveau and V. Neuville for animal care, and M. Duneau for technical assistance.
Footnotes
Competing Interests: The authors confirm that EDF fundings do not alter their adherence to all the PLoS ONE policies on sharing data and materials.
Funding: The authors' work was supported by Electricite de France, and by the Association pour la Recherche contre le Cancer (Grant 5796 and a PhD studentship to M.C.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hasegawa M, Zhang Y, Niibe H, Terry N, Meistrich M. Resistance of differentiating spermatogonia to radiation-induced apoptosis and loss in p53-deficient mice. Radiation Res. 1998;149:263–270. [PubMed] [Google Scholar]
- 2.Beumer TL, Roepers-Gajadien HL, Gademan IS, van Buul PP, Gil-Gomez G, et al. The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ. 1998;5:669–677. doi: 10.1038/sj.cdd.4400396. [DOI] [PubMed] [Google Scholar]
- 3.Coureuil M, Fouchet P, Prat M, Letallec B, Barroca V, et al. Caspase-independent death of meiotic and postmeiotic cells overexpressing p53: calpain involvement. Cell Death Differ. 2006;13:1927–1937. doi: 10.1038/sj.cdd.4401887. [DOI] [PubMed] [Google Scholar]
- 4.Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, et al. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol. 1995;15:3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richburg JH, Nanez A, Williams LR, Embree ME, Boekelheide K. Sensitivity of testicular germ cells to toxicant-induced apoptosis in gld mice that express a nonfunctional form of Fas ligand. Endocrinology. 2000;141:787–793. doi: 10.1210/endo.141.2.7325. [DOI] [PubMed] [Google Scholar]
- 6.Embree-Ku M, Venturini D, Boekelheide K. Fas is involved in the p53-dependent apoptotic response to ionizing radiation in mouse testis. Biol Reprod. 2002;66:1456–1461. doi: 10.1095/biolreprod66.5.1456. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 8.Wu GS, Burns TF, McDonald ER, 3rd, Jiang W, Meng R, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- 9.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee CM, Ye Y, Richburg JH. Testicular germ cell sensitivity to TRAIL-induced apoptosis is dependent upon p53 expression and is synergistically enhanced by DR5 agonistic antibody treatment. Apoptosis. 2006;11:2237–2250. doi: 10.1007/s10495-006-0288-1. [DOI] [PubMed] [Google Scholar]
- 11.Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM. Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Reprod. 2002;66:950–958. doi: 10.1095/biolreprod66.4.950. [DOI] [PubMed] [Google Scholar]
- 12.Kasai S, Chuma S, Motoyama N, Nakatsuji N. Haploinsufficiency of Bcl-x leads to male-specific defects in fetal germ cells: differential regulation of germ cell apoptosis between the sexes. Dev Biol. 2003;264:202–216. doi: 10.1016/s0012-1606(03)00400-7. [DOI] [PubMed] [Google Scholar]
- 13.Coultas L, Bouillet P, Loveland KL, Meachem S, Perlman H, et al. Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. Embo J. 2005;24:3963–3973. doi: 10.1038/sj.emboj.7600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiarini-Garcia H, Russell LD. High-resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 2001;65:1170–1178. doi: 10.1095/biolreprod65.4.1170. [DOI] [PubMed] [Google Scholar]
- 15.Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falciatori I, Borsellino G, Haliassos N, Boitani C, Corallini S, et al. Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. Faseb J. 2004;18:376–378. doi: 10.1096/fj.03-0744fje. [DOI] [PubMed] [Google Scholar]
- 18.Lassalle B, Bastos H, Louis JP, Riou L, Testart J, et al. ‘Side Population’ cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development. 2004;131:479–487. doi: 10.1242/dev.00918. [DOI] [PubMed] [Google Scholar]
- 19.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 20.Takubo K, Ohmura M, Azuma M, Nagamatsu G, Yamada W, et al. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 22.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27:S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, et al. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005;106:4131–4138. doi: 10.1182/blood-2005-04-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhtar RS, Geng Y, Klocke BJ, Latham CB, Villunger A, et al. BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death. J Neurosci. 2006;26:7257–7264. doi: 10.1523/JNEUROSCI.0196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 29.Jiang M, Wei Q, Wang J, Du Q, Yu J, et al. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25:4056–4066. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- 30.Gallenne T, Gautier F, Oliver L, Hervouet E, Noël B, et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravi R, Bedi A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L). Cancer Res. 2002;62:1583–1587. [PubMed] [Google Scholar]
- 32.Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat Immunol. 2003;4:255–260. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- 33.O'Flaherty J, Mei Y, Freer M, Weyman CM. Signaling through the TRAIL receptor DR5/FADD pathway plays a role in the apoptosis associated with skeletal myoblast differentiation. Apoptosis. 2006;11:2103–2113. doi: 10.1007/s10495-006-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zauli G, Secchiero P. The role of the TRAIL/TRAIL receptors system in hematopoiesis and endothelial cell biology. Cytokine Growth Factor Rev. 2006;17:245–257. doi: 10.1016/j.cytogfr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Brunetti G, Oranger A, Mori G, Tamma R, Di Benedetto A, et al. TRAIL is involved in human osteoclast apoptosis. Ann N Y Acad Sci. 2007;1116:316–322. doi: 10.1196/annals.1402.011. [DOI] [PubMed] [Google Scholar]
- 36.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, et al. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 37.Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, et al. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur J Immunol. 2002;32:2246–2254. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 39.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1999;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 40.Braghiroli L, Silvestrini B, Sorrentino C, Grima J, Mruk D, et al. Regulation of α2-macroglobulin expression in rat Sertoli cells and hepatocytes by germ cells in vitro. Biol Reprod. 1998;59:111–123. doi: 10.1095/biolreprod59.1.111. [DOI] [PubMed] [Google Scholar]
- 41.Callus BA, Moujallad DM, Silke J, Gerl R, Jabbour AM, et al. Triggering of apoptosis by Puma is determined by the threshold set by prosurvival Bcl-2 family proteins. J Mol Biol. 2008;384:313–323. doi: 10.1016/j.jmb.2008.09.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of apoptotic markers in irradiated testes. On the basis of TUNEL dose-response previously described by Hasegawa M, Wilson G, Russell LD, Meistrich ML (1997) Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiation research. 147: 457–467. Immunohistochemistry on SV129 testes, Immunostaining was performed on 5microm sections fixed in Bouin. After antigen retrieval by microwave irradiation in citrate buffer, sections were treated with 0.3% H2O2 in PBS and blocked with 3% BSA in PBS. They were then incubated with anti-cleaved caspase-9 (Asp353), -cleaved caspase-7 (Asp198) (Cell Signaling), -cytochrome c (6H2B4, Becton Dickinson) or -p53 (CM5, Novocastra) antibodies diluted in BSA overnight at 4°C. Labeling was revealed using the ABC Vectastain Kit (Vector Laboratories) and Sigma fast DAB (Sigma). Sections were counterstained with hematoxylin and analyzed under an Olympus BX51 photomicroscope equipped with an Insight QE Spot camera. (A) P53 is stabilized 4 h post-IR in spermatogonia, (B) Cytochrome c is released 7 h post-IR in some spermatogonia. Arrows indicate positive spermatogonia (bar = 20 microm). (C) Detection of dead spermatogonia 16h-post IR by TUNEL assay. (D) Cleavage of pro-caspase-9 and -7 in spermatogonia, with antibodies specifically recognizing the processed form. Arrows indicate positive spermatogonia (bar = 20 microm). (E) Processing of pro-caspase-2 and -8 followed by immunoblotting of whole lysates of MACS- alpha6+ fraction (enriched in spermatogonia) versus MACS - alpha6- fraction (including spermatocytes I, spermatocytes II and spermatids) prior to and post-IR; actin was used as a loading control. Representative results obtained from 3 male pooled testicular cells at each time point. Each experiment was performed at least twice. Membranes were hybridized with anti-caspase- 8 (1G12) and anti-caspase- 2 (11B4) (Alexis), anti-betaactin (AC15, Sigma-Aldrich) and proteins were visualized with peroxidase-coupled secondary antibodies (Pearce) using the ECL Plus System (GEHealth) on Kodak films.
(0.96 MB TIF)
Flow cytometric analysis of Dr5 labeling on testicular cell suspension. (a) Testicular suspension is analyzed according to Ho42 and PI staining; viable cells are gated in R1, and dead cells are excluded. (b) Elongated spermatids are excluded from the viable testicular cells (gate R2). (c) The SP cells are gated, and further analyzed for Dr5 labeling (d) according to the isotypic threshold (gate R4).
(0.14 MB TIF)
Analysis of the expression levels of Noxa, Bid, Bcl-XL and Bax genes. Comparison in p53+/+ or p53−/− sorted CD45- alpha6+ SP cells, by semi-quantitative RT-PCR prior to, 7 and 12 hours after irradiation. Each expression level was normalized to its value in non-irradiated cells and is indicated as a fold increase (± SEM, 3 separate experiments from pooled CD45- alpha6+ SP populations, independently purified from 5 males). On left panel, expression of the anti-apoptotic Bcl-XL gene did not vary significantly, like RNA levels of the pro-apoptotic BH3-only members Bid and Noxa, potently involved in DNA damage response. On right panel, expression of the pro-apoptotic Bax gene was significantly reduced in irradiated CD45- alpha6+ SP cells (black star p = 0.00031). The basal level in p53−/− cells was significantly increased (p = 0.0028).
(0.09 MB TIF)
Characterization of Dr5+ and Dr5- cell populations Total testicular sample was purified by MACS according to Dr5 expression. MACS cellular fractions were cytospined, fixed with 2% PFA-PBS and stained with anti-CD9, anti-Kit (Becton Dickinson) or anti-Plzf after Triton permeabilization (Santa Cruz). After incubation with a secondary antibody coupled to Alexa 488, cells were counterstained with Dapi. Kit is a marker of differentiated spermatogonia, and not of testicular stem cells. CD9 is expressed on spermatogonia and also on stem cells, which are specifically expressing plzf. Most of the Kit- spermatogonia are Plzf+.
(0.89 MB TIF)
(0.84 MB TIF)