Abstract
Background
Mosquito-borne diseases are a worldwide public health threat. Mosquitoes transmit viruses or parasites during feeding, along with salivary proteins that modulate host responses to facilitate both blood feeding and pathogen transmission. Understanding these earliest events in mosquito transmission of arboviruses by mosquitoes is essential for development and assessment of rational vaccine and treatment strategies. In this report, we compared host immune responses to chikungunya virus (CHIKV) transmission by (1) mosquito bite, or (2) by needle inoculation.
Methods and Findings
Differential cytokine expression was measured using quantitative real-time RT-PCR, at sites of uninfected mosquito bites, CHIKV-infected mosquito bites, and needle-inoculated CHIKV. Both uninfected and CHIKV infected mosquitoes polarized host cytokine response to a TH2 profile. Compared to uninfected mosquito bites, expression of IL-4 induced by CHIKV-infected mosquitoes were 150 fold and 527.1 fold higher at 3 hours post feeding (hpf) and 6 hpf, respectively. A significant suppression of TH1 cytokines and TLR-3 was also observed. These significant differences may result from variation in the composition of uninfected and CHIKV-infected mosquito saliva. Needle injected CHIKV induced a robust interferon-γ, no detectable IL-4, and a significant up-regulation of TLR-3.
Conclusions
This report describes the first analysis of cutaneous cytokines in mice bitten by CHIKV–infected mosquitoes. Our data demonstrate contrasting immune activation in the response to CHIKV infection by mosquito bite or needle inoculation. The significant role of mosquito saliva in these earliest events of CHIKV transmission and infection are highlighted.
Introduction
Mosquitoes are a significant public health problem because of their ability to transmit a variety of arboviruses and also the causative agents of malaria and filariasis to susceptible humans (www.who.int/tdr/diseases). Mosquito-borne diseases continue to emerge and re-emerge [1], [2] as demonstrated by the recent chikungunya epidemics on Indian Ocean islands and in India since 2005. Chikungunya virus (CHIKV) is an Alphavirus belonging to family Togaviridae, which is transmitted predominantly by Aedes aegypti and Ae. albopticus (www.cdc.gov/ncidod/dvbid/Chikungunya). Both mosquito species occur over vast regions of the world, including the United States, southern Europe, and tropical regions of South America, Africa, and Asia posing the very real threat that new transmission cycles could be established in these regions [3]. Since 2005, CHIK fever has been identified in an unprecedented number of travelers returning home from epidemic areas to Europe, United States, Australia, and Japan [3], [4], [5], [6], [7]. Imported CHIKV infection in returned travelers paralleled the spread of the explosive outbreaks in the Indian Ocean islands and India. In 2006, CHIKV infections were detected in Singapore among travelers returning home after visiting India and Malaysia. Those sporadic imported cases preceded the 2008 chikungunya outbreaks in Singapore, demonstrating the potential for introducing this emerging viral infection into new areas and establishing a transmission cycle with competent local vector mosquito species [8]. Thus, there is a clear risk of importing CHIKV into new ecological niches through infected travelers returning from popular tourist destinations with CHIKV epidemics.
Human infections with CHIKV occur during blood feeding by infected Aedes mosquitoes. Mosquito saliva contains a repertoire of pharmacologically important proteins/factors that modulate host haemostasis, immune response and other defenses thus facilitating blood feeding and pathogen transmission [9], [10], [11], [12], [13], [14]. Previous studies have reported that mosquito bite enhances infection with Cache Valley virus (CVV) [15], West Nile virus (WNV) [16], vesicular stomatitis virus (VSV) [17], [18], and La Crosse virus (LACV) [19]. Exposure to the bites of uninfected Ae. aegypti exacerbated mosquito transmitted WNV infection [20]. Mosquito feeding skews host T-cell immune responses away from a TH1 to a TH2 phenotype [21], [22], [23], which subsequently creates an environment that favors arbovirus transmission and infection that would otherwise be neutralized by TH1 cytokines [21], [24]. We recently showed the dynamics of dermal TH1 and TH2 cytokine expression at the bite sites of Ae. aegypti and identified an Ae. aegypti salivary gland protein that causes TH2 polarization of host CD4+ T-cells [25].
The earliest events of CHIKV transmission by mosquitoes remain poorly understood. Current knowledge of CHIKV infection comes from studies of WNV, LACV, and CVV infections. Effects of mosquito saliva on dermal cell expression of cytokines after a CHIKV-infected mosquito bite are unknown; but characterization of these mosquito-host-pathogen interactions will result in a better understanding of the earliest events in the successful transmission and establishment of CHIKV infection. Here we describe the influence of CHIKV-infected mosquito bite on the cutaneous TH1 and TH2 cytokine responses and compare the responses to that following needle injection of CHIKV.
Results
Dermal cytokine responses to uninfected or CHIKV infected mosquito bites
Mosquitoes inject saliva at the bite site during blood feeding to circumvent the host physical barriers and the complex and redundant physiological responses orchestrated by the host's haemostatic and inflammatory systems that have evolved to prevent blood loss and combat infection. Effects of CHIKV infected mosquito saliva on the dermal cell expression of cytokines at the mosquito bite site have not been reported. To characterize the influence of infected mosquito saliva on the first events of CHIKV transmission, cytokine gene expression was measured by real-time RT-PCR in skin biopsies collected at 3 and 6 hours post –feeding (hpf) by uninfected and CHIKV infected mosquitoes (Fig 1) as relative fold difference compared to naïve mice skin biopsies. In the uninfected mosquito bite samples, expression of IL-4 was up-regulated 417 fold and 76.9 fold at 3hpf and 6 hpf, respectively, while the expression of IL-2, IFN-γ and TLR-3 were down-regulated 6.6 fold, 4 fold and 3.7 fold, respectively, at 6 hpf in the same samples. In the CHIKV infected mosquito bite biopsies, expression of IL-4 was up-regulated 567 fold and 604 fold at 3 hpf and 6 hpf, respectively, while the expression ofIL-2, IFN-γ, and TLR-3 were down-regulated 2.1 fold, 1.6 fold and 5.5 fold at 6 hpf, respectively. Importantly, expression of IFN-γ was down-regulated 4.3 fold at 3 hpf in tissues exposed to CHIKV infected mosquito bites (Fig 1). Notably, compared to the uninfected mosquito bites, the expression of IL-4 was 150 fold and 527.1 fold higher in the CHIKV infected mosquito samples at 3 hpf and 6 hpf, respectively (Table 1). Overall, TH2 cytokines were significantly up-regulated while TH1 cytokines were significantly down-regulated at both study time points.
Figure 1. Comparison between uninfected mosquito (UIM) bites and CHIKV infected mosquito (CIM) bites.
Uninfected and CHIKV infected Ae. aegypti ware allowed to feed upon mouse ears, and total RNA was extracted from biopsies at the indicated times. Real-time RT-PCR was performed to measure expression of the indicated cytokine mRNAs. RNA extracted from ears of mice not exposed to mosquitoes were considered as naïve and assigned an arbitrary value of 1.0, and changes in mosquito-induced cytokine gene expression are expressed as the ratio between mosquito-fed and naïve samples. GAPDH gene was used as a normalizing control. The asterisk denotes a statistically significant difference between the means of naïve and experimental groups (*-P≤0·05; **- P≤0·001). N = 3 per group.
Table 1. Differential expression of cytokines and TLR-3 induced by CHIKV infected mosquito bites.
| Relative fold difference compared to uninfected mosquito bites | ||||
| UIM-3 hpf (naïve) | CIM-3 hpf | UIM-6 hpf (naïve) | CIM-6 hpf | |
| IL-2 | 1 | −2.6 | 1 | 4.4* |
| IL-4 | 1 | 150* | 1 | 527.1** |
| IL-10 | 1 | 0.55 | 1 | 1.01 |
| IFN-γ | 1 | −2.8* | 1 | 2.3* |
| TLR-3 | 1 | 3.64* | 1 | −1.8 |
Relative fold differences were calculated by considering uninfected mosquito bites as naïve. (UIM- uninfected mosquito bites; CIM- CHIKV infected mosquito bites).
*- P≤0.05; **- p≤0.001.
Cytokine responses to needle injected CHIKV
To understand the first immunological events in CHIKV infection without mosquito saliva, we measured the expression of cytokines at sites of intradermal needle-injected CHIKV, using real-time RT-PCR and compared with that of medium injected mouse ear biopsies (naïve). Notably, TH1 cytokines were significantly up-regulated while TH2 cytokines showed no significant change in their expression (Fig 2). Expression of TLR-3 was up-regulated 3.4 fold and 8.8 fold at 3 hpi and 6 hpi, respectively. Expression of IFN-γ was up-regulated 172 fold and 523.2 fold at 3 hpi and 6 hpi, respectively, and expression of IL-2 was up-regulated 2.1 fold at 3 hpi and 8.9 fold at 6 hpi, compared to naive.
Figure 2. Comparison between CHIKV infected mosquito (CIM) bites and needle injected CHIKV.
CHIKV infected Ae. aegypti were allowed to feed on mouse ears and total RNA was extracted from biopsies at the indicated times. In parallel, total RNA was extracted from mouse ear biopsies at sites of needle inoculation of CHIKV or medium without virus. Real-time RT-PCR was performed to measure expression of the indicated cytokine mRNAs. RNA extracted from ears of mice not exposed to mosquitoes was considered as naïve for CHIKV infected mosquito bite tissue samples. Medium-inoculated mouse biopsy samples were considered naive for needle inoculated CHIKV samples. Naive samples were assigned an arbitrary value of 1.0, and changes in mosquito-induced cytokine gene expression were expressed as the ratio between mosquito-fed and naïve samples. GAPDH gene was used as a normalizing control. The asterisk denotes a statistically significant difference between the means of naïve and experimental groups (*-P≤0·05; **- P≤0·001). N = 3 per group.
Cellular recruitment by mosquito saliva
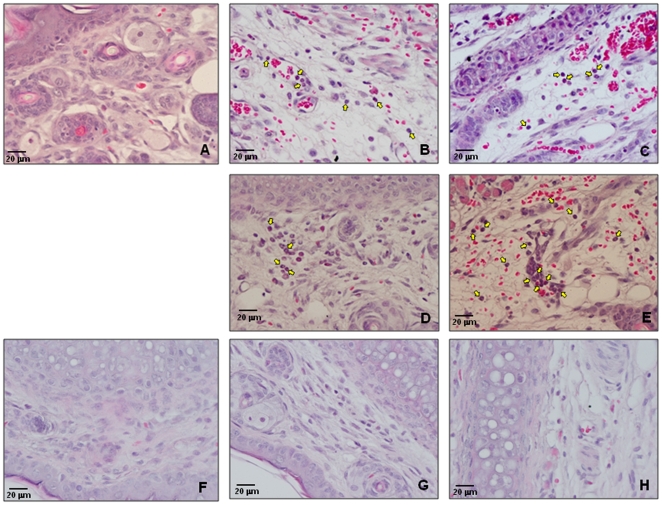
To further understand the effects of mosquito saliva in arboviral transmission, mosquito bite sites were paraffin embedded, sectioned and H&E stained. Recruitment of eosinophils was observed at both uninfected and CHIKV-infected mosquito bite sites (Fig 3 B, C, D and E). Although, a few neutrophils were observed at the CHIKV-infected mosquito bite sites, eosinophils were present in abundance. Notably, more eosinophil recruitment was observed at CHIKV- infected mosquito bite sites. Histological examination of biopsies taken at the CHIKV-injected site did not show any immune cell recruitment. There were no differences in the cellular population between naïve and CHIKV-injected sites at either 3 or 6 hpi (Fig 3 G and H).
Figure 3. Histopathological changes at the mosquito bite and CHIKV injected sites.
Biopsies obtained from mouse ear samples were fixed in 10% neutral formalin and paraffin embedded. Four to five millimeter sections were made and H&E stained. Slides were observed for cellular recruitment at the mosquito bite site or CHIKV injection site. Yellow arrows in the images point to eosinophils. A-uninfected mice (naïve); B- uninfected mosquito bite site (3hpf); C- uninfected mosquito bite site (6 hpf); D-CHIKV infected mosquito bite site (3 hpf); E-CHIKV infected mosquito bite site (6 hpf); F-medium injected site, G-needle injected CHIKV site(3 hpi); H-needle injected CHIKV site (6 hpi). N = 3 per group.
Discussion
This report provides the first analysis of selected cutaneous cytokine changes in a mouse model of CHIKV infection by mosquito bite. We also describe significant comparative differential cytokine responses of CHIKV transmitted by mosquito bite or CHIKV transmitted by intradermal needle inoculation. Mouse ears exposed to uninfected Ae. aegypti bites induced significant levels of IL-4 and suppressed IFN-γ, IL-2, and TLR-3 transcripts. These changes correlated with our earlier findings of similar responses to mosquito bites by BALB/c mice [25]. Interestingly, CHIKV-infected Ae. aegypti that fed on mouse ear induced a similar response but with a higher fold induction of IL-4. Significantly, increases of 150 fold at 3 hpf and 527.1 fold at 6 hpf were observed for CHIKV infected bite site biopsies, when compared with uninfected mosquito bite site biopsies (Table 1). Clearly, CHIKV infected mosquito bites prolong suppression of TH1 cytokine production, while inducing expression of TH2 cytokines. This skewing of host immunity towards a TH2 profile at the bite would favor infection and dissemination of CHIKV in the host due to down-regulation of anti-virus TH1 cytokines.
Modulation of host immunity towards TH2 responsiveness by mosquito saliva is reported to facilitate transmission of both CVV [15] and WNV [16]. It is plausible that CHIKV could have evolved to facilitate upregulation or downregulation of mosquito secretion of salivary proteins/factors that could favor virus replication, transmission and/or persistence in the host.
Previous studies reported that feeding efficiency of Ae. aegypti were adversely affected by arboviral [26] or malaria parasite [27] infection. These behavioral and physiologic effects are likely associated with changes in the structure of the salivary glands or in the composition of mosquito saliva. Infection of WNV in the mosquito salivary glands induced distinct morphologic and cytopathologic changes. Salivary gland function and virus transmission efficiency changed during the course of WNV infection due to pathological changes in the mosquito [28], resulting in a differential salivary gland transcript profile [29]. In Anopheles gambiae, 57 salivary gland genes were differentially regulated upon Plasmodium berghei infection [30].
Understanding the role of mosquito saliva in the earliest stages of CHIKV infection and transmission was highlighted by the dynamics of TH1 and TH2 cytokine responses when comparing needle inoculated-CHIKV with to CHIKV introduced into the host by mosquito bite. Needle inoculated-CHIKV polarized the host cytokines towards a TH1 response with significant up-regulation of IFN-γ and IL-2. Expression of IL-4 and IL-10 did not show significant change in transcript levels following needle inoculation of virus. In contrast, CHIKV infected mosquito bites skewed the host immunity towards a TH2 profile with significant IL-4 regulation. These findings clearly demonstrate that CHIKV infected mosquito feeding skews host T-cell immune responses away from a TH1 to a TH2 phenotype, which in turn can facilitate transmission of CHIKV that might otherwise be inactivated by TH1 cytokines.
Toll-like receptors (TLR) have essential roles in the initiation of innate immunity to infectious agents. In mammals, TLR family is composed of at least 12 members and each TLR acts as a primary sensor of conserved microbial components and drives the induction of specific biological responses [31]. Specific recognition of viruses by TLRs has been previously documented. The role of TLR-3 has been implicated in protective immune responses against single stranded RNA viruses such as WNV [32] and double strand RNA viruses such as Lang reovirus [33]. Our data show that needle injected CHIKV significantly up-regulated the transcription of TLR-3. In contrast, the expression of TLR-3 was down-regulated by both uninfected and CHIKV infected mosquito bites.
Expression of IFN-γ is important in defense against RNA viruses by inducing proliferation and differentiation of many cell types, activating the production of cellular proteins that prevent viral mRNA translation; and, enhancing macrophage nitric oxide production [34], [35]. Up-regulation of IFN-γ and TLR-3 in response to needle injected CHIKV suggests their role in protective immune response against this virus. These anti-viral responses are suppressed by CHIKV infected mosquito bites. The prototypical TH2 cytokine IL-4 inhibits TH1 clonal expansion including the expression of IFN-γ and activation of cytotoxic T-cells [36].
Real-time RT-PCR data reported here correlates with our histological observations. Uninfected and CHIKV-infected mosquito bites recruited eosinophils. This corresponds to up-regulation of IL-4 transcription. Also, CHIKV-infected mosquito bites recruited more eosinophils than uninfected mosquito bite sites at both 3 and 6 hpf. In contrast, needle injected CHIKV samples did not show immune cell recruitment. Also, the cell population looked similar to the naïve sample (Fig 3). It is possible that the resident cells such as keratinocytes, macrophages, and dendritic cells could be responding to virus infection by up-regulating the antiviral IFN-γ and TLR-3 transcription.
In this study, we describe, for the first time, an analysis of selected cutaneous cytokines during CHIKV infection by mosquito bite, compared with that of needle inoculated CHIKV. Our data demonstrate contrasting immune activation in response to CHIKV infection by these two different routes of transmission. This highlights a significant role of Ae. aegypti saliva in the earliest events of CHIKV transmission and infection and further confirms the importance of studying a mosquito-borne arbovirus using actual mosquito transmission of the virus, rather than needle inoculation of virus alone. Our study was performed under a controlled environment utilizing mice that were not pre-exposed to CHIKV with the objective of elucidating the earliest immune response to CHIKV infection, and to evaluate the immune response between mosquito- transmitted and needle-injected CHIKV. However, the consequences of CHIKV infection of mice pre-exposed to un-infected/infected mosquito bites or needle-injected CHIKV has not yet been investigated, and it is the subject of our future study.
Materials and Methods
Ethics statement
All experiments were conducted in an animal biosafety level 3 (ABSL-3) facility in accordance with a protocol (number: 0912070) approved by the University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee (IACUC).
Animals
Outbred CD-1 strain used in this study mice were obtained from Charles River Laboratories (Wilmington, MA). Mice were cared for in accordance with guidelines of the Committee on Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources National Research Council, Washington, DC).
Virus
Full length infectious clones of CHIKV that express GFP (CHIKV-LR 5′GFP) [31] was used in this study. This infectious clone was produced using the LR2006 OPY1 strain of CHIKV (CHIKVLR), obtained from the World Reference Center for Arboviruses at the University of Texas Medical Branch, Galveston, TX, and readily infects Ae. aegypti at a similar rate to the wild type virus, LR2006 OPY1 [37]. The presence of GFP in this clone allowed us to determine CHIKV infection in mosquitoes using epifluorescence microscopy.
In vitro growth of virus
C6/36 Ae. albopictus-derived cells were maintained at 28°C in Leibovitz L-15 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100µg/mL streptomycin [31]. Confluent monolayers of C6/36 cells were infected with CHIKV at a multiplicity of infection (moi) of 0.1 by rocking for 1 h at 25°C in 25-cm2 flasks. Cells were washed with 5 mL of L-15 medium three times and then 5.5 mL of L-15 medium was added per flask. At day 0 and at 12, 24, 48, 72, and 96 hours post-infection (hpi), a 0.5-mL sample of medium was removed and stored at −80°C. The volume of medium was restored by adding 0.5 mL of fresh medium after each sampling.
Titrations
Viral samples harvested from cell culture were quantified as tissue culture infectious dose 50 endpoint titers (log10 TCID50/mL) as described previously [37], [38]. Briefly, 100mL samples of cell culture supernatant medium was pipetted into wells of the first column of a 96-well plate seeded with C6/36 cells, serially diluted in a 10-fold series, and were incubated at 37°C for 7 days with 100 U/mL penicillin, and 100µg/mL streptomycin.
Mosquito maintenance
The Ae. aegypti Higgs White eye strain colony used in this study was maintained within the Biosafety level-3 (BSL-3) insectary at the University of Texas Medical Branch in Galveston. This colony was maintained at 28°C, with relative humidity of 70–75% under a light∶dark cycle of 14hr∶10hr with a 1h crepuscular period to simulate dawn and dusk. Mosquito eggs were maintained on semi-wet filter-paper in a humidified chamber. Eggs were placed into a plastic pan with water of approximately 1-inch depth with a small amount of food (1∶1∶1 powdered laboratory rodent diet, lactalbumin and brewers yeast) added. Under these conditions, larvae developed into the pupae stage in six to seven days. Pupae were removed, sex determined, and transferred into a small cup of water placed in a rearing cage for eclosion. Emerged adults were provided with 10% sucrose ad libitum and fed weekly on anaesthetized hamsters as per NIH guidelines for humane use of laboratory animals. Female mosquitoes were starved for 12–24 h prior to blood feeding.
Mosquito infections
Four to five day old female Ae. aegypti were intrathoracically infected with CHIKV-LR 5′GFP (6 log10 TCID50/mL) using an isolation glove box located in a BSL-3 insectary. All infections were performed in an isolation glove box. Female mosquitoes were cold-anesthetized and intrathoracically injected by using a Drummond 100µl microcapillary needle prepared with a needle puller (Narishige, Tokyo). Approimately 1µl of 6 log10 TCID50/mL of CHIKV-LR-5′GFP in L-15 medium containing 10% FBS, 100 U/mL penicillin, and 100µg/mL streptomycin was injected into each mosquito. Mosquitoes injected with only L-15 medium containing FBS and antibiotics were considered as uninfected control mosquitoes. At nine days post- infection, injected mosquitoes were used in the study.
Real-time RT-PCR to measure differential cytokine gene expression at mosquito bite sites
Twelve individual CHIKV-infected female Ae. aegypti was allowed to blood feed on the left ear of 12 two week old CD-1 mice for 30 minutes. The right ears were excluded in our experiments. Fed mosquitoes were then dissected to check for CHIKV infection by observing GFP signal in the salivary glands. Twelve other female Ae. aegypti injected with medium alone (uninfected) were allowed to feed on the left ear of 12 additional mice of the same age. In both of these experimental groups, six mice were used for each time point. Three of the mice in each group were used for cytokine expression analysis and the other three were used for histology. Punch biopsies (4 mm) were then obtained from ear bite sites at 3 hpf and 6 hpf, stored in RNALater (Ambion). Trizol reagent (Invitrogen) was used to extract total RNA. Genomic DNA contamination was eliminated by DNAse treatment. Total RNA was measured using a NanoDrop 1000 (Thermo Scientific) and RNA quality was analyzed by denaturing gel electrophoresis. First strand cDNA was synthesized from 1 µg total RNA using a Retroscript 1st Strand cDNA synthesis kit (Ambion) and subsequently used as template for real-time RT-PCR analysis. Real-time RT-PCR amplifications were performed using RT2Real-Time™ SYBR Green/Fluorescein PCR master mix (SABiosciences) in an iCycler (BioRad). The primers used in this experiment are listed in Table 2. Typically, PCR was performed by heating to 95°C for 10 min to heat-activate the HotStart Taq DNA Polymerase followed by 40 cycles of 15 sec at 94°C then 60 sec at 60°C. All reactions were performed in triplicate. Each time point sample had 3 biological replicates. GAPDH mRNA was used as a normalizing standard and RNA from mosquito non-exposed ear biopsies were considered as naïve and assigned an arbitrary value of 1.0. Changes in mosquito bite-induced cytokine gene expression were calculated as the ratio between mosquito bite and naïve samples.
Table 2. Primers used for real-time RT-PCR.
| NCBI sequence ID | Primers (5′ to 3′) | |
| GAPDH | NM_001001303 | TTGAGGTCAATGAAGGGGTC TCGTCCCGTAGACAAAATGG |
| IL-2 | NM_008366 | GTCAAATCCAGAACATGCCG AACCTGAAACTCCCCAGGAT |
| IL-4 | NM_021283 | CGAGCTCACTCTCTGTGGTG TGAACGAGGTCACAGGAGAA |
| IL-10 | NM_010548 | TGGCCTTGTAGACACCTTGG AGCTGAAGACCCTCAGGATG |
| IFN-γ | NM_008337 | GAGCTCATTGAATGCTTGGC GCGTCATTGAATCACACCTG |
| TLR-3 | NM_126166 | ATAGGGACAAAAGTCCCCCA ATGATACAGGGATTGCACCC |
Cytokine response to needle injected CHIKV
Ten micro litres of CHIKV containing 3 log10 TCID50 were intradermally injected into the left ear of CD-1 mice. The same volume of medium was injected intradermally in to mice serving as a naïve control. Punch biopsies (4 mm) were obtained from the injection sites at 3 hpi and 6 hpi. Total RNA, first strand cDNA synthesis and cytokine real-time PCR were performed as described above. All reactions were performed in triplicate. Each time point sample had 3 biological replicates. GAPDH mRNA was used as a normalizing standard and RNA from the medium injected ear biopsies was considered as naïve and assigned an arbitrary value of 1.0. Changes in mosquito bite-induced cytokine gene expression were calculated as the ratio between mosquito bite and naïve samples.
Statistics
Statistical analyses were preformed with Graph Pad 4.0 Prism software. One way nonparametric ANOVA followed by Tukey post test was performed [39].
Histology
Tissues were processed for histology as described by Ziegler et al. (2008). Briefly, 4 mm ear biopsies obtained from each mouse were fixed in 10% neutral-buffered formalin for 36 hours; transferred to 70% ethanol prior to embedding, sectioning at 4 to 5 µm and staining with hematoxylin and eosin (H&E).
Acknowledgments
We thank Jing Huang and Nicole Hausser, for their technical assistance with this study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by United States Army Medical Research and Materiel Command Award 0310075 and National Institutes of Health (NIH) Grant AI062735 to SW. This study was also supported in part by NIH contract N01-AI25489 and N01-AI30027 to RT, and NIH grant AI R21 AI073389 to SH. SZ is funded by the Sealy Center for Vaccine Development, UTMB, Galveston. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gratz NG. Emerging and resurging vector-borne diseases. Annu Rev Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 3.Simon F, Savini H, Parola P. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Med Clin North Am. 2008;92:1323–1343. doi: 10.1016/j.mcna.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Hochedez P, Hausfater P, Jaureguiberry S, Gay F, Datry A, et al. Cases of chikungunya fever imported from the islands of the South West Indian Ocean to Paris, France. Euro Surveill. 2007;12 [Google Scholar]
- 5.Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, et al. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeffer M, Loscher T. Cases of chikungunya imported into Europe. Euro Surveill. 2006;11:E060316 060312. [PubMed] [Google Scholar]
- 7.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 8.Lim PL, Oh HM, Ooi EE. Chikungunya in Singapore: imported cases among travelers visiting friends and relatives. J Travel Med. 2009;16:289–291. doi: 10.1111/j.1708-8305.2009.00313.x. [DOI] [PubMed] [Google Scholar]
- 9.Calvo E, Mans BJ, Ribeiro JM, Andersen JF. Multifunctionality and mechanism of ligand binding in a mosquito anti inflammatory protein. Proc Natl Acad Sci U S A. 2009;106:3728–3733. doi: 10.1073/pnas.0813190106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvo E, Tokumasu F, Marinotti O, Villeval JL, Ribeiro JM, et al. Aegyptin, a novel mosquito salivary gland protein, specifically binds to collagen and prevents its interaction with platelet glycoprotein VI, integrin alpha2beta1, and von Willebrand factor. J Biol Chem. 2007;282:26928–26938. doi: 10.1074/jbc.M705669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro JM, Arca B, Lombardo F, Calvo E, Phan VM, et al. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics. 2007;8:6. doi: 10.1186/1471-2164-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribeiro JM, Rossignol PA, Spielman A. Role of mosquito saliva in blood vessel location. J Exp Biol. 1984;108:1–7. doi: 10.1242/jeb.108.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro JM, Sarkis JJ, Rossignol PA, Spielman A. Salivary apyrase of Aedes aegypti: characterization and secretory fate. Comp Biochem Physiol B. 1984;79:81–86. doi: 10.1016/0305-0491(84)90081-6. [DOI] [PubMed] [Google Scholar]
- 14.Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2008;102:400–408. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards JF, Higgs S, Beaty BJ. Mosquito feeding-induced enhancement of Cache Valley Virus (Bunyaviridae) infection in mice. J Med Entomol. 1998;35:261–265. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- 16.Schneider BS, Soong L, Girard YA, Campbell G, Mason P, et al. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 2006;19:74–82. doi: 10.1089/vim.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 17.Limesand KH, Higgs S, Pearson LD, Beaty BJ. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 2000;22:461–467. doi: 10.1046/j.1365-3024.2000.00326.x. [DOI] [PubMed] [Google Scholar]
- 18.Limesand KH, Higgs S, Pearson LD, Beaty BJ. Effect of mosquito salivary gland treatment on vesicular stomatitis New Jersey virus replication and interferon alpha/beta expression in vitro. J Med Entomol. 2003;40:199–205. doi: 10.1603/0022-2585-40.2.199. [DOI] [PubMed] [Google Scholar]
- 19.Osorio JE, Godsey MS, Defoliart GR, Yuill TM. La Crosse viremias in white-tailed deer and chipmunks exposed by injection or mosquito bite. Am J Trop Med Hyg. 1996;54:338–342. doi: 10.4269/ajtmh.1996.54.338. [DOI] [PubMed] [Google Scholar]
- 20.Schneider BS, McGee CE, Jordan JM, Stevenson HL, Soong L, et al. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS ONE. 2007;2:e1171. doi: 10.1371/journal.pone.0001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider BS, Soong L, Zeidner NS, Higgs S. Aedes aegypti salivary gland extracts modulate anti-viral and TH1/TH2 cytokine responses to sindbis virus infection. Viral Immunol. 2004;17:565–573. doi: 10.1089/vim.2004.17.565. [DOI] [PubMed] [Google Scholar]
- 22.Wanasen N, Nussenzveig RH, Champagne DE, Soong L, Higgs S. Differential modulation of murine host immune response by salivary gland extracts from the mosquitoes Aedes aegypti and Culex quinquefasciatus. Med Vet Entomol. 2004;18:191–199. doi: 10.1111/j.1365-2915.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 23.Zeidner NS, Higgs S, Happ CM, Beaty BJ, Miller BR. Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 1999;21:35–44. doi: 10.1046/j.1365-3024.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- 24.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 25.Boppana VD, Thangamani S, Adler AJ, Wikel SK. SAAG-4 is a novel mosquito salivary protein that programmes host CD4 T cells to express IL-4. Parasite Immunol. 2009;31:287–295. doi: 10.1111/j.1365-3024.2009.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platt KB, Linthicum KJ, Myint KS, Innis BL, Lerdthusnee K, et al. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57:119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- 27.Rossignol PA, Ribeiro JM, Spielman A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am J Trop Med Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- 28.Girard YA, Schneider BS, McGee CE, Wen J, Han VC, et al. Salivary gland morphology and virus transmission during long-term cytopathologic West Nile virus infection in Culex mosquitoes. Am J Trop Med Hyg. 2007;76:118–128. [PubMed] [Google Scholar]
- 29.Girard YA, Mayhew GF, Fuchs JF, Li H, Schneider BS, et al. Transcriptome changes in Culex quinquefasciatus (Diptera: Culicidae) salivary glands during West Nile virus infection. J Med Entomol. 47:421–435. doi: 10.1603/me09249. [DOI] [PubMed] [Google Scholar]
- 30.Rosinski-Chupin I, Briolay J, Brouilly P, Perrot S, Gomez SM, et al. SAGE analysis of mosquito salivary gland transcriptomes during Plasmodium invasion. Cell Microbiol. 2007;9:708–724. doi: 10.1111/j.1462-5822.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi ST, Medzhitov R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 2003;4:87–94. doi: 10.1038/sj.gene.6363937. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, et al. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 34.Katze MG, Fornek JL, Palermo RE, Walters KA, Korth MJ. Innate immune modulation by RNA viruses: emerging insights from functional genomics. Nat Rev Immunol. 2008;8:644–654. doi: 10.1038/nri2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, et al. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owhashi M, Harada M, Suguri S, Ohmae H, Ishii A. The role of saliva of Anopheles stephensi in inflammatory response: identification of a high molecular weight neutrophil chemotactic factor. Parasitol Res. 2001;87:376–382. doi: 10.1007/s004360000355. [DOI] [PubMed] [Google Scholar]
- 37.Tsetsarkin K, Higgs S, McGee CE, De Lamballerie X, Charrel RN, et al. Infectious clones of Chikungunya virus (La Reunion isolate) for vector competence studies. Vector Borne Zoonotic Dis. 2006;6:325–337. doi: 10.1089/vbz.2006.6.325. [DOI] [PubMed] [Google Scholar]
- 38.Higgs S, Traul D, Davis BS, Kamrud KI, Wilcox CL, et al. Green fluorescent protein expressed in living mosquitoes–without the requirement of transformation. Biotechniques. 1996;21:660–664. doi: 10.2144/96214st03. [DOI] [PubMed] [Google Scholar]
- 39.Thangamani S, Wikel SK. Differential expression of Aedes aegypti salivary transcriptome upon blood feeding. Parasit Vectors. 2009;2:34. doi: 10.1186/1756-3305-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]