Abstract
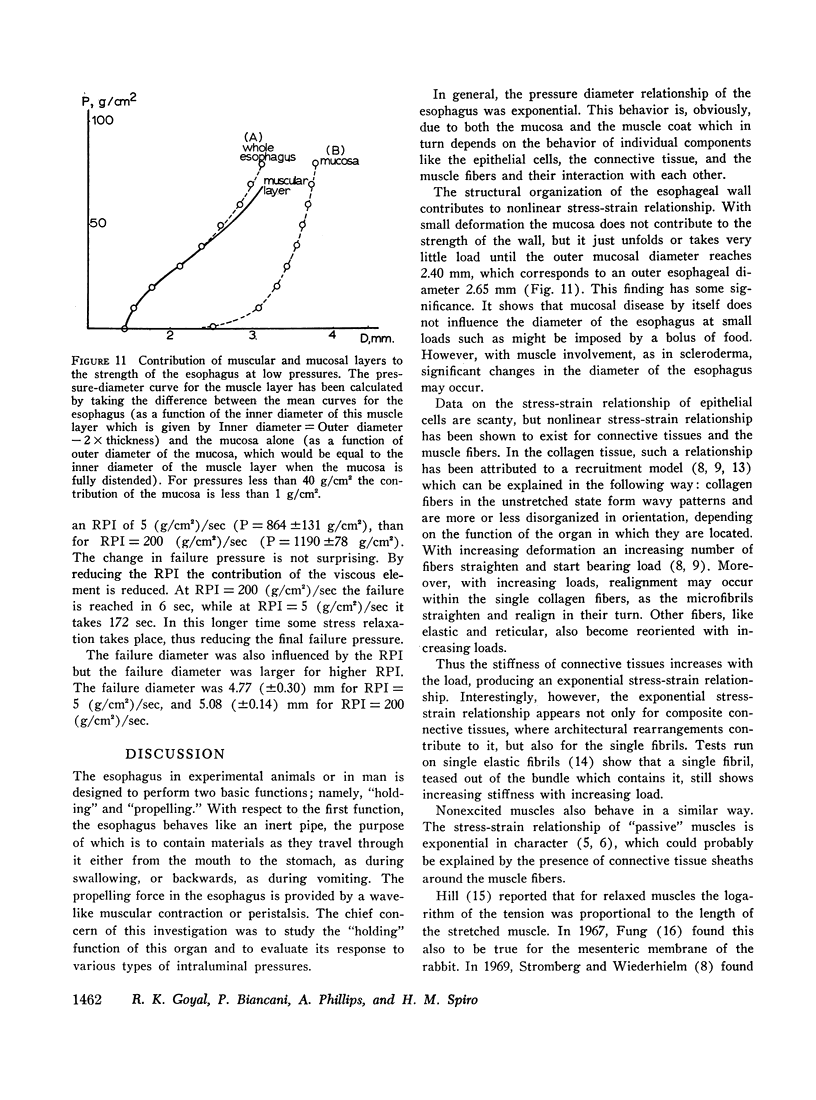
Pressure-diameter curves of the esophagus were obtained to define its mechanical properties. The mucosal contribution to the strength of the esophagus was negligible until the outer diameter almost doubled, suggesting that small intraluminal pressures are held by the muscle layer alone. For larger deformations mucosal contribution increased and at failure the mucosa held over one-half of the failure pressure of the esophagus.
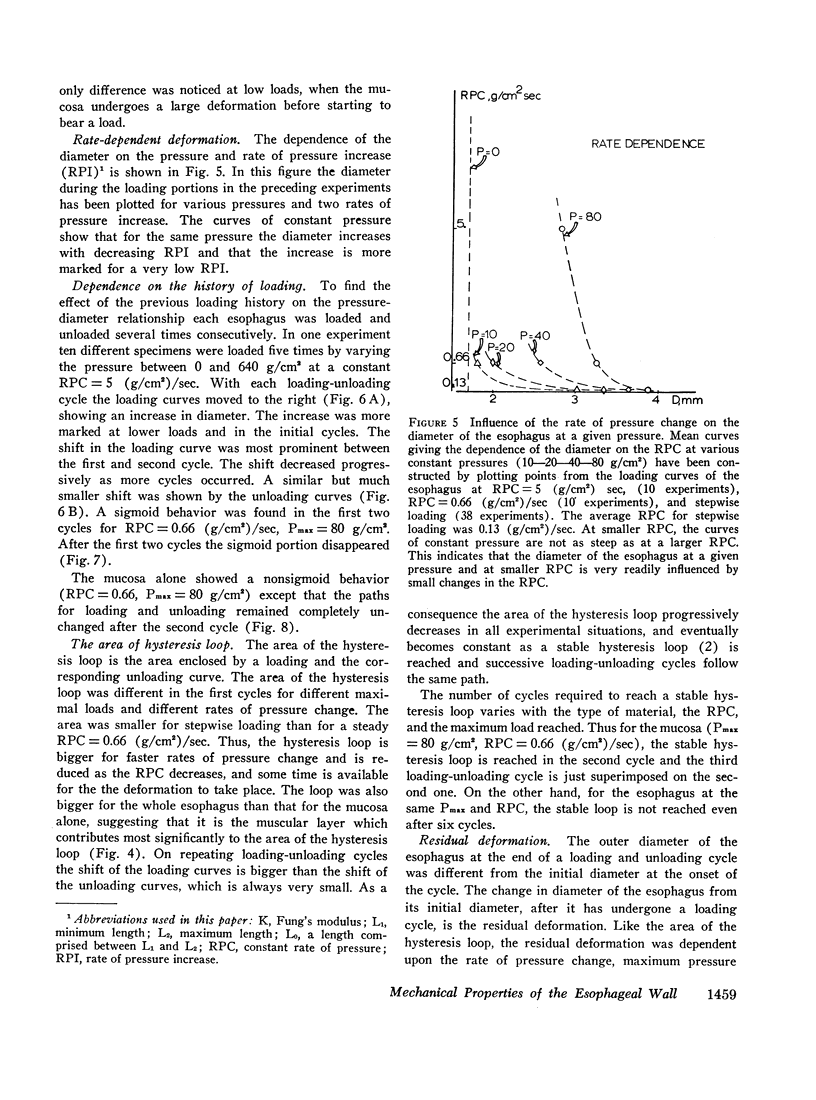
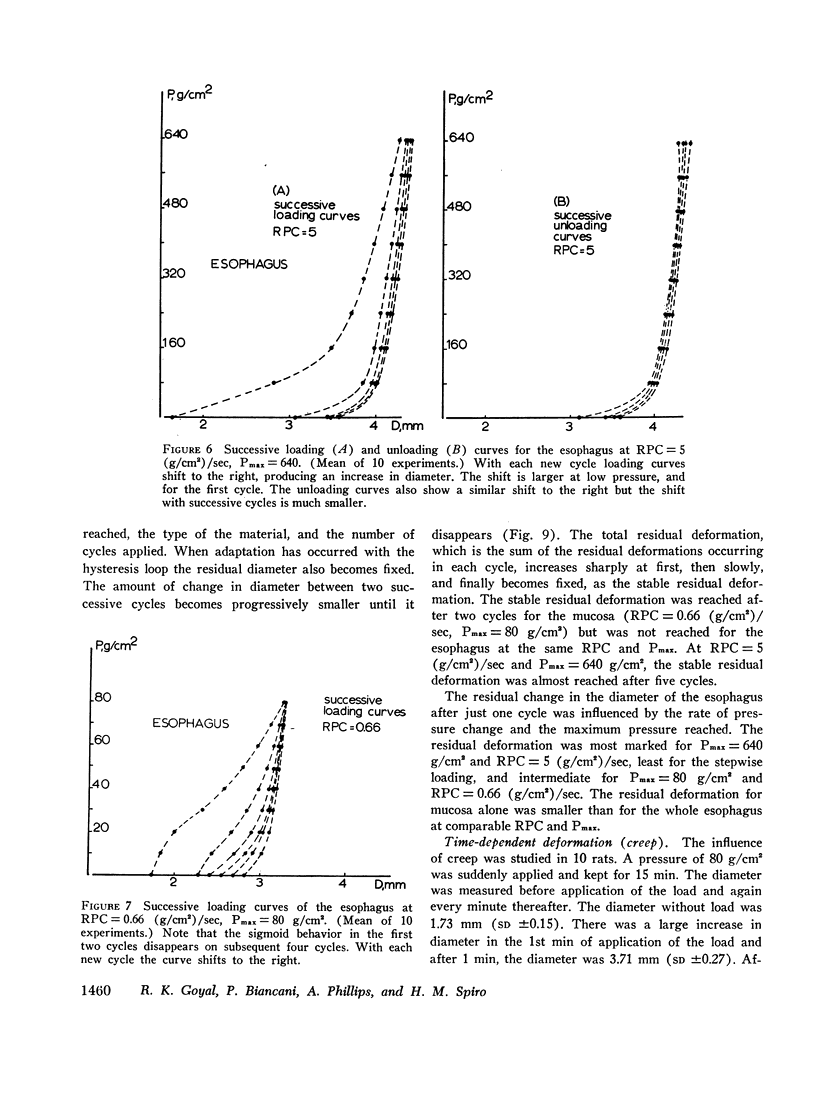
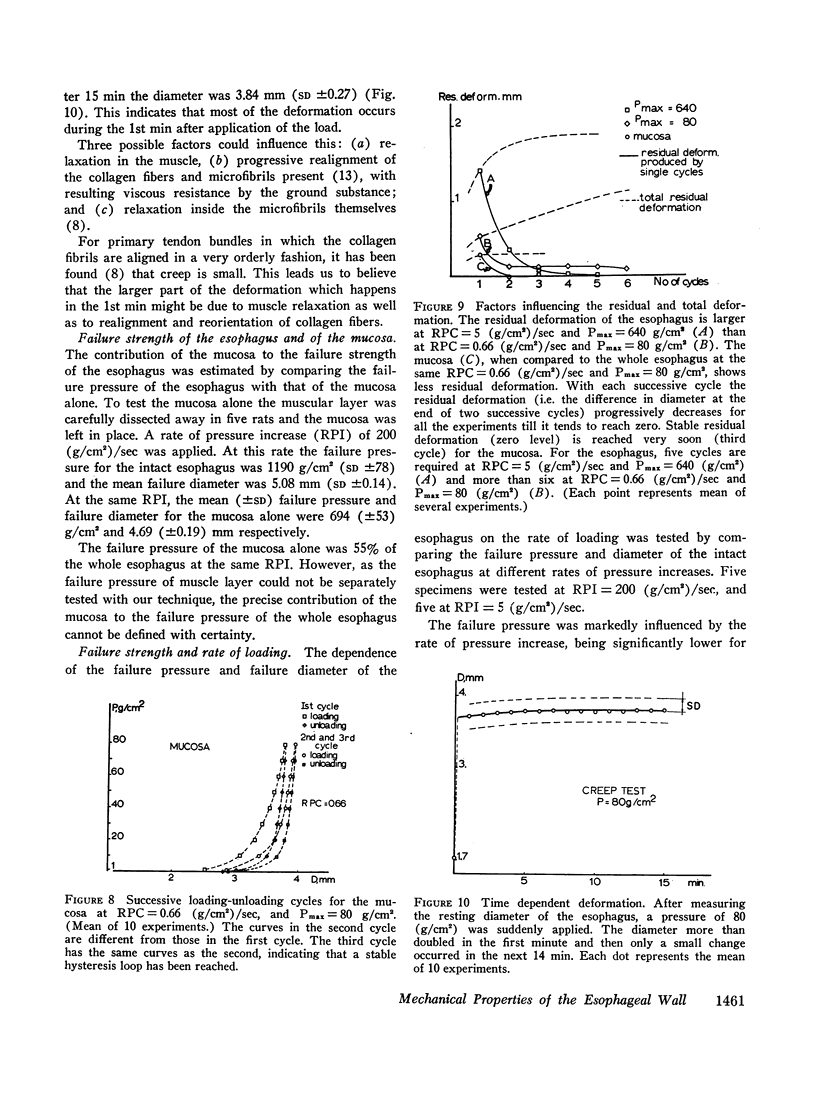
The paths followed during loading and unloading are different and exhibit hysteresis. They are influenced by the rate of pressure change, being more compliant for low rates of pressure change. They are influenced by the history of loading, being different for successive loading-unloading cycles. If enough loading-unloading cycles are repeated a stable loop is reached, which does not change thereafter.
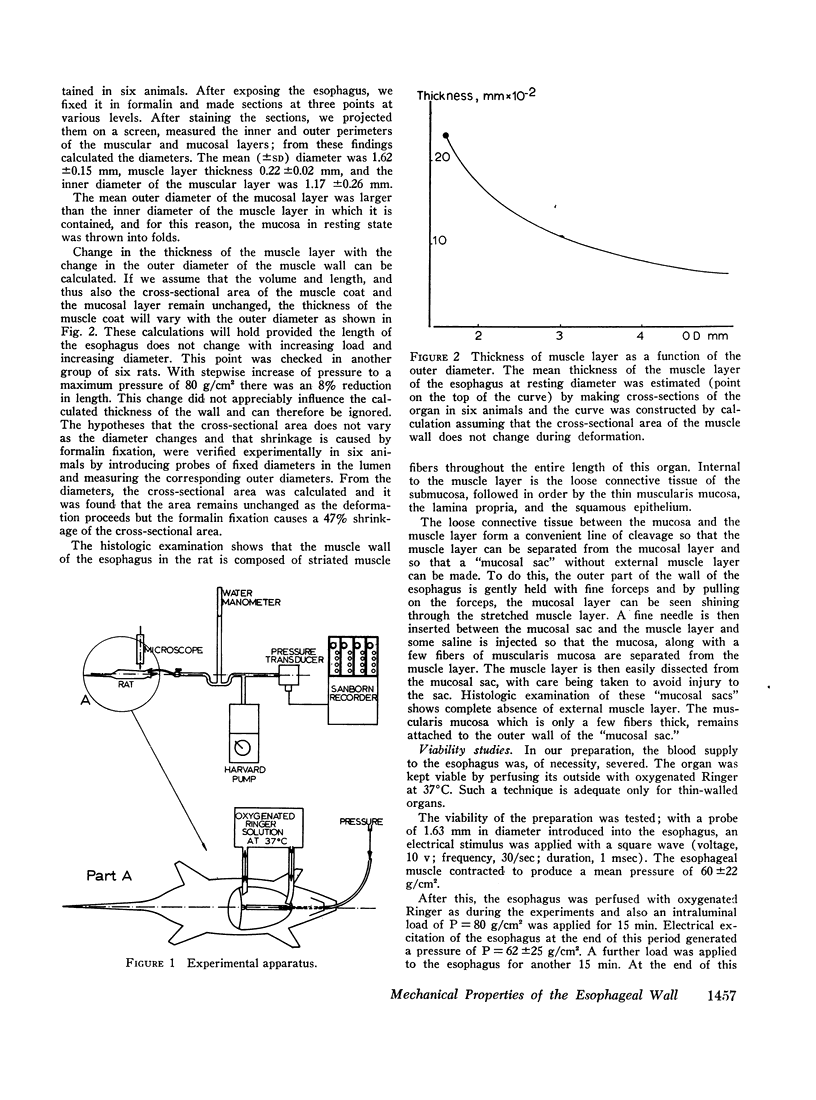
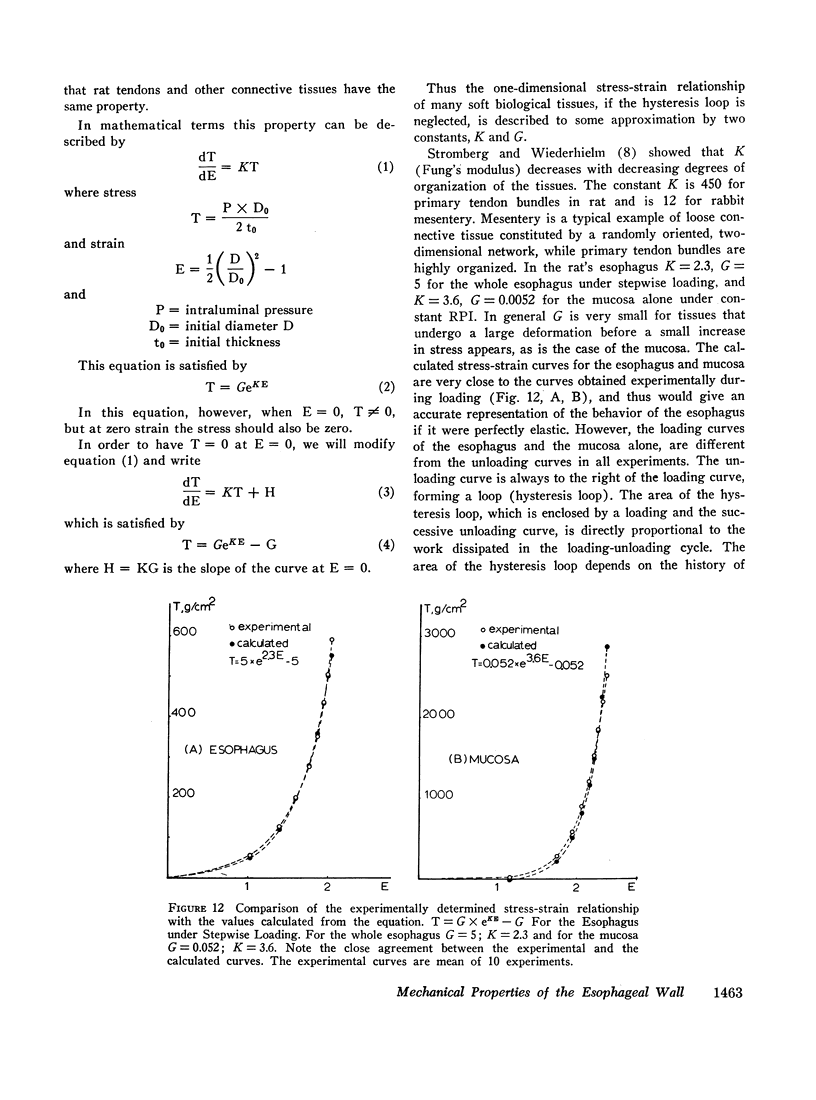
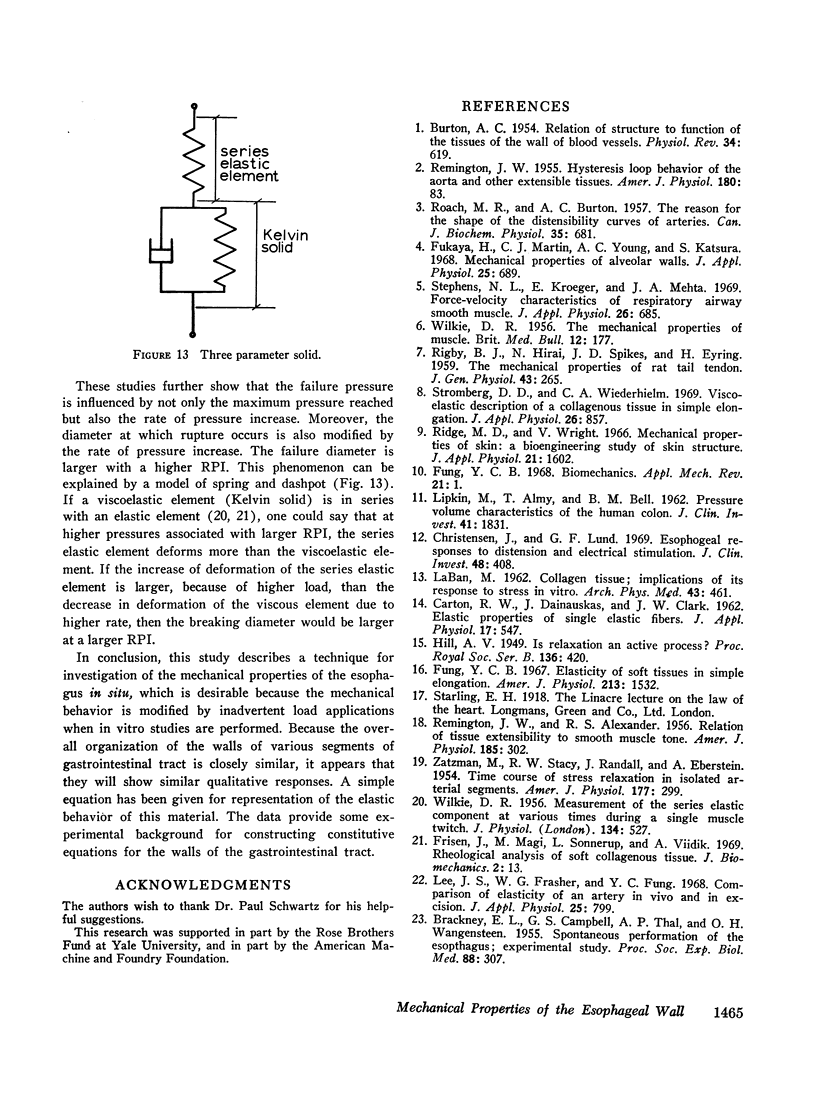
Both the mucosa and the whole esophagus show increasing stiffness with increasing pressure. This behavior can be represented by a simple exponential relationship. However, at rapid rates of pressure increases, the esophageal muscles produce sigmoid loading curves, which gradually become exponential when repeating loading.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRACKNEY E. L., CAMPBELL G. S., THAL A. P., WANGENSTEEN O. H. Spontaneous perforation of the esophagus; experimental study. Proc Soc Exp Biol Med. 1955 Feb;88(2):307–310. doi: 10.3181/00379727-88-21571. [DOI] [PubMed] [Google Scholar]
- BURTON A. C. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev. 1954 Oct;34(4):619–642. doi: 10.1152/physrev.1954.34.4.619. [DOI] [PubMed] [Google Scholar]
- CARTON R. W., DAINAUSKAS J., CLARK J. W. Elastic properties of single elastic fibers. J Appl Physiol. 1962 May;17:547–551. doi: 10.1152/jappl.1962.17.3.547. [DOI] [PubMed] [Google Scholar]
- Christensen J., Lund G. F. Esophageal responses to distension and electrical stimulation. J Clin Invest. 1969 Feb;48(2):408–419. doi: 10.1172/JCI105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya H., Martin C. J., Young A. C., Katsura S. Mechanial properties of alveolar walls. J Appl Physiol. 1968 Dec;25(6):689–695. doi: 10.1152/jappl.1968.25.6.689. [DOI] [PubMed] [Google Scholar]
- Fung Y. C. Elasticity of soft tissues in simple elongation. Am J Physiol. 1967 Dec;213(6):1532–1544. doi: 10.1152/ajplegacy.1967.213.6.1532. [DOI] [PubMed] [Google Scholar]
- LABAN M. M. Collagen tissue: implications of its response to stress in vitro. Arch Phys Med Rehabil. 1962 Sep;43:461–466. [PubMed] [Google Scholar]
- Lee J. S., Frasher W. G., Fung Y. C. Comparison of elasticity of an artery in vivo and in excision. J Appl Physiol. 1968 Dec;25(6):799–801. doi: 10.1152/jappl.1968.25.6.799. [DOI] [PubMed] [Google Scholar]
- Lipkin M., Almy T., Bell B. M. PRESSURE-VOLUME CHARACTERISTICS OF THE HUMAN COLON. J Clin Invest. 1962 Oct;41(10):1831–1839. doi: 10.1172/JCI104640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMINGTON J. W., ALEXANDER R. S. Relation of tissue extensibility to smooth muscle tone. Am J Physiol. 1956 May;185(2):302–308. doi: 10.1152/ajplegacy.1956.185.2.302. [DOI] [PubMed] [Google Scholar]
- REMINGTON J. W. Hysteresis loop behavior of the aorta and other extensible tissues. Am J Physiol. 1955 Jan;180(1):83–95. doi: 10.1152/ajplegacy.1954.180.1.83. [DOI] [PubMed] [Google Scholar]
- ROACH M. R., BURTON A. C. The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol. 1957 Aug;35(8):681–690. [PubMed] [Google Scholar]
- Ridge M. D., Wright V. Mechanical properties of skin: a bioengineering study of skin structure. J Appl Physiol. 1966 Sep;21(5):1602–1606. doi: 10.1152/jappl.1966.21.5.1602. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Kroeger E., Mehta J. A. Force-velocity characteristics of respiratory airway smooth muscle. J Appl Physiol. 1969 Jun;26(6):685–692. doi: 10.1152/jappl.1969.26.6.685. [DOI] [PubMed] [Google Scholar]
- Stromberg D. D., Wiederhielm C. A. Viscoelastic description of a collagenous tissue in simple elongation. J Appl Physiol. 1969 Jun;26(6):857–862. doi: 10.1152/jappl.1969.26.6.857. [DOI] [PubMed] [Google Scholar]
- WILKIE D. R. Measurement of the series elastic component at various times during a single muscle twitch. J Physiol. 1956 Dec 28;134(3):527–530. doi: 10.1113/jphysiol.1956.sp005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKIE D. R. The mechanical properties of muscle. Br Med Bull. 1956 Sep;12(3):177–182. doi: 10.1093/oxfordjournals.bmb.a069546. [DOI] [PubMed] [Google Scholar]
- ZATZMAN M., STACY R. W., RANDALL J., EBERSTEIN A. Time course of stress relaxation in isolated arterial segments. Am J Physiol. 1954 May;177(2):299–302. doi: 10.1152/ajplegacy.1954.177.2.299. [DOI] [PubMed] [Google Scholar]



