Abstract
Background
Hormonally active environmental agents may alter the course of pubertal development in girls, which is controlled by steroids and gonadotropins.
Objectives
We investigated associations of concurrent exposures from three chemical classes (phenols, phthalates, and phytoestrogens) with pubertal stages in a multiethnic longitudinal study of 1,151 girls from New York City, New York, greater Cincinnati, Ohio, and northern California who were 6–8 years of age at enrollment (2004–2007).
Methods
We measured urinary exposure biomarkers at visit 1 and examined associations with breast and pubic hair development (present or absent, assessed 1 year later) using multivariate adjusted prevalence ratios (PR) and 95% confidence intervals (CIs). Modification of biomarker associations by age-specific body mass index percentile (BMI%) was investigated, because adipose tissue is a source of peripubertal hormones.
Results
Breast development was present in 30% of girls, and 22% had pubic hair. High-molecular-weight phthalate (high MWP) metabolites were weakly associated with pubic hair development [adjusted PR, 0.94 (95% CI, 0.88–1.00), fifth vs. first quintile]. Small inverse associations were seen for daidzein with breast stage and for triclosan and high MWP with pubic hair stage; a positive trend was observed for low-molecular-weight phthalate biomarkers with breast and pubic hair development. Enterolactone attenuated BMI associations with breast development. In the first enterolactone quintile, for the association of high BMI with any development, the PR was 1.34 (95% CI, 1.23–1.45 vs. low BMI). There was no BMI association in the fifth, highest quintile of enterolactone.
Conclusions
Weak hormonally active xenobiotic agents investigated in this study had small associations with pubertal development, mainly among those agents detected at highest concentrations.
Keywords: biomarkers, phenols, phthalates, phytoestrogens, puberty
Over the past 50 years, a trend has been reported toward earlier pubertal development in girls, with the implication that early maturation may lead to adverse social and medical conditions, including cancer and diabetes (Schoeters et al. 2008). Race, obesity, and genetics are likely determinants of pubertal timing, but hormonally active environmental exposures may also play a role (Jacobson-Dickman and Lee 2009). Widespread exposure exists to such environmental agents. Children and minorities often have higher exposures, as demonstrated by urinary concentrations of many environmental biomarkers, compared with adults and whites [Centers for Disease Control and Prevention (CDC) 2005]. Specific chemicals that behave like estradiol include a number of phenols, such as bisphenol A. They act as hormone agonists in animal models of reproductive development, accelerating pubertal development. However, phytoestrogens and phthalates have both agonist and antagonist effects in animals, likely related to alternative mechanisms, dose levels, and exposure timing (Rasier et al. 2006).
The Breast Cancer and Environment Research Centers (BCERC) are a consortium established by the National Institute of Environmental Health Sciences and The National Cancer Institute to elucidate influences of environmental factors on timing of pubertal development in girls. For this purpose, we evaluated exposure using concurrent urinary biomarkers representing three classes of environmental agents in relation to breast and pubic hair development among the girls in this cohort. Biomarkers were selected based on a pilot study that revealed a wide range of values and high detectability in our cohort (Wolff et al. 2007). We hypothesized that phenols would be associated with earlier puberty, that phthalate biomarkers would be related to later pubertal timing, that phytoestrogens would be associated with later breast development, and that associations could be modified by obesity.
Materials and Methods
Study population
The BCERC epidemiology project is a longitudinal study of girls enrolled at 6–8 years of age and followed through puberty. It is part of a consortium of four centers with transdisciplinary research collaborations integrated across biologic, epidemiologic, and community outreach projects. Enrollment of 1,239 girls during 2004–2007 occurred at three sites: Mount Sinai School of Medicine (MSSM), which recruited in East Harlem in New York City; Cincinnati Children’s Hospital (Cincinnati), which recruited in the greater Cincinnati, Ohio, metropolitan area, and through the Breast Cancer Registry of Greater Cincinnati; and Kaiser Permanente Northern California (KPNC), which recruited members of the KPNC Health Plan in the San Francisco Bay Area. All sites obtained informed consent from parent or guardian and independently verified child assent, approved by the institutional review boards at each institution and at the CDC. Eligibility included age, female sex, no underlying endocrine medical conditions, and at MSSM, black or Hispanic race/ethnicity.
Data collection
A questionnaire was completed by the parent or guardian of the girl (usually the mother) that included medical history, product use and exposures, and demographic variables. Parents or guardians identified the girls as black, white, Asian, or other, and ethnicity as Hispanic or non-Hispanic. We assessed age, weight, height, breast and pubic hair stages at the visit when urine was collected (visit 1) and approximately 1 year later (visit 2). Visits 1 for MSSM and KPNC were at baseline. Visit 1 for Cincinnati is defined for this analysis as the visit when urine was collected, which was 6 months after the first baseline visit. Visit 2 was approximately 1 year later [details in Supplemental Material, Table 1 (doi:10.1289/ehp.0901690)]. At each visit, breast (B1–B5) and pubic hair (PH1–PH5) stages were assessed by inspection and palpation. Examiners were trained and tested by a master clinician, following a written protocol with photographs that demonstrated the maturation stages (Biro et al., in press; van Wieringen et al. 1985). Inter-rater evaluations were conducted by one pediatrician. The kappa statistic was 0.67, indicating substantial agreement; concordance was 87% (117/127 among 39 examiners) (Biro et al., in press). Height and weight were measured using calibrated scales and stadiometers at each visit. Age-specific (in months) and sex-specific body mass index percentiles (BMI%) were calculated based on CDC growth charts (CDC 2000). Pubertal stages and BMI% distribution at visit 1 were similar among girls with visit 2 breast stages (n = 985) and without (n = 166) visit 2 data; however, girls without visit 2 data were more likely to be black or Hispanic, of lower socioeconomic status, and from MSSM (Table 1).
Table 1.
Characteristics at visit 1 and by site for 1,151 girls with at least one biomarker value: BCERC cohort, 2004–2007 [n (%)].
| Variable | All sites combined | MSSM | Cincinnati | KPNC |
|---|---|---|---|---|
| Age at baseline (visit 1) (years) | ||||
| 6.0–6.9 | 293 (25.5) | 153 (37.7) | 71 (22.0) | 69 (16.4) |
| 7.0–7.9 | 571 (49.6) | 131 (32.3) | 148 (45.8) | 292 (69.2) |
| ≥ 8.0–9.4 | 287 (24.9) | 122 (30.0) | 104 (32.2) | 61 (14.5) |
| Median age | 7.50 | 7.25 | 7.64 | 7.41 |
| Age at visit 2 (years) | ||||
| 6.9–7.9 | 245 (21.3) | 105 (25.9) | 65 (20.2) | 75 (17.8) |
| 8.0–8.9 | 478 (41.5) | 97 (23.9) | 133 (14.3) | 247 (58.7) |
| ≥ 9.0–10.6 | 266 (23.1) | 103 (25.4) | 92 (28.6) | 70 (16.6) |
| Not seen including missing (1) | 162 | 101 | 32 | 29 |
| Median age | 8.50 | 8.41 | 8.66 | 8.50 |
| Child race/ethnicity | ||||
| White | 391 (34.0) | 0 (0.0) | 220 (68.1) | 171 (40.5) |
| Black | 353 (30.7) | 164 (40.4) | 97 (30.0) | 92 (21.8) |
| Hispanic, non-black | 344 (29.9) | 242 (59.6) | 1 (0.3) | 101 (23.9) |
| Asian | 53 (4.6) | 0 (0.0) | 4 (1.2) | 49 (11.6) |
| Other | 10 (0.9) | 0 (0.0) | 1 (0.3) | 9 (2.1) |
| BMI age- and sex-specific percentile | ||||
| < 50th | 394 (34.2) | 133 (32.8) | 101 (31.3) | 160 (37.9) |
| 50–85th | 388 (33.7) | 114 (28.1) | 132 (40.9) | 142 (33.6) |
| ≥ 85th | 369 (32.1) | 159 (39.2) | 90 (27.9) | 120 (28.4) |
| Parent or guardian education | ||||
| ≤ High school | 342 (29.7) | 236 (58.1) | 29 (9.0) | 77 (18.2) |
| > High school | 765 (66.5) | 162 (39.9) | 261 (80.8) | 342 (81.0) |
| Missing | 44 | 8 | 33 | 3 |
| Season of urine collection | ||||
| Spring (March–May) | 393 (34.1) | 112 (27.6) | 163 (50.5) | 118 (28.0) |
| Summer (June–August) | 322 (28.0) | 94 (23.2) | 48 (14.9) | 71 (16.8) |
| Fall (September–November) | 213 (18.5) | 90 (22.2) | 64 (19.8) | 168 (39.8) |
| Winter (December–February) | 223 (19.4) | 110 (27.1) | 48 (14.9) | 65 (15.4) |
| Interval between visits 1 and 2 | ||||
| < 14 months | 893 (90.5) | 239 (78.4) | 288 (99.0) | 367 (93.6) |
| ≥ 14 months | 94 (9.5) | 66 (21.6) | 3 (1.0) | 25 (6.4) |
| Breast stage at visit 1 | ||||
| B1 | 943 (81.9) | 317 (78.1) | 237 (73.4) | 389 (92.2) |
| B2+ | 206 (17.9) | 89 (21.9) | 86 (26.6) | 31 (7.4) |
| Missing | 2 | 0 | 0 | 2 |
| Breast stage at visit 2 | ||||
| B1 | 688 (59.8) | 194 (47.8) | 167 (51.7) | 327 (77.5) |
| B2+ | 297 (25.8) | 111 (27.3) | 124 (38.4) | 62 (14.7) |
| Not seen including missing (5) | 166 | 101 | 32 | 33 |
| Pubic hair stage at visit 1 | ||||
| PH1 | 979 (85.1) | 334 (82.3) | 275 (85.1) | 370 (87.7) |
| PH2+ | 151 (13.1) | 71 (17.5) | 43 (13.3) | 37 (8.8) |
| Missing | 21 | 1 | 5 | 15 |
| Pubic hair stage at visit 2 | ||||
| PH1 | 756 (65.7) | 216 (53.2) | 230 (71.2) | 310 (73.5) |
| PH2+ | 211 (18.3) | 88 (21.7) | 61 (18.9) | 62 (14.7) |
| Not seen including missing (23) | 184 | 102 | 32 | 50 |
| Total n | 1,151 | 406 | 323 | 422 |
Urinary biomarker measurements
Samples collected at visit 1 were analyzed at the National Center for Environmental Health laboratories at CDC for nine phthalate metabolites [n = 1,149; monoethylphthalate (MEP), mono-butyl phthalate, mono-iso-butyl phthalate, mono-benzyl phthalate, mono-3-carboxypropyl phthalate, mono-2-ethyl-5-carboxypentyl phthalate, mono-(2-ethyl-5-hydroxylhexyl) phthalate, mono-(2-ethyl-5-oxohexyl) phthalate, and mono-(2-ethylhexyl) phthalate (MEHP)], seven phenols (benzophenone-3, bisphenol A, 2,5-dichlorophenol, triclosan; n = 1,149; methyl-, butyl-, and propyl- parabens, n = 1,059), and three phytoestrogens (daidzein, genistein, enterolactone; n = 1,150). Parabens were not measured early in the study. At least one urinary biomarker measurement was available among 1,151 girls, 985 with breast stages. We substituted limit of detection (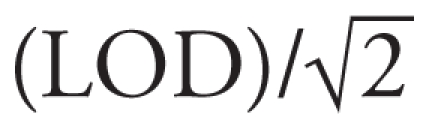 for results below the LOD. Adjustment for urine dilution was accomplished using creatinine, to account for difference in sampling (spot specimens at MSSM and KPNC, early-morning samples at Cincinnati) and interindividual variation in urine dilution. We included log-creatinine in models using continuous log-biomarker variables, and we created quintile cut points from creatinine-corrected concentrations (micrograms per gram creatinine). As previously described, to reduce multiple comparisons we combined the phthalate metabolites into two groups that represent similar sources and similar biologic activity, low- (< 250 Da) and high-molecular-weight (> 250 Da) phthalate metabolites (low MWP and high MWP) [details in Supplemental Material, Table 2 (doi:10.1289/ehp.0901690)]. We expressed high MWP molar sum as MEHP (molecular weight 278) and the low MWP as MEP (molecular weight 194) so that units were the same as the other analytes (micrograms per liter). Similarly, a molar sum of the paraben metabolites was created (paraben sum) expressed as propyl paraben (molecular weight 180.2). Models with the individual phthalate and paraben metabolites were consistent with the molar sum variables. Results using di(2-ethylhexyl)phthalate (DEHP)-sum metabolites were almost identical to those for the high MWP, and they represented 75% ± 16% (mean ± SD) of the high MWP biomarkers. Therefore, only the latter models are presented.
for results below the LOD. Adjustment for urine dilution was accomplished using creatinine, to account for difference in sampling (spot specimens at MSSM and KPNC, early-morning samples at Cincinnati) and interindividual variation in urine dilution. We included log-creatinine in models using continuous log-biomarker variables, and we created quintile cut points from creatinine-corrected concentrations (micrograms per gram creatinine). As previously described, to reduce multiple comparisons we combined the phthalate metabolites into two groups that represent similar sources and similar biologic activity, low- (< 250 Da) and high-molecular-weight (> 250 Da) phthalate metabolites (low MWP and high MWP) [details in Supplemental Material, Table 2 (doi:10.1289/ehp.0901690)]. We expressed high MWP molar sum as MEHP (molecular weight 278) and the low MWP as MEP (molecular weight 194) so that units were the same as the other analytes (micrograms per liter). Similarly, a molar sum of the paraben metabolites was created (paraben sum) expressed as propyl paraben (molecular weight 180.2). Models with the individual phthalate and paraben metabolites were consistent with the molar sum variables. Results using di(2-ethylhexyl)phthalate (DEHP)-sum metabolites were almost identical to those for the high MWP, and they represented 75% ± 16% (mean ± SD) of the high MWP biomarkers. Therefore, only the latter models are presented.
Laboratory techniques and quality control protocols are identical to those reported previously in a pilot study (Wolff et al. 2007). Briefly, urine undergoes an automated cleanup with enzymatic deconjugation, followed by high-performance liquid chromatography-isotope dilution tandem mass spectrometry quantification (Kato et al. 2005; Rybak et al. 2008; Ye et al. 2005, 2006). In addition to the internal CDC quality control procedures, we incorporated approximately 10% masked quality control specimens (n = 101) from a single urine pool. The coefficients of variation (SD/mean concentration) were < 10% for 13 analytes and were between 10% and 21% for the remaining six biomarkers.
Statistical analyses
We examined relationships among pubertal stages, biomarkers, and study characteristics using nonparametric statistics (Spearman or Kruskal–Wallis) and multivariate linear regression (version 9.1.3; SAS Institute Inc., Cary, NC). We conducted multivariate analyses using Proc Genmod (SAS) with modified Poisson regression, which provides robust error variance estimates and is appropriate for outcomes that are not rare (Zou 2004). We computed prevalence ratios (PRs) and 95% confidence intervals (CIs) for any development [breast stage 2 or higher (B2+); pubic hair stage 2 or higher (PH2+)] versus no development (B1 or PH1) in relation to biomarker exposures. We considered covariates related to pubertal development and urinary biomarker concentrations. Variables that did not alter any biomarker estimate by > 10% were backward-eliminated from the models. Excluding observations with very dilute biospecimens (n = 58 with creatinine < 0.2 g/L) did not materially change biomarker estimates; therefore, they were retained in the analyses. We assessed the trend of estimates for biomarker quintiles using their ordinal values with the contrast option in Proc Genmod. Models with continuous log-biomarker variable were consistent with results of models using quintiles. We report results for pubertal stages assessed at visit 2, one year after urine collection, when the proportions with B2+ and PH2+ were greater and site variations were less pronounced. There were 985 girls with biomarkers and visit 2 breast stage and 967 with visit 2 pubic hair stages.
We hypothesized that exogenous exposures were likely to operate jointly with BMI, a strong endogenous hormonal risk factor for pubertal development (Kaplowitz 2008). This hypothesis was examined by adding to models an interaction term for the product of the biomarker variable (log-continuous or ordinal quintile values) times BMI dichotomized at the median of the age-specific percentiles in our study population (low BMI/high BMI).
Results
BCERC cohort girls were 6–8 years of age and mainly black, Hispanic, and white race/ethnicities (Table 1). Median ages differed by < 0.5 years across the sites, being youngest at MSSM and oldest at Cincinnati at both visits. Every site had > 20% blacks, but there were no whites from MSSM and very few Hispanics at Cincinnati. All characteristics including pubertal stages differed by site (Table 1; p < 0.01). Compared with normative national data for BMI, 65% of girls were above the 50th percentile at visit 1 (i.e., 15% more than expected), 32% were above the 85th percentile (overweight), and 17% were also above the 95th percentile (obese) (CDC 2000). BMI% was highest among MSSM (median 75th percentile of the norm) and lowest among Cincinnati girls (median 64th percentile).
As expected, the environmental biomarkers were detected in almost all of the urine samples [Supplemental Material, Table 2 (doi:10.1289/ehp.0901690)]. Overall, phenol median concentrations were < 100 μg/L, and urinary phytoestrogen and phthalate biomarker medians were > 400 μg/L. Geometric mean concentrations (micrograms per liter) of urinary exposure biomarkers are presented in Table 2 mutually adjusted for covariates. Benzophenone-3 (BP3), a sunscreen, was higher in samples collected in the summer and among white children, compared with blacks and Hispanics. 2,5-Dichlorophenol (2,5-DCP) was lower in whites and higher in MSSM participants; it is the metabolite of 1,4-dichlorobenzene used in mothballs and room deodorizers. Parabens are found as preservatives in many personal care products; their levels were higher among blacks and in samples collected in summer. Parabens often occur together with BP3 and low MWP in consumer products. Low-MWP biomarkers were higher in blacks and Hispanics than whites and Asians. The isoflavones daidzein and genistein, which are found in soy products, were somewhat higher among Asians. Enterolactone, a gut metabolite of lignans found in flax, beans, grains, and berries, tended to be higher among blacks and whites.
Table 2.
Geometric means (95% CIs) of environmental urinary biomarkers at baseline examination in relation to covariates, mutually adjusted and for ln-urinary creatinine (1,102 girls with all characteristics, 2004–2007).
| Phenols (μg/L) |
Phthalate monoesters, μg/L |
Phytoestrogens (μg/L) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BP3 | BPA | 2,5-DCP | Triclosan | Paraben sum | Low MWP | High MWP | Daidzein | Genistein | Enterolactone | |
| Age at baseline exam (years) | ||||||||||
| 6.0–6.9 | 33 (24–45) | 2.1 (1.8–2.4) | 13 (10–17) | 17 (13–23) | 78 (61–101) | 169 (145–199) | 183 (158–212) | 143 (106–194) | 66 (49–90) | 307 (248–381) |
| 7.0–7.9 | 28 (21–37) | 1.9 (1.7–2.2) | 14 (11–18) | 17 (13–23) | 63 (50–79) | 161 (140–186) | 178 (156–202) | 106 (81–139) | 49 (37–64) | 323 (267–390) |
| ≥ 8.0 | 28 (20–38) | 1.8 (1.5–2.1) | 14 (11–19) | 17 (13–23) | 63 (48–81) | 156 (133–182) | 157 (136–182) | 112 (83–151)* | 50 (37–67)* | 320 (259–396) |
| Race/ethnicity | ||||||||||
| White | 51 (41–63) | 2.0 (1.8–2.3) | 6.0 (5.1–7.5) | 14 (11–17) | 39 (33–47) | 114 (102–127) | 179 (162–199) | 118 (96–146) | 56 (45–69) | 396 (341–461) |
| Black | 12 (10–15) | 2.2 (2.0–2.4) | 22 (18–26) | 16 (13–19) | 170 (143–201) | 211 (190–233) | 150 (137–165) | 96 (79–116) | 40 (33–49) | 351 (306–403) |
| Hispanic, non-black | 22 (17–27) | 2.1 (1.9–2.3) | 20 (17–24) | 15 (12–19) | 83 (69–99) | 190 (170–212) | 178 (160–197) | 80 (65–98) | 38 (31–47) | 273 (235–318) |
| Asian | 32 (20–52) | 1.5 (1.2–2.0) | 14 ( 9.0–21) | 14 (9.1–23) | 55 (37–82) | 117 (91–149) | 174 (139–219) | 203 (127–324) | 118 (74–188) | 269 (193–375) |
| Other | 52 (17–160)** | 1.9 (1.1–3.3) | 15 (5.5–38)** | 32 (11–90) | 46 (19–113)** | 211 (120–369)** | 182 (109–305)* | 133 (46–384)** | 47 (16–137)** | 311 (146–663)** |
| BMI age-specific percentile at baseline | ||||||||||
| < 50th | 31 (23–42) | 2.0 (1.7–2.3) | 13 (9.8–16) | 19 (15–26) | 70 (55–89) | 153 (132–178) | 180 (157–206) | 116 (87–153) | 53 (40–71) | 376 (308–460) |
| 50–85th | 27 (20–36) | 2.0 (1.7–2.3) | 14 (11–18) | 15 (12–20) | 61 (48–78) | 157 (136–182) | 167 (146–192) | 121 (91–161) | 52 (39–69) | 345 (282–421) |
| ≥ 85th | 31 (23–42) | 1.9 (1.6–2.2) | 15 (12–20) | 17 (13–22) | 72 (57–93) | 177 (152–206) | 170 (148–196) | 121 (91–162) | 58 (43–77) | 245 (199–301)** |
| Caregiver education | ||||||||||
| 12 years | 24 (18–33) | 1.9 (1.6–2.2) | 15 (11–20) | 18 (14–24) | 63 (49–81) | 174 (149–203) | 178 (154–205) | 117 (87–157) | 55 (41–74) | 288 (233–356) |
| ≥ 13 years | 36 (28–47)** | 2 (1.7–2.3) | 13 (10–17) | 16 (13–21) | 72 (58–90) | 151 (132–173)* | 167 (147–189) | 122 (95–158) | 54 (42–70) | 347 (289–417)* |
| Study site | ||||||||||
| MSSM | 22 (16–30) | 2.3 (1.9–2.7) | 46 (34–60) | 15 (11–20) | 70 (54–92) | 201 (171–237) | 242 (209–281) | 89 (65–120) | 42 (31–57) | 287 (230–357) |
| Cincinnati | 20 (14–28) | 1.9 (1.6–2.2) | 13 (9.6–17) | 28 (21–39) | 66 (50–88) | 163 (138–194) | 154 (131–180) | 135 (97–186) | 60 (43–82) | 252 (200–318) |
| KPNC | 58 (44–78)** | 1.7 (1.5–2.0)** | 4.7 (3.7–6.1)** | 12 (9.0–16)** | 66 (52–83) | 129 (112–149)** | 137 (120–157)** | 143 (109–188)** | 64 (49–84)* | 439 (361–534)** |
| Season of urine collection | ||||||||||
| Spring | 20 (15–27) | 1.8 (1.5–2.0) | 13 (10–17) | 17 (13–22) | 66 (51–84) | 154 (132–179) | 169 (147–194) | 134 (101–178) | 64 (48–85) | 310 (253–380) |
| Summer | 125 (92–172) | 2.4 (2.1–2.8) | 13 (10–17) | 18 (14–24) | 93 (72–121) | 208 (177–243) | 185 (160–214) | 98 (73–132) | 45 (33–60) | 271 (219–335) |
| Fall | 22 (16–30) | 2.0 (1.7–2.4) | 18 (14–24) | 15 (11–21) | 54 (41–70) | 145 (123–171) | 175 (151–204) | 124 (91–170) | 57 (42–77) | 349 (280–436) |
| Winter | 14 (10–19)** | 1.6 (1.4–2.0)** | 13 (9.9–18)* | 18 (13–25) | 63 (48–83)** | 149 (126–176)** | 161 (138–188) | 125 (91–171) | 55 (40–76)* | 343 (273–430) |
When any adjusted geometric mean differed from one or more of the others within a characteristic, the bottom value is marked with an asterisk. BMI% is sex- and age-specific (CDC 2000). Spring is March, April, May; other seasons follow sequentially. Paraben sum is the molar sum of methyl-, butyl-, and propyl-parabens, expressed as propylparaben (MW 180.2). Low MWP is the molar sum of MEP, MBP (monobutyl phthalate), MIBP expressed as MEP (MW = 194). High MWP is the molar sum of MBZP (mono-benzyl phthalate), MCPP [mono(3-carboxypropyl) phthalate], MEHP, MEOHP [mono-(2-ethyl-5-oxohexyl) phthalate], MEHHP [mono-(2-ethyl-5-hydroxyhexyl) phthalate], and MECPP [mono(2-ethyl-5-carboxypentyl) phthalate] expressed as MEHP (MW = 278). DEHP metabolite sum, which was 75 ± 16% of high MWP and had almost identical means, is omitted from the table. Details are in Supplemental Material, Table 2 (doi:10.1289/ehp.0901690).
p < 0.05,
p < 0.01.
One year after urine samples were collected for biomarker measurements, breast development was present in 30% and pubic hair in 22% of girls (Table 1). As shown in Table 3, the frequency of any breast and pubic hair development increased or decreased across quintiles of most biomarkers, but the adjusted PRs were close to null. Low MWP had weak positive associations with both breast and pubic hair stages. For breast development, the adjusted PR was 1.06 (95% CI, 0.99–1.14; p-trend = 0.087) in the fifth versus first quintile. The isoflavones daidzein and genistein had weak inverse associations with breast stage; for daidzein, the adjusted PR was 0.96 for the fifth versus first quintile (95% CI, 0.90–1.02; p-trend = 0.061). Triclosan had a suggestive inverse association with pubic hair development, but the trend was not monotonic, and the CIs were similar for every quintile (Table 3). An inverse relationship with pubic hair development was seen for high MWP.
Table 3.
PRs and 95% CIs for breast development stage (B2+ vs. B1) and pubic hair stage (PH2+ vs. PH1) in relation to urinary environmental biomarkers measured 1 year earlier. BCERC Cohort, 2004–2008.
| Quintiles of creatinine-corrected biomarker concentrations (C) |
||||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | p-Trend | |
| Breast development | ||||||
| Phenols | ||||||
| BP3 | ||||||
| Quintile median μg/gC | 3.6 | 11.1 | 30.4 | 93.6 | 867 | |
| n B2+/n total | 76/196 | 65/197 | 64/196 | 52/197 | 40/196 | |
| PR (95% CI) | 1 (Ref) | 0.96 (0.89–1.03) | 0.96 (0.89–1.02) | 0.91 (0.85–0.98) | 0.87 (0.81–0.93) | < 0.0001 |
| Adj PR (95% CI) | 1 (Ref) | 1.00 (0.94–1.06) | 1.01 (0.95–1.07) | 0.99 (0.93–1.06) | 1.01 (0.94–1.09) | 0.847 |
| BPA | ||||||
| Quintile median μg/gC | 1.0 | 1.6 | 2.4 | 3.7 | 8.7 | |
| n B2+/total | 69/196 | 59/197 | 54/196 | 61/197 | 54/196 | |
| PR (95% CI) | 1 (Ref) | 0.96 (0.90–1.03) | 0.94 (0.88–1.01) | 0.97 (0.90–1.04) | 0.94 (0.88–1.01) | 0.171 |
| Adj PR (95% CI) | 1 (Ref) | 0.96 (0.90–1.02) | 0.95 (0.89–1.01) | 0.97 (0.91–1.03) | 0.97 (0.91–1.03) | 0.533 |
| 2,5-DCP | ||||||
| Quintile median μg/gC | 1.6 | 4.0 | 10.7 | 37.2 | 179 | |
| n B2+/total | 34/196 | 51/197 | 62/196 | 74/197 | 76/196 | |
| PR (95% CI) | 1 (Ref) | 1.07 (1.00–1.15) | 1.12 (1.05–1.20) | 1.17 (1.10–1.25) | 1.18 (1.11–1.26) | < .0001 |
| Adj PR (95% CI) | 1 (Ref) | 0.99 (0.93–1.06) | 0.97 (0.91–1.04) | 1.04 (0.96–1.12) | 1.02 (0.94–1.10) | 0.416 |
| Triclosan | ||||||
| Quintile median μg/gC | 2.6 | 6.9 | 15 | 38 | 170 | |
| n B2+/total | 57/196 | 49/197 | 59/196 | 65/197 | 67/196 | |
| PR (95% CI) | 1 (Ref) | 0.97 (0.90–1.04) | 1.01 (0.94–1.08) | 1.03 (0.96–1.10) | 1.04 (0.97–1.11) | 0.077 |
| Adj PR (95% CI) | 1 (Ref) | 0.99 (0.93–1.05) | 0.97 (0.92–1.03) | 1.01 (0.95–1.07) | 1.03 (0.97–1.10) | 0.242 |
| Paraben sum | ||||||
| Quintile median μg/gC | 15 | 36 | 84 | 198 | 839 | |
| n B2+/total | 45/180 | 50/181 | 58/181 | 45/181 | 68/180 | |
| PR (95% CI) | 1 (Ref) | 1.02 (0.95–1.10) | 1.06 (0.98–1.14) | 1.00 (0.93–1.07) | 1.10 (1.03–1.18) | 0.036 |
| Adj PR (95% CI) | 1 (Ref) | 1.01 (0.95–1.07) | 1.02 (0.96–1.09) | 0.98 (0.92–1.04) | 1.03 (0.96–1.10) | 0.653 |
| Phthalates | ||||||
| High MWP | ||||||
| Quintile median μg/gC | 84 | 138 | 204 | 326 | 616 | |
| n B2+/total | 67/196 | 62/197 | 53/196 | 40/197 | 75/196 | |
| PR (95% CI) | 1 (Ref) | 0.98 (0.91–1.05) | 0.95 (0.88–1.02) | 0.90 (0.84–0.96) | 1.03 (0.96–1.10) | 0.716 |
| Adj PR (95% CI) | 1 (Ref) | 0.98 (0.92–1.04) | 0.98 (0.92–1.04) | 0.94 (0.88–1.00) | 1.03 (0.97–1.10) | 0.781 |
| Low MWP | ||||||
| Quintile median μg/gC | 66 | 112 | 173 | 272 | 721 | |
| n B2+/total | 46/196 | 54/197 | 55/196 | 64/197 | 78/196 | |
| PR (95% CI) | 1 (Ref) | 1.03 (0.96–1.11) | 1.04 (0.97–1.11) | 1.07 (1.00–1.15) | 1.13 (1.06–1.21) | 0.000 |
| Adj PR (95% CI) | 1 (Ref) | 1.03 (0.97–1.09) | 1.01 (0.95–1.07) | 1.04 (0.98–1.11) | 1.06 (0.99–1.14) | 0.087 |
| Phytoestrogens | ||||||
| Daidzein | ||||||
| Quintile median μg/gC | 20 | 48 | 104 | 281 | 1,359 | |
| n B2+/total | 59/196 | 65/197 | 67/197 | 58/196 | 47/196 | |
| PR (95% CI) | 1 (Ref) | 1.02 (0.95–1.10) | 1.03 (0.96–1.10) | 1.00 (0.93–1.07) | 0.95 (0.89–1.02) | 0.121 |
| Adj PR (95% CI) | 1 (Ref) | 1.05 (0.99–1.11) | 1.02 (0.96–1.08) | 1.00 (0.94–1.06) | 0.96 (0.90–1.02) | 0.061 |
| Genistein | ||||||
| Quintile median μg/gC | 9.4 | 20 | 45 | 127 | 610 | |
| n B2+/total | 62/196 | 65/197 | 65/196 | 54/197 | 50/196 | |
| PR (95% CI) | 1 (Ref) | 1.01 (0.94–1.08) | 1.01 (0.94–1.08) | 0.97 (0.90–1.04) | 0.95 (0.89–1.02) | 0.081 |
| Adj PR (95% CI) | 1 (Ref) | 1.02 (0.96–1.08) | 1.00 (0.94–1.06) | 0.97 (0.91–1.03) | 0.97 (0.92–1.03) | 0.103 |
| Enterolactone | ||||||
| Quintile median μg/gC | 97 | 283 | 522 | 899 | 1,922 | |
| n B2+/total | 66/196 | 62/197 | 66/196 | 57/197 | 45/196 | |
| PR (95% CI) | 1 (Ref) | 0.98 (0.92–1.05) | 1.00 (0.93–1.07) | 0.96 (0.90–1.03) | 0.92 (0.86–0.99) | 0.018 |
| Adj PR (95% CI) | 1 (Ref) | 1.02 (0.96–1.08) | 1.03 (0.97–1.09) | 1.00 (0.94–1.07) | 1.03 (0.97–1.10) | 0.462 |
| Pubic hair development | ||||||
| Phenols | ||||||
| BP3 | ||||||
| Quintile median μg/gC | 3.7 | 11 | 30 | 94 | 853 | |
| n PH2+/n total | 48/195 | 54/195 | 40/191 | 35/194 | 33/189 | |
| PR (95% CI) | 1 (Ref) | 1.02 (0.96–1.10) | 0.97 (0.91–1.04) | 0.95 (0.89–1.01) | 0.94 (0.88–1.01) | 0.01 |
| Adj PR (95% CI) | 1 (Ref) | 1.06 (1.00–1.14) | 1.02 (0.96–1.09) | 1.02 (0.95–1.09) | 1.06 (0.98–1.15) | 0.40 |
| BPA | ||||||
| Quintile median μg/gC | 1.0 | 1.6 | 2.4 | 3.8 | 8.8 | |
| n PH2+/total | 39/194 | 44/196 | 52/190 | 38/191 | 37/193 | |
| PR (95% CI) | 1 (Ref) | 1.02 (0.95–1.09) | 1.06 (0.99–1.14) | 1.00 (0.93–1.07) | 0.99 (0.93–1.06) | 0.67 |
| Adj PR (95% CI) | 1 (Ref) | 1.03 (0.96–1.09) | 1.06 (0.99–1.13) | 0.99 (0.92–1.05) | 1.00 (0.94–1.07) | 0.57 |
| 2,5-DCP | ||||||
| Quintile median μg/gC | 1.6 | 4.0 | 11 | 37 | 179 | |
| n PH2+/total | 32/189 | 32/192 | 40/195 | 57/194 | 49/194 | |
| PR (95% CI) | 1 (Ref) | 1.00 (0.94–1.06) | 1.03 (0.97–1.10) | 1.11 (1.03–1.18) | 1.07 (1.00–1.15) | 0.00 |
| Adj PR (95% CI) | 1 (Ref) | 0.95 (0.89–1.02) | 0.95 (0.89–1.02) | 1.00 (0.92–1.08) | 0.93 (0.86–1.01) | 0.40 |
| Triclosan | ||||||
| Quintile median μg/gC | 2.6 | 6.9 | 15 | 38 | 170 | |
| n PH2+/total | 56/191 | 37/192 | 44/192 | 32/195 | 41/194 | |
| PR (95% CI) | 1 (Ref) | 0.92 (0.86–0.99) | 0.95 (0.89–1.02) | 0.90 (0.84–0.96) | 0.94 (0.87–1.00) | 0.06 |
| Adj PR (95% CI) | 1 (Ref) | 0.93 (0.87–0.99) | 0.93 (0.87–0.99) | 0.89 (0.83–0.94) | 0.94 (0.88–1.00) | 0.02 |
| Paraben sum | ||||||
| Quintile median μg/gC | 15 | 436 | 84 | 197 | 837 | |
| n PH2+/total | 33/178 | 25/177 | 37/178 | 44/174 | 50/179 | |
| PR (95% CI) | 1 (Ref) | 0.96 (0.90–1.03) | 1.02 (0.95–1.09) | 1.06 (0.98–1.13) | 1.08 (1.01–1.16) | 0.00 |
| Adj PR (95% CI) | 1 (Ref) | 0.94 (0.89–1.01) | 0.95 (0.89–1.02) | 1.02 (0.96–1.10) | 0.97 (0.90–1.04) | 0.80 |
| Phthalates | ||||||
| High MWP | ||||||
| Quintile median μg/gC | 84 | 138 | 203 | 327 | 613 | |
| n PH2+/total | 55/193 | 44/195 | 37/191 | 35/192 | 39/193 | |
| PR (95% CI) | 1 (Ref) | 0.95 (0.89–1.02) | 0.93 (0.87–0.99) | 0.92 (0.86–0.98) | 0.94 (0.87–1.00) | 0.04 |
| Adj PR (95% CI) | 1 (Ref) | 0.98 (0.92–1.04) | 0.95 (0.90–1.01) | 0.95 (0.89–1.01) | 0.94 (0.88–1.00) | 0.04 |
| Low MWP | ||||||
| Quintile median μg/gC | 66 | 112 | 173 | 272 | 718 | |
| n PH2+/total | 30/190 | 30/192 | 50/194 | 44/195 | 56/193 | |
| PR (95% CI) | 1 (Ref) | 1.00 (0.94–1.06) | 1.09 (1.02–1.16) | 1.06 (0.99–1.13) | 1.11 (1.04–1.19) | 0.00 |
| Adj PR (95% CI) | 1 (Ref) | 1.00 (0.94–1.06) | 1.05 (0.98–1.12) | 1.03 (0.97–1.10) | 1.06 (0.98–1.13) | 0.08 |
| Phytoestrogens | ||||||
| Daidzein | ||||||
| Quintile median μg/gC | 20 | 48 | 104 | 281 | 1,347 | |
| n PH2+/total | 49/195 | 46/192 | 39/196 | 31/190 | 44/191 | |
| PR (95% CI) | 1 (Ref) | 0.99 (0.92–1.06) | 0.96 (0.90–1.02) | 0.93 (0.87–0.99) | 0.98 (0.92–1.05) | 0.18 |
| Adj PR (95% CI) | 1 (Ref) | 1.01 (0.95–1.08) | 0.97 (0.91–1.03) | 0.95 (0.89–1.01) | 1.00 (0.94–1.07) | 0.39 |
| Genistein | ||||||
| Quintile median μg/gC | 9.5 | 20 | 46 | 129 | 607 | |
| n PH2+/total | 48/193 | 45/194 | 45/193 | 26/194 | 45/190 | |
| PR (95% CI) | 1 (Ref) | 0.99 (0.92–1.06) | 0.99 (0.92–1.06) | 0.91 (0.85–0.97) | 0.99 (0.92–1.06) | 0.19 |
| Adj PR (95% CI) | 1 (Ref) | 1.00 (0.94–1.06) | 1.01 (0.95–1.07) | 0.93 (0.88–0.99) | 1.03 (0.96–1.09) | 0.76 |
| Enterolactone | ||||||
| Quintile median μg/gC | 97 | 282 | 522 | 899 | 1,832 | |
| n PH2+/total | 45/195 | 37/193 | 48/194 | 40/195 | 39/187 | |
| PR (95% CI) | 1 (Ref) | 0.97 (0.91–1.04) | 1.01 (0.95–1.09) | 0.98 (0.92–1.05) | 0.98 (0.92–1.05) | 0.77 |
| Adj PR (95% CI) | 1 (Ref) | 0.99 (0.93–1.05) | 1.02 (0.96–1.08) | 0.98 (0.92–1.05) | 1.03 (0.96–1.10) | 0.47 |
Abbreviations: Adj, adjusted; C, creatinine; Ref, referent. Adjusted models included age, race/ethnicity, sex- and age-specific BMI% (CDC 2000), guardian education, season of urine collection, and site. There were fewer paraben measurements than other biomarkers because they were not measured early in the study. Quintile cut points were based on 982 girls with breast stage data, biomarkers, and creatinine. Quintile medians are among girls with breast or pubic stages. Breast stages were available for 981 girls with all biomarkers (963 for pubic hair); one additional girl with phthalate/phenol biomarkers was B1, PH2+ (982 total breast, 964 PH); one additional girl with phytoestrogens was B2+, PH1 (982 total breast, 964 PH stages).
Enterolactone modified the association of BMI with breast development. The proportion of low-BMI girls at pubertal stages B2+ increased from 15 to 20% across enterolactone quintiles (see note to Table 4). Among girls with high BMI, the proportion in the first quintile (48% B2+) decreased to 25% B2+ in the fifth quintile. This inverse association among high-BMI girls supported our hypothesis, and it remained after further adjustment for covariates (Table 4). The difference in the enterolactone PRs between low- and high-BMI groups suggests that higher enterolactone exposures may attenuate the BMI association. Specifically, in the first quintile of enterolactone in Table 4, the PR for pubertal stage B2+ versus B1 was 1.34 (95% CI, 1.23–1.45) for high BMI compared with low-BMI girls, the referent. This difference, the “BMI association,” diminished steadily so that in the fifth quintile of enterolactone, the PRs for B2+ versus B1 were comparable for low BMI and high BMI (PR = 1.14 and 1.20, respectively, with similar CIs) (Table 4). Thus, breast development differed among low- and high-BMI girls with low enterolactone exposure, but development was similar at the highest exposure levels regardless of BMI. These enterolactone associations with breast stage were similar in models stratified by BMI and further adjusted for BMI%; results were also consistent by race/ethnicity and by site.
Table 4.
Adjusted PRs (95% CIs) for any breast development stage (B2+ vs. B1) at visit 2 in relation to urinary environmental biomarkers and age-specific BMI measured at visit 1.
| Urinary enterolactone quintile [median creatinine (C) corrected biomarker concentration (μg/gC) for quintile] |
||||||
|---|---|---|---|---|---|---|
| 1st (97 μg/gC) | 2nd (283 μg/gC) | 3rd (522 μg/gC) | 4th (899 μg/gC) | 5th (1,922 μg/gC) | p-Trend | |
| n B2+ / n total | 12/84 | 14/82 | 17/99 | 20/102 | 27/124 | |
| Adjusted PR (95% CI) among low-BMI girls (BMI% range, < 1 to 68%; median = 36.0%) | 1.0 (ref) | 1.06 (0.96–1.15) | 1.05 (0.96–1.15) | 1.11 (1.01–1.22) | 1.15 (1.05–1.26) | 0.0016 |
| n B2+ / n total | 54/112 | 48/115 | 49/97 | 37/95 | 18/72 | |
| Adjusted PR (95% CI) among high-BMI girls (BMI% range, 68.5 to > 99%; median = 90.1%) | 1.34 (1.23–1.45) | 1.30 (1.20–1.42) | 1.35 (1.24–1.48) | 1.24 (1.13–1.36) | 1.21 (1.10–1.33) | 0.0105 |
PRs were computed from model with interaction term (ordinal values of enterolactone biomarker quintiles times 2-level BMI), adjusted for age at visit 2, race/ethnicity, site, caregiver education, season of urine collection. p-Interaction = 0.0006.
Apparent modification by BMI% of three other biomarkers in relation to pubertal stages could be explained by residual confounding of race or BMI% [p-interactions < 0.1; see Supplemental Material, Table 3 (doi:10.1289/ehp.0901690)]. In addition, genistein had a borderline interaction, but neither BMI stratum exhibited a trend of the adjusted PRs across biomarker quintiles. 2,5-DCP exhibited a positive association with breast development among high-BMI girls (p-interaction = 0.071; n = 948; p-trend 0.009), but in models stratified by BMI% further adjusted for BMI%, the association among high-BMI girls was null. The resulting attenuated estimate was similar to that of the main effect model for 2,5-DCP shown in Table 3. High MWP (p-interaction = 0.039) did not exhibit a dose–response relationship with breast development in low- or high-BMI groups (p-trends > 0.80). BP3 exhibited patterns similar to those of enterolactone. The BP3 association with breast stage had a positive direction in low-BMI girls and an inverse relationship in high-BMI girls (p-interaction 0.088). However, no dose–response relationship was evident. In BMI-stratified models, among girls with low-BMI the adjusted PR was 1.08 for the fifth versus first quintile of BP3 (95% CI, 0.97–1.20; p-trend 0.15; n =4 69); in high-BMI girls the p-trend was 0.38 (not shown).
Discussion
In this group of 1,151 girls, we examined concurrent exposures from three chemical classes that possess known or likely hormonal activity in relation to pubertal development. Biologically relevant levels of the biomarkers existed among girls in the cohort. Most biomarkers were ubiquitously detected, and maximal concentrations were in the range known to elicit effects experimentally (e.g., > 10 μmol). Overall, biomarker concentrations were similar to those reported in the National Health and Nutrition Examination Survey (NHANES) for children 6–11 years of age (CDC 2005), as were those in the pilot study (Wolff et al. 2007). These urinary metabolites are derived from common personal and household products or dietary sources, and absorption may occur through ingestion, inhalation, or dermal routes (National Science Foundation 2008).
Associations of concurrent exposure biomarkers with breast and pubic hair development in these girls were not strong, but those observed were among the chemicals with greatest exposure levels. The strongest finding was attenuation by enterolactone exposure of the BMI association with breast development. Along with the inverse relationships of daidzein and genistein with breast development and high MWP with pubic hair stage, the results were consistent with our a priori hypotheses and the experimental literature. Comparable associations of phytoestrogens with breast stage were seen in an earlier small study (Wolff et al. 2008). Phytoestrogens including enterolactone are known to possess hormonal activity (Adlercreutz 2002). A protective effect for puberty might be consistent with counteracting the influence of obesity (Horn-Ross 1995) or by reducing adiposity (Cederroth et al. 2007). In contrast, associations were positive, albeit very weak, for low MWP with both breast and hair development. It is not clear why low- and high-MWP metabolites could have opposite associations with developmental stages, yet various reports of such exposures in humans and animals show divergent hormonal associations, depending on timing and intensity of exposure or treatment and on rodent strain (Adlercreutz 2002; Rasier et al. 2006; Schoeters et al. 2008; Shen et al. 2009). In addition, patterns and density of ambient exposures no doubt differ for the low MWPs and high MWPs (Adibi et al. 2008).
Residual confounding or misclassification of exposures and outcomes remain possible explanations for our results. Collinearity of covariates, such as that among BMI, race/ethnicity, urinary creatinine, and urinary biomarkers and their variation by study site, are potential difficulties. We used methods with robust variance handling in an effort to minimize such problems. A potential explanation for the lack of strong associations is overadjustment of the models due to the inclusion of certain covariates (Greenland et al. 1999; Weinberg 1993). For example, BMI may be both a confounder and on the pathway between exposure and pubertal development. Considering the interrelationships among our variables, the models presented are the most appropriate. For our main analyses, we used creatinine-corrected biomarker concentrations to create quintile cut points. Creatinine correction may be inadequate for some or all analytes. However, we did not measure specific gravity, an alternate measure of urine dilution (Hauser et al. 2004; Miller et al. 2004). Other methods, such as the regression normalization procedures (Heavner et al. 2006), may not be appropriate for all urinary metabolites. Besides exposure misclassification, there is potential error in the outcome measurement of pubertal stage because of inter-rater variability in pubertal stage assessment. Therefore, both exposure and outcome measurements may be subject to nondifferential misclassification bias, which would likely shift the estimates toward the null. Additional considerations, including genetic and racial differences in exposure and development, would require considerably larger sample size. We estimated that for the main effects we had adequate power (80%) to detect PRs of 1.1 in 479 girls, if B2+ or PH2+ were > 20% in the fifth quintile (alpha 0.05), and a PR of 0.94 with 948 girls; these values are similar to the strongest associations we observed. Our effect estimates also may be conservative, because we used Poisson models instead of logistic regression models. For example, for the PR of 0.94 (95% CI, 0.88–1.00, high MWP and PH2+) in Table 3, we computed an odds ratio (OR) of 0.60 (95% CI, 0.34–1.06). However, measures of association using ORs may be over- or underestimated (Zou 2004). Finally, some or all of our findings may be due to chance; > 100 comparisons were made for the models presented in Tables 3 and 4. Associations of hormonal exposures in this study were small, which may be consistent with their relatively weak biological activity compared with endogenous hormones (Fang et al. 2000; Shen et al. 2009). Small effect estimates may be more realistic than those in previous studies that had small sample sizes (Wolff et al. 2008). There will be greater power to detect associations in longitudinal analyses that can also better reflect causal relationships than cross-sectional analyses; we plan to undertake such analyses as the cohort matures. The reports of delayed pubertal development in relation to blood lead concentrations in the NHANES population are informative for our findings, as the lead effects appear stronger than those we observed. Selevan et al. (2003) observed among black girls an OR of 0.62 (95% CI, 0.41–0.96, multivariate adjusted) for PH2+ versus PH1 among girls with blood lead > 3 μg/dL, quartile of exposure, compared with those having blood lead < 1 μg/dL, approximately the upper versus first quartile of their exposures. For the same NHANES population, Wu et al. (2003) found ORs for PH2+ of 0.27 (95% CI, 0.08–0.93) in the top exposure group (≥ 5 μg/dL) compared with ≤ 2 μg/dL blood lead. The proportion of PH2+ in the low exposure stratum was 81% versus 44% at high exposure. By comparison, the prevalence of PH2+ was 28% in the first compared with 20% in the fifth quintile of high MWP in Table 3, and the adjusted PR was 0.94 (95% CI, 0.88–1.00). High-MWP medians were 7-fold different between these quintiles compared with 3-fold differences in the lead exposure categories.
An additional consideration is that the peripubertal period is likely not the only critical window of exposure for pubertal development. Both animal and epidemiology studies suggest that prenatal and perinatal exposures also exert effects on later development (Rasier et al. 2006; Schoeters et al. 2008). Exposures during different windows may affect different molecular targets, including prenatal imprinting, the hypothalamic-pituitary axis, gonadotropin-releasing hormone neurons, hormone receptors, and aromatase action (Schoeters et al. 2008). Effective exposure ranges for these mechanisms may also differ widely, that is, toxic equivalents. Environmental agents in our study are cleared rapidly; it is possible that a single biomarker measurement is inadequate to quantify exposure relevant to pubertal development. However, single measurements of urinary biomarkers of phenols and phthalates were fairly representative of 6–12 months of exposure in children this age (Teitelbaum et al. 2008), likely because of common use and continuous exposure to many chemicals. Time-integrated multiple childhood exposure measures prenatally and prepubertally may be possible in alternate study designs. An important additional direction is to evaluate multiple exposures, including the extremes of exposure, multiple high exposures, early life exposures, and/or extremes of development (very late vs. very early changes) (Chou et al. 2009).
As we have mentioned, these environmental biomarkers were considered important for pubertal development because their concentrations are higher and in some cases their bioassay potency is greater than commonly studied environmental agents such as lead and 1,1′-dichloro-2,2′-bis(4-chlorophenyl)ethylene (DDE). Although the suggestive associations we observed are small, within 10% of null, a small effect size could affect a significant proportion of the population because of the ubiquity of these exposures and by their high levels (micromolar) observed in urine of the BCERC cohort.
Appendix 1.
We greatly appreciate the advice, support and collaboration of colleagues at NIH, including G. Collman, D. Winn, E. Maull, L. Reinlib, S. Lynch, G. Ellison, and C. Dilworth. We acknowledge the Avon Foundation for support and donations. We thank the study investigators and staff at the three medical centers involved in this research including M. Galvez, B. Brenner, J. Wetmur, J. Chen, P. Sheffield, N. Vangeepurum, J. Forman, L. Boguski, J. Britton, S. Peter, A. Mejia, A. Richiez, J. Montana, E. Chae, R. Osborne, E. Moshier, C. Zhu, and K. McGovern (MSSM); C. Dahl, C. Baker, S. Myatt, K. Ford, B. Bornschein, L. Yaghjyan, S. Roda (Cincinnati); L. Greenspan, B. Sternfeld, C. Ambrosone, J. Deardorff, S. Stewart, K. Balke, C.Ashley, C.Bonnell, A. Beeck, C. Chan, D. Davis, E. Landaverde, S. Burleson, R. Lum, A. Mirabedi, and M. Trotter (Kaiser Permanente). We are grateful to CDC colleagues M. Silva, E. Samandar, J. Preau, X. Ye, A. Bishop, J. Reidy, C. Pfeiffer, D. Parker, P. Olive, M. Kimberly, and C. Dodson.
Fox Chase Cancer Center and Mount Sinai acknowledge their community clinical collaborators, including North General Pediatric Clinic, Settlement Health Center, Children’s Aid Society, Little Sisters of the Assumption, Mount Sinai Pediatric Clinic, and our Community Outreach and Translation Core (COTC) partners (L. Claudio, S. Williams, D. Duncan, A. Fonfa). UCSF/Kaiser Permanente acknowledges their institutional and community collaborators, including Marin Breast Cancer Watch, California Department of Public Health, Marin County Department of Health and Human Services, San Francisco Department of Public Health, University of California Davis, University of Michigan, Roswell Park Cancer Institute, and our COTC partners (J. Barlow, K. Koblick). University of Cincinnati/Cincinnati Children’s Hospital acknowledges institutional and community collaborators, including the Breast Cancer Alliance of Greater Cincinnati, the Children’s Hospital Medical Center Clinical Research Center, the Breast Cancer Registry of Greater Cincinnati, Winton Woods School District, Fort Thomas School District, and our COTC partners (K. Brown, K. Ball).
Footnotes
Financial support was provided by grants ES/CA012770, 012771, 012800, 012801 from the National Institute of Environmental Health Sciences (NIEHS) and the National Cancer Institute (NCI); NIEHS (ES009584, ES012645); U.S. Environmental Protection Agency (R827039, RD831711); Agency for Toxic Substances and Disease Registry (ATU 300014); NCI (CA93447); and National Center for Research Resources (MO1-RR-00071, UL1-RR024131).
Supplemental Material is available online (doi:10.1289/ehp.0901690 via http://dx.doi.org/).
We gratefully acknowledge our collaborators within the Breast Cancer and Environment Research Centers, including the Fox Chase Cancer Center, Michigan State University, University of Cincinnati/Cincinnati Children’s Hospital, University of California San Francisco Comprehensive Cancer Center, Kaiser Permanente, Lawrence Berkeley National Laboratory, Mount Sinai School of Medicine, and University of Alabama Birmingham. See Appendix for details.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, the National Institutes of Health, the Centers for Disease Control and Prevention, or the California Department of Public Health. Individuals are listed in Appendix 1.
References
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116:467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlercreutz H. Phytoestrogens and breast cancer. J Steroid Biochem Mol Biol. 2002;83:113–118. doi: 10.1016/s0960-0760(02)00273-x. [DOI] [PubMed] [Google Scholar]
- Biro FM, Galvez M, Greenspan L, Succop P, Vangeepuram N, Pinney S, et al. Pubertal assessment methodology and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. doi: 10.1542/peds.2009-3079. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) CDC Growth Charts: United States. 2000. [[accessed 10 March 2010]]. Available: http://www.cdc.gov/growthcharts/
- CDC (Centers for Disease Control and Prevention) National Report on Human Exposure to Environmental Chemicals. 2005. [[accessed 10 March 2010]]. Available: http://www.cdc.gov/exposurereport/
- Cederroth CR, Vinciguerra M, Gjinovci A, Kühne F, Klein M, Cederroth M, et al. A phytoestrogen-rich diet increases energy expenditure and decreases adiposity in mice. Environ Health Perspect. 2007;115:1467–1473. doi: 10.1289/ehp.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Huang PC, Lee CC, Wu MH, Lin SJ. Phthalate exposure in girls during early puberty. J Pediatr Endocrinol Metab. 2009;22:69–77. doi: 10.1515/jpem.2009.22.1.69. [DOI] [PubMed] [Google Scholar]
- Fang H, Tong W, Perkins R, Soto AM, Prechtl NV, Sheehan DM. Quantitative comparisons of in vitro assays for estrogenic activities. Environ Health Perspect. 2000;108:723–729. doi: 10.1289/ehp.00108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environ Health Perspect. 2004;112:751–753. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavner DL, Morgan WT, Sears SB, Richardson JD, Byrd GD, Ogden MW. Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers’ spot and 24-h urines. J Pharm Biomed Anal. 2006;40:928–942. doi: 10.1016/j.jpba.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Horn-Ross PL. Phytoestrogens, body composition, and breast cancer. Cancer Causes Control. 1995;6:567–573. doi: 10.1007/BF00054166. [DOI] [PubMed] [Google Scholar]
- Jacobson-Dickman E, Lee MM. The influence of endocrine disruptors on pubertal timing. Curr Opin Endocrinol Diabetes Obes. 2009;16:25–30. doi: 10.1097/med.0b013e328320d560. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208–S217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, et al. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50:924–932. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- National Science Foundation. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. National Academies Press; 2008. [PubMed] [Google Scholar]
- Rasier G, Toppari J, Parent AS, Bourguignon JP. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: a review of rodent and human data. Mol Cell Endocrinol. 2006;254–255:187–201. doi: 10.1016/j.mce.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Rybak ME, Parker DL, Pfeiffer CM. Determination of urinary phytoestrogens by HPLC-MS/MS: a comparison of atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI) J Chromatogr B Analyt Technol Biomed Life Sci. 2008;861:145–150. doi: 10.1016/j.jchromb.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Schoeters G, Den HE, Dhooge W, van LN, Leijs M. Endocrine disruptors and abnormalities of pubertal development. Basic Clin Pharmacol Toxicol. 2008;102:168–175. doi: 10.1111/j.1742-7843.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. Blood lead concentration and delayed puberty in girls. N Engl J Med. 2003;348:1527–1536. doi: 10.1056/NEJMoa020880. [DOI] [PubMed] [Google Scholar]
- Shen O, Du G, Sun H, Wu W, Jiang Y, Song L, et al. Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol Lett. 2009;191:9–14. doi: 10.1016/j.toxlet.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- van Wieringen JC, Roede MJ, Wit JM. Growth diagrams for patient care [in Dutch] Tijdschr Kindergeneeskd. 1985;53:147–152. [PubMed] [Google Scholar]
- Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;137:1–8. doi: 10.1093/oxfordjournals.aje.a116591. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Britton JA, Boguski L, Hochman S, Maloney N, Serra N, et al. Environmental exposures and puberty in inner-city girls. Environ Res. 2008;107:393–400. doi: 10.1016/j.envres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Buck GM, Mendola P. Blood lead levels and sexual maturation in U.S. girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Environ Health Perspect. 2003;111:737–741. doi: 10.1289/ehp.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:53–59. doi: 10.1016/j.jchromb.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]


