Abstract
Background
Black carbon (BC) is a marker of traffic pollution that has been associated with blood pressure (BP), but findings have been inconsistent. MicroRNAs (miRNAs) are emerging as key regulators of gene expression, but whether polymorphisms in genes involved in processing of miRNAs to maturity influence susceptibility to BC has not been elucidated.
Objectives
We investigated the association between BC and BP, as well as potential effect modification by single nucleotide polymorphisms (SNPs) in miRNA processing genes.
Methods
Repeated measures analyses were performed using data from the VA Normative Aging Study. Complete covariate data were available for 789 participants with one to six study visits between 1995 and 2008. In models of systolic and diastolic BP, we examined SNP-by-BC interactions with 19 miRNA-related variants under recessive models of inheritance. Mixed-effects models were adjusted for potential confounders including clinical characteristics, lifestyle, and meteorologic factors.
Results
A 1-SD increase in BC (0.415 μg/m3) was associated with 3.04 mmHg higher systolic (95% confidence interval (CI), 2.29–3.79) and 2.28 mmHg higher diastolic BP (95% CI, 1.88–2.67). Interactions modifying BC associations were observed with SNPs in the DICER, GEMIN4, and DiGeorge critical region-8 (DGCR8) genes, and in GEMIN3 and GEMIN4, predicting diastolic and systolic BP, respectively.
Conclusions
We observed evidence of effect modification of the association between BP and 7-day BC moving averages by SNPs associated with miRNA processing. Although the mechanisms underlying these associations are not well understood, they suggest a role for miRNA genesis and processing in influencing BC effects.
Keywords: black carbon, blood pressure, epigenetic mechanisms, gene-environment interactions, genetic polymorphisms
Exposure to particulate air pollution has been associated with cardiovascular morbidity and mortality in numerous epidemiologic studies (Brook 2008; Pope et al. 2004). Black carbon (BC), a combustion by-product, is a widely used marker of traffic pollution and has been linked to cardiac and ventricular arrhythmias (Rich et al. 2005), ST-segment depression (Gold et al. 2000), decreased flow-mediated vascular reactivity (O’Neill et al. 2005), lowered heart rate variability (Schwartz et al. 2005), and increased cardiovascular mortality (Maynard et al. 2007). Growing evidence suggests that traffic-related pollution, including BC, may be driving the cardiotoxic effects observed in response to air pollution exposures (Hoffmann et al. 2007). A few recent studies have examined associations between particles and blood pressure (BP), and although positive associations have been observed (Auchincloss et al. 2008; Ibald-Mulli et al. 2001; Zanobetti et al. 2004), both inverse (Harrabi et al. 2006) and null (Jansen et al. 2005; Madsen and Nafstad 2006) associations have also been reported.
MicroRNAs (miRNAs) are small, noncoding RNAs that repress or inhibit gene expression by targeting messenger RNA (mRNA) (Mattick and Makunin 2006; Zhang 2008). Evidence suggests that miRNAs affect pathogenic pathways including angiogenesis (Suarez and Sessa 2009), redox signaling (Brewer and Shah 2009; Urbich et al. 2008), and stress response (van Rooij et al. 2007). Much of the existing miRNA-related literature has focused on cancer outcomes (Esquela-Kerscher and Slack 2006; Hu et al. 2008, 2009; Luthra et al. 2008; Yang et al. 2008). However, there is growing evidence that the dysregulation of cell-signaling pathways associated with miRNA is a key factor affecting heart disease (Chen et al. 2008; Cheng et al. 2007; Divakaran and Mann 2008; Ikeda et al. 2007; Zhang 2008). Additionally, studies in controlled environments have demonstrated cardiovascular effects due to loss of function of miRNA processing genes (Asada et al. 2008; da Costa Martins et al. 2008; Suarez et al. 2007). For the current study, we genotyped participants for potentially functional single nucleotide polymorphisms (SNPs) involved in the processing and formation of miRNAs. We then investigated the association between air pollution and BP as well as potential differences in susceptibility by SNP carrier status. We hypothesized that polymorphisms in genes that regulate miRNA processing could modify effects of BC on BP, a marker of autonomic function and cardiovascular health.
Materials and Methods
Study population
Our study participants were members of the Veterans Affairs Normative Aging Study (NAS). This is an ongoing longitudinal study of aging established in 1963, details of which have been published previously (Bell et al. 1966). Briefly, the NAS began as a closed cohort of 2,280 male volunteers from the greater Boston area, 21–80 years of age at entry, who enrolled after an initial health screening determined that they were free of known chronic medical conditions. All participants provided informed consent. Collection of blood samples for genetic analysis began in the late 1990s; 942 participants were still actively participating and provided BP and blood samples for some or all miRNA-related SNPs, which were successfully genotyped. Participants were reevaluated every 3–5 years using detailed on-site physical examinations and questionnaires.
Physical parameters and medical history
Study center visits occurred after an overnight fast and abstention from smoking. Physical examination included measurement of height and weight, with body mass index (BMI) calculated as weight (kilograms)/height (meters squared). Questionnaires evaluated lifestyle factors and medication use. Type 2 diabetes was classified based on physician’s diagnosis or fasting blood glucose > 126 mg/dL.
BP measurements
At each clinical visit, a physician measured BP using a standard mercury sphygmomanometer with a 14-cm cuff. Systolic BP (SBP) and fifth-phase diastolic BP (DBP) were measured in each arm to the nearest 2 mmHg while the participant was seated. The means of the right and left arm measurements were used as the BP measurement of each participant for analytical purposes. Although there was no specific rest period prior to measurement of BP, SBP and DBP were measured immediately after a complete patient history was taken with the subject seated.
SNP selection and genotyping
SNPs were selected based on previously published work investigating associations between genes involved in miRNA processing and disease (Horikawa et al. 2008; Yang et al. 2008). These SNPs were chosen because of overlap in pathways involved in cancer processes related to autonomic function through cell signaling, apoptosis, angiogenesis, and inflammation. Genotyping was performed using multiplex polymerase chain reaction assays designed with Sequenom SpectroDESIGNER software (Sequenom, Inc., San Diego, CA). The extension product was then spotted onto a 384-well spectroCHIP before analysis in the MALDI-TOF mass spectrometer (Sequenom, Inc.). Duplication was performed on 5% of the samples. The 24 SNPs analyzed for this study were all successfully genotyped. After genotyping, we excluded those SNPs for which fewer than 10 participants were homozygous variant carriers [rg1106042 in HIWI, rs3742330 in DICER, rs417309 in DiGeorge critical region-8 (DGCR8), rs636832 in Argonaute 1 (AGO1)] and also those with a Hardy–Weinberg p-value < 0.05 (rs10719 in DROSHA), leaving a total of 19 SNPs in 10 genes.
BC and meteorologic measurements
Continuous BC was measured at a Harvard School of Public Health monitoring site located at Countway Library (10 Shattuck Street, Boston, MA, USA), 1 km from the clinical examination site, and was averaged by hour before BP measurement using an aethalometer (Magee Scientific, Berkeley, CA, USA). We obtained temperature and relative humidity measures from the Boston airport weather station.
Statistical methods
Because repeated measures of BP were available for many of these study participants with both BC measurements and genotyping, we were able to obtain greater power by using multiple measures. We evaluated SBP and DBP as dependent variables and analyzed their associations with BC in linear mixed-effects models. Previous studies have suggested that longer averaging times are more relevant to the associations between particles and BP (Zanobetti et al. 2004), and recent sensitivity analysis within the NAS found 7-day moving averages as being most strongly associated with BP outcomes for short-term time windows (1 hr to 1 week) (Mordukhovich et al. 2009). Therefore, we used 7-day moving averages of ambient BC concentrations matched on the time of BP measurement for each participant, and we evaluated SBP and DBP as dependent variables.
We examined associations between BP and BC using two different approaches to address potential confounding. In model 1, we adjusted for age, BMI, BC, and apparent temperature (a marker of perceived temperature) as continuous variables as well as smoking status (never, current, former) and season of clinical visit (spring: March–May; summer: June–August; fall: September–November; winter: December–February). In model 2, we adjusted for covariates included in model 1 as well as blood urea nitrate, pulse, and median income treated as continuous variables in addition to education (≤ 12 years, 13–16 years, and > 16 years), alpha blocker, beta blocker, calcium channel blocker, angiotensin receptor blocker, angiotensin-converting enzyme (ACE) inhibitor, diuretic use, two or more alcoholic drinks per day, and diabetes diagnosis. We chose to examine recessive models of inheritance only (rather than dominant and additive) to limit the number of associations tested. Covariates from model 2 were selected a priori to be included in the regression models, regardless of statistical significance. We then examined the associations between the 19 SNPs in miRNA processing genes as well as SNP-by-BC cross-product terms to assess interactions. The default α level was defined as p = 0.05.
We performed sensitivity analyses to determine whether our results were robust to changes in the covariates adjusted for in the models and also whether there were significant effects due to missing data. To address attrition in the study, we used inverse probability weighting to determine whether reweighting these observations with only one or two repeated measures significantly changed our results (Hernan et al. 2006). This was done by modeling the probability of having only one measurement or only two measurements using the covariates described in model 2 and treating those participants with three or more visits as the reference group. We then used the inverse of the predicted values as the weights. We also examined associations adjusting only for covariates selected in model 1 to determine whether the significance and or magnitude of our adjusted results changed. Linkage disequilibrium (LD) between SNPs in the same gene that met the significance criterion were assessed using the LDPlotter tool (Innate Immunity in Heart Lung and Blood Disease; http://www.pharmgat.org/IIPGA2/Bioinformatics/).
Results
Of the 2,280 men who originally entered the cohort in 1963, complete covariate data were available on participants who took part in one to six examinations during the study period. Because the NAS comprises over 95% white participants, we restricted our analysis to white individuals based on self-report. There were 942 participants with some or all microRNA-processing genotyping data and BP measurements. Of these, BC data were available for 799 participants who reside in Massachusetts. All visits occurred between 1995, when pollutant monitoring began, and 2008. Our full models (model 2) include data from the 789 participants with complete covariate data and some or all genotyping data. In this group, 645 (82%) participants had at least two study visits from 1995 to 2008; 475 (60%) had three or more visits. Our study population was composed entirely of males, most of whom were former cigarette smokers (Table 1). The mean age (± SD) of study participants was 72.3 ± 7.5 years and mean BMI was 28.0 ± 4.1 kg/m2. Average SBP and DBP were 132 ± 18.4 mmHg and 76.8 ± 10.9 mmHg, respectively. We evaluated the association of SBP and DBP with ambient BC and expressed the results as the mmHg change associated with a 1-SD increase in BC (equivalent to 0.415 μg/m3) (Table 2). In our fully adjusted models (model 2), we found that a 1-SD increase in BC concentration was associated with 3.04-mmHg higher SBP (95% CI, 2.29–3.79; p = 0.003) and a 2.28-mmHg higher DBP (95% CI, 1.88–2.67; p < 0.001). These associations were attenuated compared with our model 1 analyses, which were adjusted for a subset of potential confounders. In model 1 analyses, we observed that a 1-SD change in BC was associated with 3.52-mmHg (95% CI, 2.77–4.26) and 2.72-mmHg (95% CI, 2.31–3.12) changes in SBP and DBP, respectively.
Table 1.
Descriptive statistics at baseline for participants included in analysis (n = 789).a
| Study characteristic | Value |
|---|---|
| BP measurements | |
| SBP (mmHg) | 132.0 ± 18.4 |
| DBP (mmHg) | 76.8 ± 10.9 |
| Clinical measures | |
| Age at visit (years) | 72.3 ± 7.5 |
| BMI (kg/m2) | 28.0 ± 4.1 |
| Pulse (beats/min) | 71.1 ± 8.1 |
| Hypertension diagnosis | 542 (81.8) |
| Hypertension medication use | 478 (72.1) |
| Two or more drinks per day | 199 (25.2) |
| Diabetesb | 182 (23.1) |
| Education (years) | |
| ≤ 12 | 280 (35.5) |
| 13–16 | 372 (47.2) |
| > 16 | 137 (17.3) |
| Smoking status | |
| Never | 237 (30.0) |
| Current | 47 (6.0) |
| Former | 505 (64.0) |
| Air pollution and weather data | |
| Seven-day moving average BC (μg/m3) | 0.98 ± 0.415 |
Values are mean ± SD or no. (%).
A total of 2,349 observations (one to six study visits) from 789 NAS participants were included in these analyses.
Diabetes was classified as physician-diagnosed diabetes mellitus or fasting blood glucose > 126 mg/dL.
Table 2.
| Model 1c mmHg (95% CI) | Model 2d mmHg (95% CI) | |
|---|---|---|
| SBP | 3.52 (2.77–4.26) | 3.04 (2.29–3.79) |
| DBP | 2.72 (2.31–3.12) | 2.28 (1.88–2.67) |
Corresponding to a 0.415-μg/m3 increase in 7-day average BC concentrations.
A total of 2,349 observations from 789 NAS participants were included in these analyses.
Model 1 was adjusted for age, BMI, smoking status, season, 7-day moving average of BC, and 7-day moving average of apparent temperature.
Model 2 was adjusted for education, blood urea nitrate, age, BMI, pulse, smoking status, pack-years, median income, apparent temperature, alpha blocker use, beta blocker use, calcium channel blocker use, angiotensin receptor blocker use, ACE inhibitor use, diuretics, season, two or more drinks per day, and diabetes diagnosis.
The complete list of the 19 SNPs analyzed is described in Table 3. We genotyped 24 SNPs and excluded those SNPs that, in fewer than 10 study participants, were homozygous carriers of the variant allele (rg1106042 in HIWI, rs3742330 in DICER, rs417309 in DGCR8, rs636832 in AGO1) and those in which Hardy–Weinberg equilibrium was not met at the 0.05 level (rs10719 in DROSHA), leaving a total of 19 SNPs in 10 genes. First, we examined the main effects of the SNPs of interest within this population. In our fully adjusted models, none of these SNPs were associated with BP at the 0.05 level. We examined BC-by-SNP interactions, and results are reported in Table 4. In models of SBP, interactions with BC were observed for two SNPs. For homozygous variant carriers of rs197414, a 1-SD change in BC was associated with 9.82-mmHg lower DBP (95% CI, −18.68 to −0.95), whereas in wild-type individuals and heterozygous carriers, we observed a 3.07-mmHg increase in BP (95% CI, 2.32–3.82); however, there were only 10 homozygous carriers of this variant, and the CI of these carriers is very wide. We also observed 5.58-mmHg higher BP (95% CI, 3.01–8.14) among GEMIN4 rs1062923 homozygous recessive carriers in response to a 1-SD change in BC, but only 2.87 (95% CI, 2.10–3.65) in heterozygotes and homozygous wild-type carriers. Because this SNP had a Hardy–Weinberg p-value of 0.05, we approach interpretation of these results with caution.
Table 3.
SNPs in miRNA processing genes included in analysis.
| Genea | RS number | SNP position | Alleles | Role | Amino acid change | MAF (%) |
|---|---|---|---|---|---|---|
| Gem-associated protein 3 | rs197414 | chr1:112110646 | C/A | Coding exon | Arginine/serine | 0.12 |
| (GEMIN3) (DDX20) | rs197388 | chr1:112099005 | T/A | Promoter | 0.18 | |
| rs197412 | chr1:112110476 | T/C | Coding exon | Isoleucine/threonine | 0.38 | |
| AGO1 (EIF2C1) | rs595961 | chr1:36140367 | A/G | Intron | 0.15 | |
| DROSHA (RNASEN) | rs6877842 | chr5:31568395 | G/C | Promoter | 0.18 | |
| Exportin 5 (XPO5) | rs11077 | chr6:43598925 | C/A | 3′ UTR | 0.40 | |
| AGO2 (EIF2C2) | rs4961280 | chr8:141716596 | C/A | Promoter | 0.18 | |
| Tar-RNA binding protein 2 (TARBP) | rs784567b | chr12:52180732 | C/T | Promoter | 0.49 | |
| DICER (DICER1) | rs13078 | chr14:94626500 | T/A | 3′ UTR | 0.18 | |
| Gem-associated protein 4 (GEMIN4) | rs7813 | chr17:594936 | T/C | Coding exon | Cysteine/arginine | 0.43 |
| rs1062923c | chr17:595817 | T/C | Coding exon | Isoleucine/threonine | 0.19 | |
| rs3744741 | chr17:595982 | C/T | Coding exon | Arginine/glutamine | 0.11 | |
| rs4968104 | chr17:596255 | T/A | Coding exon | Valine/glutamic acid | 0.27 | |
| rs910925 | chr17:596297 | G/C | Coding exon | Glycine/alanine | 0.42 | |
| rs2740348 | chr17:596685 | G/C | Coding exon | Glutamic acid/glutamine | 0.15 | |
| rs910924 | chr17:602670 | C/T | Promoter | 0.27 | ||
| DiGeorge syndrome critical region gene 8 (DGCR8) | rs3757 | chr22:18479331 | G/A | 3′ UTR | 0.25 | |
| rs1640299 | chr22:18478359 | G/T | 3′ UTR | 0.48 | ||
| Ras-related nuclear protein (RAN) | rs14035 | chr12:129927194 | C/T | 3′ UTR | 0.28 |
Abbreviations: HWE, Hardy–Weinberg equilibrium; MAF, minor allele frequency; RS, reference SNP.
Gene name (abbreviation), official gene symbol.
HWE p = 0.08.
HWE p = 0.05.
Table 4.
Effect modification of the association between a 1-SDa increase in BC concentrations and BP by gene variants related to miRNA processing under recessive models.b
| Sensitivity analyses |
|||||
|---|---|---|---|---|---|
| Results Δ mmHg (95% CI) | Model 2 p-interactionc | Model 1 p-interaction | IPW p-interactiond | ||
| DBP | |||||
| DGCR8 rs1640299 | |||||
| Wild-type and heterozygotes | 590 | 2.57 (2.12 to 3.02) | 0.006 | 0.002 | 0.004 |
| Homozygous carriers | 189 | 1.48 (0.78–2.18) | |||
| DICER rs13078 | |||||
| Wild-type and heterozygotes | 738 | 2.32 (1.91 to 2.72) | 0.017 | 0.03 | 0.008 |
| Homozygous carriers | 25 | 0.13 (−1.65 to 1.91) | |||
| GEMIN4 rs7813 | |||||
| Wild-type and heterozygotes | 638 | 2.44 (2.00 to 2.87) | 0.02 | 0.04 | 0.006 |
| Homozygous carriers | 136 | 1.40 (0.60 to 2.21) | |||
| GEMIN4 rs910925 | |||||
| Wild-type and heterozygotes | 647 | 2.44 (2.02 to 2.87) | 0.024 | 0.04 | 0.006 |
| Homozygous carriers | 134 | 1.43 (0.61 to 2.25) | |||
| SBP | |||||
| GEMIN3 rs197414 | |||||
| Wild-type and heterozygotes | 769 | 3.07 (2.32 to 3.82) | 0.005 | 0.01 | 0.01 |
| Homozygous carriers | 10 | −9.82 (−18.68 to −0.95) | |||
| GEMIN4 rs1062923 | |||||
| Wild-type and heterozygotes | 739 | 2.87 (2.10 to 3.65) | 0.045 | 0.04 | 0.027 |
| Homozygous carriers | 37 | 5.58 (3.01 to 8.14) | |||
A 1-SD increment in BC concentration is equal to 0.415 μg/m3.
Adjusted for age (years), BMI, cigarette smoking status (never, current, former), pack-years smoked, blood urea nitrate, type 2 diabetes diagnosis, two or more alcoholic drinks per day, and current antihypertensive medication use (including angiotensin II receptor-, alpha-adrenoceptor, beta- or calcium channel blockers, diuretics, or ACE inhibitors) as well as apparent temperature, and season of clinical visit (spring: March–May; summer: June–August; autumn: September–November; winter: December–February).
Two-sided p-value for interaction from SNP-by-BC coefficient.
p-Value for interaction from SNP-by-BC coefficient for sensitivity analysis using IPW.
We observed statistically significant interactions for four SNP-by-BC interactions predicting DBP. In all of these analyses, smaller changes in BP were observed for carriers of the homozygous variant genotype. A 1-SD change in BC was associated with 1.48-mmHg higher DBP (95% CI, 0.78–2.18) for homozygous variant carriers and 2.57 mmHg (95% CI, 2.12– 3.02) in others. The rs13078 DICER SNP was associated with 0.13-mmHg higher BP in carriers of the homozygous variant (95% CI, −1.65 to 1.91) and 2.32 (95% CI, 1.91–2.72) in others. Under a recessive model, homozygous carriers of the variant alleles of rs7813 and rs910925, both in GEMIN4, were both associated with lower BP in response to a 1-SD increase in BC compared with others. Both these SNPs produced very similar magnitudes of effect and were found to be in high LD (r2 = 0.9). As a sensitivity analysis, we also investigated whether there were significant associations in models adjusted only for model 1 variables, and the same SNPs achieved significant results. We also tested our associations using inverse probability weighting (IPW) to address attrition and found that our most significant results for both SBP and DBP became more significant, although changes associated with the IPW analysis were modest. We report the model 1 p-values and IPW p-values for sensitivity additionally in Table 4.
Discussion
We observed that BC concentration averaged over the 7 days preceding each study center visit was positively associated with SBP and DBP in a cohort of elderly men. Furthermore, SNPs in miRNA processing genes modified the BC associations with BP that we observed. Our analysis focused on potentially functional SNPs located in genes involved in the processing of miRNAs. To our knowledge, this is the first study to investigate the role of polymorphisms in miRNA processing genes to be identified as effect modifiers of an association between air pollution and cardiovascular response.
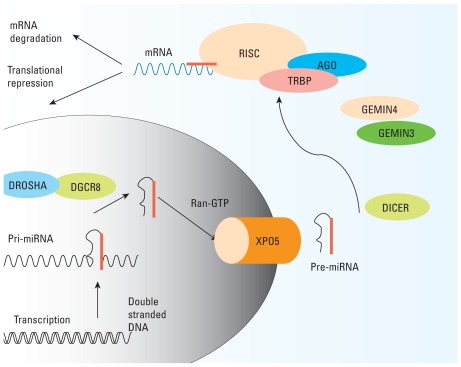
We selected these SNPs because of their involvement in the biogenesis and processing of miRNA. The transcription and processing of miRNAs has been described in detail elsewhere (Bartel 2004). Briefly, miRNAs are processed in a multistep pathway (Figure 1). First, long primary transcripts coded within the introns of protein-coding genes are transcribed into primary miRNAs (pri-miRNAs) approximately 100 nucleotides (nt) in length. Through a series of steps mediated by DROSHA and DGCR8, the pre-miRNA stem-loop (approximately 70–100 nt) is formed. After export from the nucleus via the RAN-GTP complex and XPO5, these pre-miRNAs undergo modification by DICER within the cytoplasm to generate an ~ 22-nt duplex from the loop complex, which comprises miRNA and its complement. AGO2, GEMIN3, and GEMIN4 then interact with miRNA to form a ribonucleoprotein, which guides the miRNA into the RNA-induced silencing complex (RISC), where the miRNA strand anneals to the 3′ untranslated regions (UTRs) of target mRNAs, promoting translational repression or mRNA degradation. More than 700 human miRNA have been identified in the miRBase Registry (http://microrna.sanger.ac.uk/) (Griffiths-Jones et al. 2008).
Figure 1.
Major steps in miRNA processing. Long primary transcripts coded within the introns of protein-coding genes are transcribed into primary miRNAs (pri-miRNAs). Then DROSHA and the DGCR8 complex mediate the formation of the pre-miRNA stem-loop. After export from the nucleus via the RAN-GTP complex and Exportin5, these pre-miRNAs undergo modification by DICER within the cytoplasm to generate an ~ 22-nt duplex from the loop complex, which comprises the miRNA and its complement. AGO2, GEMIN3, and GEMIN4 then interact with miRNA to form a ribonucleoprotein that guides the miRNA into the RISC, where the miRNA strand anneals to the 3′ UTRs of target mRNAs, promoting translational repression or mRNA degradation.
It has been shown that miRNAs, which are generally associated with silencing or suppression of target gene transcripts (negative regulation), may become activators of gene expression during stress (Rocha 2007; Yang and Paschen 2008). Animal studies have also shown that miRNA expression is downregulated in lungs of rats exposed to cigarette smoke, which is consistent with the hypothesis that alterations of the miRNA control mechanism can occur even after relatively short periods of exposure (Izzotti et al. 2009). Other forms of epigenetic regulation, such as DNA methylation, have been associated with exposure to traffic particles (Baccarelli et al. 2009). Recently, miRNA processing genes, such as DICER, have been associated with endothelial function and angiogenesis (Suarez et al. 2007).
None of the interactions identified were significantly predictive of both SBP and DBP. Although BC interactions with SNPs in DGCR8, DICER, and GEMIN4 were observed in models of DBP, only interactions with single SNPs in GEMIN3 and GEMIN4 were predictive of SBP. The DICER and DGCR8 SNPs for which we observed interactions predicting DBP are both located within 3′ UTRs. These regions are not translated directly into the protein; however, they are thought to play a role in mRNA stability. Additionally, these polymorphisms may affect miRNA binding to the 3′ UTR of target mRNAs. Previously, DICER knockdown increased activation of the endothelial nitric oxide synthase pathway (Suarez et al. 2007). DICER is thought to be more generally involved in miRNA processing, whereas DGCR8 is thought to be more specific to particular miRNAs (Han et al. 2006). DGCR8 is required for the maturation of miRNA primary transcripts at the initial stages. A recent study found that deletion of DGCR8 in cardiomyocytes leads to left ventricular malfunction progressing to a dilated cardiomyopathy and premature lethality (Rao et al. 2009), which suggests a central role of this gene in cardiovascular function. Lack of DICER activity in knockout studies has been suggested to be associated with defective blood vessel formation (Bernstein et al. 2003; Yang et al. 2005). However, no associations predicting SBP have been observed for the particular SNPs that we observed interacting with BC.
In our analyses, we found that BC interactions with SNPs in GEMIN4 were associated with SBP and DBP. Interactions with one SNP in GEMIN3, rs197414, was also associated with SBP. These genes code for proteins associated with the survival motor neuron (SMN) complex. GEMIN3 is a DEAD-box RNA helicase that binds directly with the SMN, and GEMIN4 is thought to play a role as cofactor in this response (Charroux et al. 2000). Together, GEMIN3 and GEMIN4 play a critical role in the processing of pre-miRNA by introducing the miRNA precursor to the RISC (Murashov et al. 2007). Along with the AGO proteins, these genes form a complex with the miRNA to create a ribonucleoprotein (Maniataki and Mourelatos 2005). Previously, the GEMIN3 SNP rs197414, for which we observed much lower BP among the few carriers, has been associated with higher bladder cancer rates (Yang et al. 2008). A study found that the GEMIN4 rs7813 variant was associated with in vitro hepatocellular carcinoma cell line growth inhibition compared with the wild-type allele, suggesting that the amino acid change caused by this SNP might have physiologic significance (Wan et al. 2004).
Some mechanisms of BC effects may be different for SBP and DBP. For example, other studies have observed stronger associations between ambient pollutant exposures and DBP as opposed to SBP in human (Fakhri et al. 2009) and animal studies (Bartoli et al. 2009) in controlled environments. The mechanisms for these differences are not well understood. Systolic pressure rises with age; by contrast, diastolic pressure increases until around age 50 years and falls with increasing years (Burt et al. 1995). The role of SBP and DBP in predicting downstream health consequences remains controversial. Some recent reports have suggested that SBP should be the primary risk factor reported in older individuals (Williams et al. 2008). However, within the Framingham Heart Study, isolated diastolic hypertension was a cardiovascular risk factor (Franklin et al. 2009). Subjects with this condition were found to have about two times the cardiovascular risk with normal BP, and increased cardiovascular risk among subjects with isolated diastolic hypertension was confirmed in a multivariate-adjusted model.
We previously examined genetic polymorphisms in two specific pathways as modifiers of the effects of air pollution within the NAS: oxidative stress and metal processing. We chose these pathways because prior work suggested a) that particulate air pollution generated oxidative stress, and b) that the soluble metal component of particulate air pollution was responsible for part of the effect. Specifically, we examined SNPs in genes related to oxidative stress as modifiers of the effects of particles on heart rate variability (Chahine et al. 2007), on endothelial markers (Madrigano et al. 2009), and on plasma homocysteine (Ren et al. 2010). We have also examined them as modifiers of the effects of ozone on lung function (Alexeeff et al. 2007), and we have examined associations with SNPs in metal-processing genes and heart rate variability (Park et al. 2006; Ren et al. 2010). Recently, we reported that polymorphisms in oxidative defense genes did not significantly modify the association of BC with BP, although there were trends in that direction (Mordukhovich et al. 2009). Taken together, these studies suggest that these pathways may be important for air pollution effects, although confirmation for other related outcomes and in other cohorts will be required.
In another recent paper, we demonstrated that BC exposure is associated with epigenetic changes—specifically, a reduction in LINE-1 methylation (Baccarelli et al. 2009). miRNAs are other important epigenetic mechanisms of gene expression control. Here we explored a novel pathway, polymorphisms in genes responsible for processing miRNAs, and found evidence for effect modification of BC on BP. Again, it will be important to determine whether these same polymorphisms modify effects on other end points and in other cohorts.
Although we believe our findings suggest altered susceptibility to BC exposures resulting from alterations in miRNA-processing related genotypes, we acknowledge that there are some limitations to our analysis. Despite these observations, we are not able to ascertain whether the polymorphisms described here modify the associations with BC because they are more susceptible to downregulation or because their basal activity makes individuals more susceptible to downstream changes in biological function. It is possible that both mechanisms may be at work. Additionally, it is also possible that observed associations could be attributable to LD between the genotyped SNPs and some other causal variant. For each of the SNP-by-BC interactions examined, we limited our investigation to associations under a recessive model of inheritance to maximize power to detect interactions, and therefore we may have overlooked some true associations that would only be observed under other additive or dominant models of inheritance. On the other hand, we also face the problem of multiple comparisons, as we examined multiple SNPs in the miRNA pathway. We tested interactions with 19 SNPs in this study, and multiple testing is subject to false positives. However, whereas studies of large numbers of genes incorporate corrections for multiple testing, we did not test separate independent hypotheses. Rather, we tested consistency of the pattern of association examined and not just the significance of the most significant association for the 10 genes examined.
We also acknowledge that there may be some misclassification of exposure in our analysis. Our study uses stationary measures of air pollution to represent personal exposures. Prior research indicates that when looking at longitudinal air pollution, most error is of the Berkson type. To the extent that this error is classical, simulation studies have shown that it is highly unlikely to bias away from the null even in the presence of covariates. This indicates that this exposure misclassification may lead to an underestimation of the health effects of air pollution (Zeger et al. 2000). In addition, several studies, including one conducted in the greater Boston area, have found that longitudinal measures of ambient particulate concentrations are representative of longitudinal variation in personal exposures (Rojas-Bracho et al. 2000). BC concentrations are spatially heterogeneous because of the numerous local (mobile) sources. Therefore, measurement error in our BC exposure metric would likely attenuate the true association. Given that we found significant positive associations for BC, it is unlikely that this error would affect our conclusions. Our study population is homogenous, consisting entirely of elderly men, and we have restricted our analysis to white individuals. The median distance of the participant homes from the central site monitoring station was 17.6 km. However, our findings are consistent with studies of particulate air pollution conducted in more heterogeneous populations.
Conclusions
We have used a pathway approach to investigate how the processing of miRNAs may be a susceptibility factor for traffic particle–induced cardiovascular effects. Individual miRNAs may have multiple targets; more research is needed to address the relation between BC exposure and BP among diverse study populations and to clarify the mechanisms underlying the association between BC and BP. These results provide support for the hypothesis of the toxic effects of traffic pollution as measured by BC and contribute to growing understanding about the role of epigenetic modification and response to environmental stresses such as exposure to BC. In cardiovascular research, these results may be used to explore interactions with BC. Further investigations are needed to replicate our findings in other large cohort studies.
Footnotes
Funding for this work was provided by National Institute of Environmental Health Sciences grants ES 14663, ES 15172, ES 00002, P01 ES09825, EPA R832416, T32-07069, and T32-HL007374-30. The VA Normative Aging Study, a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts, is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs.
References
- Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure and lung function: effect modified by obesity and airways hyperresponsiveness in the VA Normative Aging Study. Chest. 2007;132(6):1890–1897. doi: 10.1378/chest.07-1126. [DOI] [PubMed] [Google Scholar]
- Asada S, Takahashi T, Isodono K, Adachi A, Imoto H, Ogata T, et al. Downregulation of Dicer expression by serum withdrawal sensitizes human endothelial cells to apoptosis. Am J Physiol Heart Circ Physiol. 2008;295(6):H2512–H2521. doi: 10.1152/ajpheart.00233.2008. [DOI] [PubMed] [Google Scholar]
- Auchincloss AH, Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the multi-ethnic study of atherosclerosis (MESA) Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, et al. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ Health Perspect. 2009;117:361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of healthy aging. Gerontologist. 1966;6(4):179–184. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nat Genet. 2003;35(3):215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- Brewer AC, Shah AM. Redox signalling and miRNA function in cardiomyocytes. J Mol Cell Cardiol. 2009;47(1):2–4. doi: 10.1016/j.yjmcc.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond) 2008;115(6):175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25(3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, et al. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroux B, Pellizzoni L, Perkinson RA, Yong J, Shevchenko A, Mann M, et al. Gemin4. A novel component of the SMN complex that is found in both gems and nucleoli. J Cell Biol. 2000;148(6):1177–1186. doi: 10.1083/jcb.148.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, et al. Targeted deletion of dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA. 2008;105(6):2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170(6):1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, et al. Conditional Dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008;118(15):1567–1576. doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- Divakaran V, Mann DL. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res. 2008;103(10):1072–1083. doi: 10.1161/CIRCRESAHA.108.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Fakhri AA, Ilic LM, Wellenius GA, Urch B, Silverman F, Gold DR, et al. Autonomic effects of controlled fine particulate exposure in young healthy adults: effect modification by ozone. Environ Health Perspect. 2009;117:1287–1292. doi: 10.1289/ehp.0900541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, et al. Single versus combined blood pressure components and risk for cardiovascular disease: The Framingham Heart Study. Circulation. 2009;119(2):243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008;36(database issue):D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, et al. Molecular basis for the recognition of primary microRNAs by the drosha-DGCR8 complex. Cell. 2006;125(5):887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Harrabi I, Rondeau V, Dartigues JF, Tessier JF, Filleul L. Effects of particulate air pollution on systolic blood pressure: a population-based approach. Environ Res. 2006;101(1):89–93. doi: 10.1016/j.envres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol. 2006;98(3):237–242. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Moebus S, Mohlenkamp S, Stang A, Lehmann N, Dragano N, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116(5):489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- Horikawa Y, Wood CG, Yang H, Zhao H, Ye Y, Gu J, et al. Single nucleotide polymorphisms of microRNA machinery genes modify the risk of renal cell carcinoma. Clin Cancer Res. 2008;14(23):7956–7962. doi: 10.1158/1078-0432.CCR-08-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L, et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008;118(7):2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Liang J, Wang Z, Tian T, Zhou X, Chen J, et al. Common genetic variants in pre-microRNAs were associated with increased risk of breast cancer in Chinese women. Hum Mutat. 2009;30(1):79–84. doi: 10.1002/humu.20837. [DOI] [PubMed] [Google Scholar]
- Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001;91(4):571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, et al. Altered microRNA expression in human heart disease. Physiol Genomics. 2007;31(3):367–373. doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009;23(3):806–812. doi: 10.1096/fj.08-121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KL, Larson TV, Koenig JQ, Mar TF, Fields C, Stewart J, et al. Associations between health effects and particulate matter and black carbon in subjects with respiratory disease. Environ Health Perspect. 2005;113:1741–1746. doi: 10.1289/ehp.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra R, Singh RR, Luthra MG, Li YX, Hannah C, Romans AM, et al. MicroRNA-196a targets annexin A1: a microRNA-mediated mechanism of annexin A1 downregulation in cancers. Oncogene. 2008;27(52):6667–6678. doi: 10.1038/onc.2008.256. [DOI] [PubMed] [Google Scholar]
- Madrigano J, Baccarelli A, Wright R, Suh H, Sparrow D, Vokonas P, et al. Air pollution, obesity, genes, and cellular adhesion molecules. Occup Environ Med. 2009 doi: 10.1136/oem.2009.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen C, Nafstad P. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO) Eur J Epidemiol. 2006;21(7):485–491. doi: 10.1007/s10654-006-9025-x. [DOI] [PubMed] [Google Scholar]
- Maniataki E, Mourelatos Z. A human, ATP-independent, RISC assembly machine fueled by pre-miRNA. Genes Dev. 2005;19(24):2979–2990. doi: 10.1101/gad.1384005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet 15 Spec No. 2006;1:R17–R29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- Maynard D, Coull BA, Gryparis A, Schwartz J. Mortality risk associated with short-term exposure to traffic particles and sulfates. Environ Health Perspect. 2007;115:751–755. doi: 10.1289/ehp.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Wilker EH, Suh H, Wright RO, Sparrow D, Vokonas PS, et al. Black carbon exposure, oxidative stress genes, and blood pressure in a repeated measures study. Environ Health Perspect. 2009;117:1767–1772. doi: 10.1289/ehp.0900591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashov AK, Chintalgattu V, Islamov RR, Lever TE, Pak ES, Sierpinski PL, et al. RNAi pathway is functional in peripheral nerve axons. FASEB J. 2007;21(3):656–670. doi: 10.1096/fj.06-6155com. [DOI] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111(22):2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Park SK, O’Neill MS, Wright RO, Hu H, Vokonas PS, Sparrow D, et al. HFE genotype, particulate air pollution, and heart rate variability: a gene-environment interaction. Circulation. 2006;114(25):2798–2805. doi: 10.1161/CIRCULATIONAHA.106.643197. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Rao PK, Toyama Y, Chiang HR, Gupta S, Bauer M, Medvid R, et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res. 2009;105(6):585–594. doi: 10.1161/CIRCRESAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Park SK, Vokonas PS, Sparrow D, Wilker E, Baccarelli A, et al. Air pollution and homocysteine: more evidence that oxidative stress-related genes modify effects of particulate air pollution. Epidemiology. 2010;21(2):198–206. doi: 10.1097/EDE.0b013e3181cc8bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, et al. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161(12):1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- Rocha S. Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem Sci. 2007;32(8):389–397. doi: 10.1016/j.tibs.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Rojas-Bracho L, Suh HH, Koutrakis P. Relationships among personal, indoor, and outdoor fine and coarse particle concentrations for individuals with COPD. J Expo Anal Environ Epidemiol. 2000;10(3):294–306. doi: 10.1038/sj.jea.7500092. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60(6):455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100(8):1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104(4):442–454. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79(4):581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316(5824):575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- Wan D, He M, Wang J, Qiu X, Zhou W, Luo Z, et al. Two variants of the human hepatocellular carcinoma-associated HCAP1 gene and their effect on the growth of the human liver cancer cell line Hep3B. Genes Chromosomes Cancer. 2004;39(1):48–58. doi: 10.1002/gcc.10293. [DOI] [PubMed] [Google Scholar]
- Williams B, Lindholm LH, Sever P. Systolic pressure is all that matters. Lancet. 2008;371(9631):2219–2221. doi: 10.1016/S0140-6736(08)60804-1. [DOI] [PubMed] [Google Scholar]
- Yang H, Dinney CP, Ye Y, Zhu Y, Grossman HB, Wu X. Evaluation of genetic variants in microRNA-related genes and risk of bladder cancer. Cancer Res. 2008;68(7):2530–2537. doi: 10.1158/0008-5472.CAN-07-5991. [DOI] [PubMed] [Google Scholar]
- Yang W, Paschen W. Conditional gene silencing in mammalian cells mediated by a stress-inducible promoter. Biochem Biophys Res Commun. 2008;365(3):521–527. doi: 10.1016/j.bbrc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280(10):9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110(15):2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. MicroRNAs: role in cardiovascular biology and disease. Clin Sci (Lond) 2008;114(12):699–706. doi: 10.1042/CS20070211. [DOI] [PubMed] [Google Scholar]