Abstract
Background
Elevated left ventricular mass (LVM) is a strong predictor of negative cardiovascular outcomes, including heart failure, stroke, and sudden cardiac death. A relationship between close (< 50 m compared with > 150 m) residential proximity to major roadways and higher LVM has previously been described, but the mechanistic pathways that are involved in this relationship are not known. Understanding genetic factors that influence susceptibility to these effects may provide insight into relevant mechanistic pathways.
Objective
We set out to determine whether genetic polymorphisms in genes affecting vascular and autonomic function, blood pressure, or inflammation influence the relationship between traffic proximity and LVM.
Methods
This was a cross-sectional study of 1,376 genotyped participants in the Multi-Ethnic Study of Atherosclerosis, with cardiac magnetic resonance imaging performed between 2000 and 2002. The impact of tagged single-nucleotide polymorphisms (tagSNPs) and inferred haplotypes in 12 candidate genes (ACE, ADRB2, AGT, AGTR1, ALOX15, EDN1, GRK4, PTGS1, PTGS2, TLR4, VEGFA, and VEGFB) on the relationship between residential proximity to major roadways and LVM was analyzed using multiple linear regression, adjusting for multiple potential confounders.
Results
After accounting for multiple testing and comparing homozygotes, tagSNPs in the type 1 angiotensin II receptor (AGTR1, rs6801836) and arachidonate 15-lipoxygenase (ALOX15, rs2664593) genes were each significantly (q < 0.2) associated with a 9–10% difference in the association between residential proximity to major roadways and LVM. Participants with suboptimal blood pressure control demonstrated stronger interactions between AGTR1 and traffic proximity.
Conclusions
Common polymorphisms in genes responsible for vascular function, inflammation, and oxidative stress appear to modify associations between proximity to major roadways and LVM. Further understanding of how genes modify effects of air pollution on CVD may help guide research efforts into specific mechanistic pathways.
Keywords: AGTR1; ALOX15; cardiac structure; cardiac MRI; gene-environment interactions; left ventricular mass; traffic, air pollution
Long-term exposure to ambient air pollution has been associated with cardiovascular morbidity and mortality in numerous epidemiologic studies (Dockery et al. 1993; Miller et al. 2007; Pope et al. 2004), but the mechanisms responsible for its observed effects remain poorly understood (Bhatnagar 2006). A variety of hypothesized pathways have been implicated in human and animal studies, including an effect of air pollution on endothelial function and vascular tone, autonomic function, thrombosis, blood pressure, and oxidative stress. Recent reviews of the studies implicating these pathways have been published (Brook 2007; Mills et al. 2009; Pope and Dockery 2006; Simkhovich et al. 2008).
Among studies that have demonstrated increased cardiovascular morbidity and mortality associated with air pollution exposures, traffic-related air pollution in particular has been increasingly recognized as an important source of cardiovascular disease (CVD)-modifying exposures. Recent work in the Multi-Ethnic Study of Atherosclerosis (MESA) has demonstrated a link between close (< 50 m) residential major roadway proximity, an indicator of high traffic exposure, and higher left ventricular mass (LVM) index (Van Hee et al. 2009). Increased LVM is associated with the subsequent development of depressed ejection fraction (Drazner et al. 2004), and left ventricular hypertrophy is associated with heart failure, arrhythmia, and sudden cardiac death (Bluemke et al. 2008; Levy et al. 1990). Efforts to elucidate the ways in which air pollution might relate to higher LVM may provide not only greater understanding of the underlying CVD process itself but also potential strategies for intervention.
Several genes and genetic pathways have been noted to have important functions in the postulated mechanisms responsible for the impact of air pollution on CVD. Components of the renin–angiotensin system, including angiotensinogen (AGT), angiotensin-converting enzyme (ACE), and type 1 angiotensin II receptor (AGTR1) genes, have well-described impacts on inflammation, blood pressure, and vasoconstriction (Gradman 2009) that are mediated in part via G protein–coupled receptor kinases (GRKs), including GRK4 (Felder and Jose 2006). Prostaglandin-endoperoxide synthase 1 and 2 genes (PTGS1 and PTGS2), as well as 15-lipoxygenase gene (ALOX15), play important roles in vascular inflammation and oxidative stress that leads to CVD (Dogné et al. 2005; Mochizuki and Kwon 2008; Wittwer and Hersberger 2007). Endothelin-1 (EDN1) is a potent vasoconstrictor that activates signaling pathways leading to atherosclerosis (Ivey et al. 2008). The beta-2 adrenergic receptor (ADRB2) affects cardiac autonomic function and the development of heart failure (Triposkiadis et al. 2009). Together with ALOX15, vascular endothelial growth factors A and B (VEGFA and VEGFB) promote vascular growth, which contributes to the development of atherosclerotic plaque and plaque instability (Mochizuki and Kwon 2008; Sluimer and Daemen 2009). Toll-like receptor 4 (TLR4) affects the inflammatory response that influences initiation and progression of atherosclerosis (Pasterkamp et al. 2004).
We conducted a study to determine whether polymorphisms in these prespecified genes involved in the regulation of vascular tone, blood pressure, autonomic function, and oxidative stress modify the previously observed relationship between proximity to major roadways and LVM in MESA.
Materials and Methods
Population and sampling
MESA is a prospective cohort study designed to examine the progression of subclinical CVD; it enrolled 6,814 men and women 44–85 years of age who were free of clinical CVD at entry. The participants were recruited from six U.S. communities: Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan, New York; and St. Paul, Minnesota. Details of the sampling, recruitment, and data collection have been previously reported (Bild et al. 2002). A subcohort of 2,880 unrelated MESA subjects were selected for genetic studies from subjects who gave informed consent for DNA extraction and use in genetic studies and had sufficient DNA available for this study. Priority was given to subjects who were included in a 1,000-person subset of participants with additional blood biomarker measurements, supplemented by random selection from remaining participants to fulfill balanced ethnic group representation (720 African American, 720 Hispanic, 720 Chinese, and 720 European American) and equality by sex.
For the present study, we included all participants in the genetic study database (n = 2,847) who underwent cardiac magnetic resonance imaging (MRI) at examination 1 (n = 2,152), had accurate address information for exposure assignment, and represented the groups with the greatest contrast in traffic proximity exposure (living within 50 m compared with ≥ 150 m from a major roadway) and the largest LVM association from our prior study (Van Hee et al. 2009), leaving 1,376 participants for analysis.
Study procedures
Medical history, address, anthropometric measurements, physical examination, and laboratory data for the present study were taken from the first examination of the MESA cohort, July 2000 to August 2002 (Bild et al. 2002). Information about age, sex, ethnicity, and medical history were obtained by questionnaires administered at the screening and the first examination. Institutional review boards at all participating centers approved the protocol, ensuring conformance with the Declaration of Helsinki, and all participants gave informed consent.
Geocoding and exposure assessment
Participants’ residential addresses were assigned geographic coordinates using ArcGIS 9.1 software (ESRI, Redlands, CA) in conjunction with the Dynamap/2000 street network and geocoding database (Tele Atlas, Boston, MA). We calculated proximity to traffic by measuring the distance from geocoded home address to the nearest major roadway (interstate, state, or county highway or major arterial), with a maximum search radius of 150 m. Exposure groups were divided into 0–50 m and > 150 m from the nearest major roadway. These two groups were chosen a priori based upon the results of our prior study, which demonstrated the largest associations between traffic exposure and LVM comparing the group residing within 50 m of a major roadway with the group living > 150 m away (Van Hee et al. 2009). This exposure contrast is consistent with the observed distribution of near-roadway air pollutants, with many pollutants rapidly approaching background levels by 150–200 m from large roadways (Hoek et al. 2002; Smargiassi et al. 2005; Zhu et al. 2002).
Cardiac MRI imaging
LVM was obtained by cardiac MRI. Images were acquired by 1.5-T MRI scanners (Signa LX and CVi, GE Healthcare, Waukesha, WI; and Somatom Vision and Sonata, Siemens Medical Solutions, Berlin, Germany) using a protocol previously described (Natori et al. 2006). All MRI data were submitted to the MESA MRI Reading Center at Johns Hopkins Hospital for centralized processing using MASS software, version 4.2 (Medis, Leiden, Netherlands).
DNA extraction
DNA was extracted from peripheral leukocytes isolated from packed cells of anticoagulated blood by use of a commercially available DNA isolation kit (Puregene; Gentra Systems, Minneapolis, MN). The DNA was quantified by determination of absorbance at 260 nm followed by PicoGreen analysis (Molecular Probes, Inc., Eugene, OR). Two vials of DNA were stored per participant at −70°C and subsequently aliquoted for use.
Selection of candidate genes
Twelve genes were selected from among genotyped MESA candidate genes: ACE, ADRB2, AGT, AGTR1, ALOX15, EDN1, GRK4, PTGS1, PTGS2, TLR4, VEGFA, and VEGFB. Genes were chosen based upon hypothesized mechanisms responsible for the effect of air pollutants on LVM as described above.
Selection of single-nucleotide polymorphisms
Single-nucleotide polymorphisms (SNPs) were selected in candidate gene loci according to the following criteria: a) within the proximal and distal 10-kb regions 5′ and 3′ to the given candidate gene (NCBI Build 35; National Center for Biotechnology Information 2004); b) compatibility with the Illumina GoldenGate technology (Fan et al. 2006) as determined by the Assay Design Tool (TechSupport, Illumina, San Diego, CA); and c) minor allele frequency (MAF) > 0.05 or a tag (r2 > 0.8) for another SNP with MAF > 0.05 as determined by applying the multilocus or “aggressive” “Tagger” option of Haploview version 3 (Barrett et al. 2005) using International HapMap project data for CEPH (Caucasian) and Yoruban populations (release 19; International HapMap Consortium 2003). In some cases a complete set of tagged SNPs (tagSNPs) for a given candidate gene was not possible because of these competing criteria. Additional SNPs were added from a) LDselect analysis of resequencing information from the SeattleSNPs project if available (Carlson et al. 2004; Nickerson 2003); b) nonsynonymous SNPs from dbSNP release 124 (National Center for Biotechnology Information 2005); and c) SNPs with prior reports of association proposed by a MESA investigator.
Selection of ancestry informative markers
Ancestry informative markers (AIMs) were selected from an Illumina proprietary SNP database to maximize the difference in allele frequencies between any pair of ethnic groups: European American versus African American, European American versus Chinese American, and African American versus Chinese American. Additional markers informative for Mexican-American ancestry were selected from published lists (Choudhry et al. 2006; Collins-Schramm et al. 2004). All subjects were genotyped for the 199 total AIMs selected for inclusion. Principal components of AIMs were calculated using Stata (version 10.1; StataCorp LP, College Station, TX).
Genotyping
Genotyping was performed by Illumina Genotyping Services (Illumina Inc.) using their proprietary GoldenGate assay. Of 150 SNPs initially typed, 11 failed quality control measures and were excluded. After excluding 24 SNPs with an overall MAF < 2%, 115 common tagSNPs in the 12 genes under study were chosen for the present analysis. We inferred haplotypes using the expectation-maximization algorithm of PHASE 2.1 (Stephens and Scheet 2005). Haplotype uncertainty was estimated using the diplotype probability. Within a gene, haplotypes with probability-weighted frequencies < 2.5% were grouped together in a gene-specific haplotype bin assigned “others.” A total of 85 common haplotypes were investigated. Supplemental Material, Table 1 (doi:10.1289/ehp.0901535.), summarizes the tagSNPs and haplotypes examined.
Data quality control
Illumina performed initial quality control to identify samples and SNPs that failed genotyping according to proprietary protocols and sporadic failed genotypes with GenCall (Illumina) quality score < 99.99%. After removal of failed SNPs and samples, the genotype calling rate was 99.93%, with maximum missing data rate per sample of 2.1% and maximum missing per SNP of 4.98%. The cohort genetic data was checked for cryptic sample duplicates and discrepancies in genetically predicted sex (using X markers) versus study database reported sex. Samples with unresolved duplicate and sex discrepancies were removed from the genetic study database.
Statistical methods
We produced descriptive statistics using frequencies and percentages for categorical variables and means and SDs for continuous variables. The distributions of covariates were compared across proximity to major roadway categories and log-transformed LVM using analysis of variance methods for continuous variables and chi-square tests for categorical variables. SNP genotype frequencies were tested for race-stratified Hardy–Weinberg equilibrium using exact tests. Race-stratified linkage disequilibrium was assessed for each of the 12 genes under analysis.
A total of 1,139 participants had no missing data on any outcomes or covariates. An initial analysis was performed without imputation and using models with reduced sets of covariates that required no imputation. Subsequently, iterative multiple imputation procedures were performed using switching regression and chained equations for the full 1,376 participants, including data on cotinine levels for 1,082 of 1,376 participants to impute secondhand smoking data (Royston 2007). This process resulted in the full sample of 1,376 adults with some imputed values. The regression analysis was repeated on the imputed sample, yielding overall results similar to those estimated initially. All reported findings are from the imputed data set because the precision of the estimates is improved by the increased sample size, and the full data set is less likely to be subject to bias (Greenland and Finkle 1995; van der Heijden et al. 2006).
We first performed SNP-specific analyses to examine interactions between SNPs and very close (< 50 m) residential proximity to major roadway for log-transformed LVM. We then performed haplotype analyses to also examine interactions between haplotypes and very close proximity to major roadways for LVM. We fitted linear, additive (1 degree of freedom) models separately for each individual SNP (coded 0, 1, and 2 for minor allele copies) and for each haplotype (coded 0, 1, and 2 for number of haplotype copies). Diplotype probabilities were used as weights in the haplotype analyses clustered on participant identifier. Individual F-tests for the product term of SNP × proximity < 50 m and haplotype × proximity < 50 m were calculated to obtain p-values for interactions. The false discovery rate (FDR) correction (q-value) was used to account for multiple testing (115 tests in each SNP model and 85 haplotypes in each haplotype model) (Hochberg and Benjamini 1990; Storey 2002). A q-value of 0.2, corresponding to a 20% FDR, was selected as a threshold for acceptable level of significance (Smith et al. 2007). To account for potential confounding by population stratification, we adjusted for the first five principal components of AIMs in all analyses. All models were additionally adjusted for age, sex, height, and weight.
Additional potential confounders for the full model, chosen a priori based upon a prior study of the relationship between traffic exposure and LVM (Van Hee et al. 2009), and results of the initial descriptive analyses included household income, highest educational attainment level, systolic and diastolic blood pressure, medication use, low- (LDL) and high- (HDL) density-lipoprotein cholesterol, physical activity, alcohol use, smoking and pack-year history of smoking, secondhand smoke exposure, and diabetes status by fasting blood glucose criteria or medication for diabetes. Medication use was modeled using indicator variables for ACE inhibitors, angiotensin-receptor blockers (ARBs), diuretics, beta-blockers, lipid-lowering medications, aspirin, nonsteroidal antiinflammatory drugs (NSAIDs), and cyclooxygenase (COX) inhibitors. Because several of these covariates (particularly blood pressure) may lie in the causal biological pathway between the exposure and outcome, and as a sensitivity analysis, we also examined several partially adjusted models with reduced sets of covariates. Confidence intervals (CIs) were calculated at an alpha value of 0.05. We then conducted several sensitivity analyses, including stratification by race, blood pressure, and medication use. Subpopulation effects in all interaction models were calculated using linear combinations of predictors in each model (lincom command in Stata).
Data were analyzed using Stata and R (version 2.9; R Development Core Team 2009). The authors had full access to the data and take responsibility for the integrity of the data.
Results
Descriptive statistics
Table 1 indicates the overall distribution of participant characteristics. The 1,376 participants in this study ranged in age from 44 to 84 years. Although all MESA participants were free of clinical CVD at baseline, a substantial percentage had cardiac risk factors (Table 1). On bivariate analyses, race, sex, site, antihypertensive medication use, body mass index (BMI), secondhand smoke exposure, and income were at least moderately associated (p < 0.2) with proximity to major roadway (data not shown). African Americans, Hispanics, women, participants in New York and Baltimore, those on antihypertensive medication and specifically ACE inhibitor therapy, and those with higher BMIs, higher secondhand smoke exposures, and lower income tended to reside nearer to major roadways. Associations between LVM and nearly every covariate were strong. African-American race, St. Paul study site, male sex, younger age, hypertension, antihypertensive medication use, BMI, blood pressure, cigarette smoke exposure, fasting blood glucose, income, education, lower HDL cholesterol, and higher height and weight were all associated with higher LVM on bivariate analysis. After adjustment for height and weight, LVM was positively associated with age (data not shown).
Table 1.
Population characteristics at baseline examination: MESA, 2002 (n = 1,376).
| Characteristic | n (%) |
|---|---|
| Distance to major roadway (m) | |
| > 150 | 919 (66.8) |
| < 50 | 457 (33.2) |
| Site/center | |
| Baltimore, MD | 179 (13.0) |
| Chicago, IL | 229 (16.6) |
| Los Angeles, CA | 444 (32.3) |
| New York, NY | 205 (14.9) |
| St. Paul, MN | 150 (10.9) |
| Winston-Salem, NC | 169 (12.3) |
| Sex | |
| Female | 722 (52.5) |
| Race/ethnicity | |
| African American | 322 (23.4) |
| Chinese | 394 (28.6) |
| European American | 352 (25.6) |
| Hispanic | 308 (22.4) |
| Age (years) | |
| 44–54 | 454 (33.0) |
| 55–64 | 397 (28.9) |
| 65–74 | 386 (28.1) |
| 75–84 | 139 (10.1) |
| Highest education level completed | |
| Less than high school | 266 (19.3) |
| Completed high school or some college | 437 (31.8) |
| Technical school or associate degree | 178 (12.9) |
| Bachelor’s degree | 255 (18.5) |
| Graduate/professional degree | 237 (17.2) |
| Gross family income | |
| < $25,000 | 463 (33.6) |
| $25,000 to $49,999 | 347 (25.2) |
| $50,000 to $74,999 | 223 (16.2) |
| $75,000 to $99,999 | 120 (8.7) |
| > $100,000 | 183 (13.3) |
| BMI (kg/m2) | |
| < 23 | 256 (18.6) |
| 23–27.5 | 540 (39.2) |
| 27.6–40 | 559 (40.6) |
| > 40 | 21 (1.5) |
| Fasting blood glucose (mg/dL) | |
| < 100 | 813 (59.1) |
| 100–125 | 381 (27.7) |
| > 125, untreated | 56 (4.1) |
| > 125, treated | 124 (9.0) |
| Cigarette smoking status | |
| Never | 791 (57.5) |
| Former | 415 (30.2) |
| Current | 170 (12.4) |
| Pack-years smoking | |
| < 10 | 1,016 (73.8) |
| 10–19.9 | 123 (8.9) |
| ≥ 20 | 230 (16.7) |
| Secondhand smoke exposure (hr/week) | |
| 0 | 768 (55.8) |
| 1 | 158 (11.5) |
| 2–5 | 130 (9.5) |
| 6–10 | 50 (3.6) |
| > 10 | 92 (6.7) |
| Alcohol use | |
| Never | 367 (26.7) |
| Former | 287 (20.9) |
| Current | 709 (51.5) |
| Blood pressure (mmHg) | |
| < 130/85 | 863 (62.7) |
| 130–139/85–89 | 193 (14) |
| 140–159/90–99 | 241 (17.5) |
| > 159/99 | 79 (5.7) |
| Hypertension medication | 473 (34.4) |
| Angiotensin type 2 antagonists | 45 (3.3) |
| Combinations of angiotensin II antagonists plus diuretics | 26 (1.9) |
| ACE inhibitors without diuretics | 146 (10.6) |
| ACE inhibitors with diuretics | 11 (0.8) |
| Any lipid-lowering medication | 209 (15.2) |
| Aspirin | 295 (21.4) |
| Beta-blockers without diuretics | 123 (8.9) |
| Beta-blockers with diuretics | 8 (0.6) |
| COX-2 inhibitors | 89 (6.5) |
| NSAIDs | 183 (13.3) |
| LDL (mg/dL) | |
| < 100 | 390 (28.3) |
| 100–129 | 535 (38.9) |
| 130–159 | 320 (23.3) |
| 160–189 | 94 (6.8) |
| > 189 | 17 (1.2) |
| HDL (mg/dL) | |
| < 40 | 291 (21.1) |
| 40–59 | 768 (55.8) |
| > 59 | 315 (22.9) |
| LVMa | |
| Grams | 140.2 ± 37.8 |
| Log LVM | 4.9 ± 0.3 |
Multiple imputation using all data including measured cotinine levels for 1,082 participants was used to impute missing covariates (179 secondhand smoke values, 40 income level measures, 20 LDL and 2 HDL values, 13 alcohol use and 7 pack-years smoking values, and 3 educational level, 3 exercise level, and 2 diabetes status measures).
Mean ± SD.
We noted no significant deviations from race-stratified Hardy–Weinberg equilibrium, such as those associated with genotyping error, on exact testing at an alpha level of 0.05 with Bonferroni correction for 115 tests. Supplemental Material, Table 1 (doi:10.1289/ehp.0901535), lists the selected SNPs and inferred haplotypes for this study, with the frequencies (for tagSNPs) or probability-weighted frequencies (for inferred haplotypes) of each. The principal components of AIMs showed generally good discrimination between self-categorized races, with Hispanics demonstrating the most diverse ancestry and showing some ancestral overlap with other racial groups [Supplemental Material, Figure 2 (doi:10.1289/ehp.0901535)].
Primary SNP interaction analyses
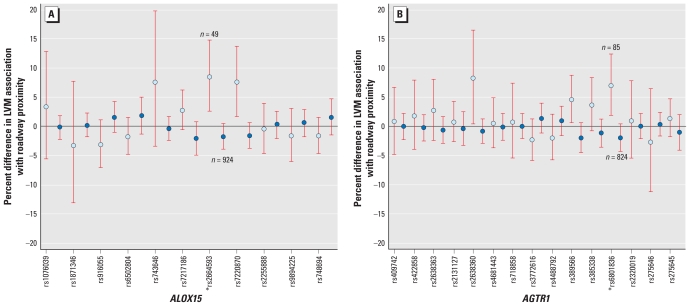
All SNP–traffic proximity interaction models tested demonstrated significant evidence of interactions for two of the 12 genes tested, ALOX15 and AGTR1 [Table 2; see also Supplemental Material, Figure 1 (doi:10.1289/ehp.0901535)]. The minimally adjusted model (model 1) containing only study site and principal components of AIMs, age, sex, height, and weight showed significant (q < 0.2) evidence of interactions for rs389566 and rs6801836 in AGTR1, with a 9% (95% CI, 2–16%) difference in the impact of traffic proximity (< 50 m residential proximity compared with > 150 m residential proximity) on LVM comparing homozygotes for rs389566 and an 11% (95% CI, 3–19%) difference comparing homozygotes for rs6801836. The full model (model 5) comprising all covariates showed significant evidence of interactions for rs2664593 in ALOX15 [10% (95% CI, 2–19%) difference in the association between traffic proximity and LVM comparing homozygotes] and rs6801836 in AGTR1 [9% (95% CI, 2–17%) difference comparing homozygotes]. Complete results of all SNP–roadway proximity interaction tests for all genes are included in Supplemental Material, Table 2 (doi:10.1289/ehp.0901535).
Table 2.
SNP–traffic interactions on LVM, top four interactions by model: percent change in LVM associated with close (< 50 m) residential proximity to major roadway compared with > 150 m, by tagSNP homozygote.
| Model/Gene | tagSNP | p-Value for interaction | Genotype | Percent difference in LVM associated with traffic proximity (95% CI) |
|---|---|---|---|---|
| Model 1: study site, age, sex, AIMs, height, weight | ||||
| AGTR1 | rs389566 | 0.001* | TT | 6.3 (2.1 to 10.8) |
| AA | −2.9 (−5.5 to −0.2) | |||
| AGTR1 | rs6801836 | 0.001* | GG | 8.6 (3.2 to 14.3) |
| AA | −2.5 (−5 to −0.1) | |||
| ALOX15 | rs2664593 | 0.011 | CC | 7.5 (1.3 to 14.2) |
| GG | −1.6 (−3.9 to 0.7) | |||
| AGTR1 | rs385338 | 0.012 | CC | 5.5 (0.8 to 10.5) |
| GG | −1.9 (−4.4 to 0.6) | |||
| Model 2: model 1 + diabetes, lipids, education, income, alcohol use, and physical activity | ||||
| AGTR1 | rs389566 | 0.001* | TT | 6.1 (1.8 to 10.5) |
| AA | −2.9 (−5.5 to −0.2) | |||
| AGTR1 | rs6801836 | 0.001* | GG | 8.5 (3 to 14.2) |
| AA | −2.6 (−5 to −0.1) | |||
| ALOX15 | rs2664593 | 0.013 | CC | 7.3 (1 to 13.9) |
| GG | −1.7 (−4 to 0.7) | |||
| AGTR1 | rs385338 | 0.018 | CC | 5.1 (0.4 to 10.1) |
| GG | −1.9 (−4.4 to 0.7) | |||
| Model 3: model 2 + smoking status, pack-years, and secondhand smoke exposure | ||||
| AGTR1 | rs389566 | 0.002* | TT | 6 (1.7 to 10.4) |
| AA | −2.8 (−5.4 to −0.2) | |||
| AGTR1 | rs6801836 | 0.001* | GG | 8.2 (2.8 to 14) |
| AA | −2.5 (−5 to −0.1) | |||
| ALOX15 | rs2664593 | 0.013 | CC | 7.3 (1 to 13.9) |
| GG | −1.7 (−4 to 0.7) | |||
| AGTR1 | rs385338 | 0.019 | CC | 5.1 (0.3 to 10) |
| GG | −1.9 (−4.4 to 0.7) | |||
| Model 4: model 3 + blood pressure | ||||
| AGTR1 | rs6801836 | 0.002* | GG | 7.7 (2.6 to 13.1) |
| AA | −2 (−4.3 to 0.4) | |||
| ALOX15 | rs2664593 | 0.004* | CC | 8.3 (2.4 to 14.7) |
| GG | −1.5 (−3.7 to 0.8) | |||
| AGTR1 | rs389566 | 0.007 | TT | 5.1 (1.1 to 9.3) |
| AA | −2 (−4.5 to 0.6) | |||
| ALOX15 | rs7220870 | 0.013 | AA | 7.3 (1.4 to 13.5) |
| CC | −1.3 (−3.5 to 1) | |||
| Model 5 (a priori model): model 4 + medication use | ||||
| ALOX15 | rs2664593 | 0.003* | CC | 8.5 (2.6 to 14.9) |
| GG | −1.7 (−3.9 to 0.5) | |||
| AGTR1 | rs6801836 | 0.004* | GG | 7 (1.9 to 12.4) |
| AA | −1.9 (−4.2 to 0.4) | |||
| ALOX15 | rs7220870 | 0.008 | AA | 7.5 (1.7 to 13.8) |
| CC | −1.5 (−3.7 to 0.7) | |||
| AGTR1 | rs389566 | 0.013 | TT | 4.6 (0.6 to 8.8) |
| AA | −1.9 (−4.4 to 0.6) | |||
Model 1 (minimally adjusted model) contains each tagSNP, proximity to roadway categories, interaction terms between SNP and proximity, study site, age, sex, height, weight, and the principal components of AIMs. Model 2 contains covariates from model 1, plus additional CVD risk factors (diabetes, lipids, education, income, alcohol use, and physical activity) except smoking. Model 3 contains covariates from model 2 plus smoking. Model 4 adds blood pressure to model 3. Model 5, the fully adjusted model, adds medication use (ACE inhibitors, ARBs, diuretics, beta-blockers, lipid-lowering medications, aspirin, NSAIDs, and COX inhibitors) to model 4.
Interactions meeting q-value threshold for statistical significance (q < 0.2).
In all cases of significant interactions, the minor allele represented the deleterious allele. The MAF of polymorphisms conferring apparent susceptibility to traffic proximity ranged from 23% (rs6801836) to 33% (rs389566) for AGTR1 and from 19% (rs2664593) to 21% (rs7220870) for ALOX15. The top four SNPs representing greatest evidence for interaction overall in models 4 and 5 consist of one group of two adjacent tagSNPs on ALOX15 (rs2664593 at position 7 and rs7220870 at position 8) and two nearby tagSNPs on AGTR1 (rs389566 at position 10 and rs6801836 at position 12). Figure 1 represents the relative location of each evaluated tagSNP on the two genes with significant findings. In the Supplemental Material, Figures 3 and 4 (doi:10.1289/ehp.0901535) show linkage disequilibrium maps for each racial group for ALOX15 and AGTR1. SNP positions 7 and 8 in ALOX15 and 10–12 in AGTR1 show strong linkage disequilibrium across all racial groups.
Figure 1.
Percent difference in LVM associated with living within 50 m of a major roadway compared with living > 150 m away (fully adjusted model), by all tagSNP homozygotes in ALOX15 (A) and AGTR1 (B). SNPs are arranged in their positional order along the chromosome. Data points indicate estimates for individuals homozygous for the minor allele (light blue) and for the major allele (dark blue). Numbers represent the number of participants in the significant genotype categories. Error bars are 95% CIs.
*Interactions meeting q-value threshold for statistical significance (q < 0.2).
Haplotype interaction results
After rare haplotypes were collapsed into a single category, remaining haplotypes containing the deleterious SNPs in AGTR1 and ALOX15 had low probability-weighted haplotype frequencies [3% for AGTR1-E and AGTR1-F, 9% for ALOX15-C, and 3% for ALOX15-I; see Supplemental Material, Table 1 (doi:10.1289/ehp.0901535)]. Despite this reduced power to detect interactions for haplotypes compared with individual SNPs, we found significant (q < 0.2) interactions for AGTR1-F after taking into account multiple testing. In model 5, proximity to roadway had no significant association with LVM in individuals without this haplotype, whereas the presence of a single haplotype conferred an 8.6% (95% CI, 3.0–14.6%) positive difference in LVM (p-value for interaction = 0.002) associated with close (< 50 m) proximity to a major roadway compared with living farther away (> 150 m). All five models showed consistent impacts on susceptibility, with the presence of AGTR1-F showing increased susceptibility to traffic proximity on LVM. Complete results of all haplotype–roadway proximity interaction tests for all genes and all models are included in Table 3 of the Supplemental Material (doi:10.1289/ehp.0901535).
Sensitivity analyses
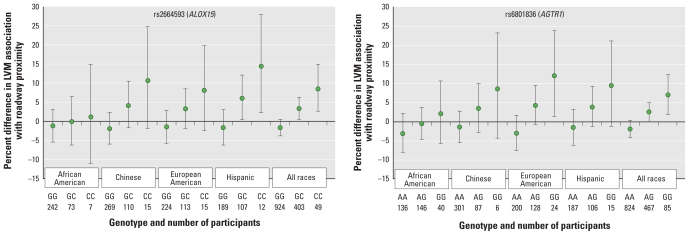
As described above and shown in Table 2 and Supplemental Material, Figure 1 (doi:10.1289/ehp.0901535), results were not particularly sensitive to model selection. To investigate the impact of racial structure on the findings, we performed stratification by race (using a three-way interaction among SNP, proximity to roadway, and race) for the two significant (q < 0.2) interactive SNPs in the full model, an analysis that includes adjustment for the principal components of AIMs (Figure 2). Although we found no significant evidence of a three-way interaction between rs6801836 (p-value for three-way interaction = 0.7) or rs2664593 (p-value for three-way interaction = 0.6), roadway proximity, and race, the overall results suggest less pronounced gene–environment interactions for African Americans compared with other racial groups.
Figure 2.
Race-stratified percent difference in LVM associated with living within 50 m of a major roadway compared with living > 150 m away (fully adjusted model) for tagSNPs showing significant evidence of interactive effects: rs2664593 in ALOX15 (A) and rs6801836 in AGTR1 (B). Error bars are 95% CIs.
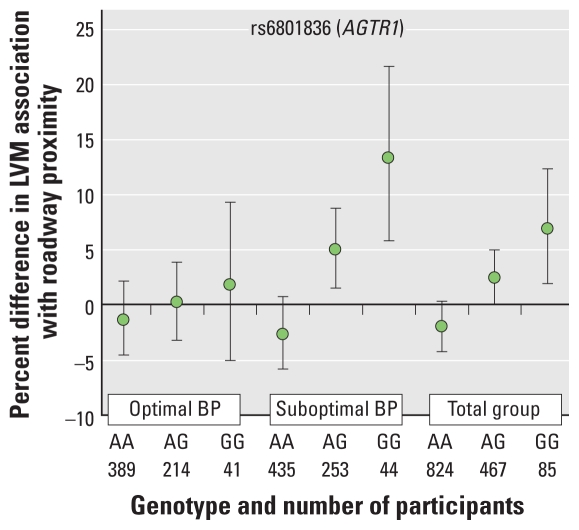
We also explored stratification by blood pressure categories [Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII) categories for all participants, binary optimal/suboptimal blood pressure, binary hypertensive/not hypertensive] (Chobanian et al. 2003) and use of medications important in the renin-angiotensin system or inflammatory pathways (ACE inhibitors, ARBs, aspirin, and NSAIDs) to determine whether these were groups particularly susceptible to this interaction. Among these analyses, we found significant evidence of interaction among rs6801836, proximity to traffic, and optimal versus suboptimal blood pressure categories. The difference in the LVM change associated with roadway proximity for individuals with greater numbers of the deleterious (G) allele in rs6801836 was significantly larger among individuals with suboptimal blood pressure by JNC VII criteria (systolic blood pressure ≥ 120 mmHg or diastolic blood pressure ≥ 80 mmHg, n = 732 participants) than among those with optimal blood pressure (p-value for three-way interaction = 0.05; Figure 3). We found no other significant three-way interactions for the medication use or blood pressure categories tested.
Figure 3.
Percent difference in LVM associated with living within 50 m of a major roadway compared with living > 150 m away, by blood pressure (BP) categories (optimal vs. suboptimal) according to JNC VII criteria and rs6801836 (AGTR1) genotype. Model comprises SNP, proximity to roadway categories, interaction terms between SNP and proximity, study site, age, sex, height, weight, principal components of AIMs, diabetes, lipids, education, income, alcohol use, physical activity, and smoking. Error bars are 95% CIs.
Discussion
We found significant evidence of gene–traffic interactions in MESA, with polymorphisms in AGTR1 and ALOX15, genes important in vascular function and inflammation/oxidative stress, associated with substantial alterations in the association between traffic proximity and LVM. Exploratory analyses additionally revealed that the interaction associated with the common polymorphism in the AGTR1 gene (rs6801836) is larger for individuals with suboptimal blood pressure (> 120 mmHg systolic or > 80 mmHg diastolic) than for those with optimal blood pressure control. Haplotype analyses, although likely underpowered in this setting, provided additional support for SNP findings in AGTR1.
AGTR1 is a well-known regulator of blood pressure and common target of specific pharmacologic intervention (ARB) in hypertension. The specific tagSNP locus (rs6801836) found to demonstrate interactive effects here has not yet been observed to show main effects in MESA or other studies (Bahrami et al. 2008). However, polymorphisms in AGTR1, and particularly the A1166C polymorphism (rs5186), have been associated with inflammation (Suchankova et al. 2009) and left ventricular hypertrophy (Smilde et al. 2007). Additionally, an association between the A1166C polymorphism and hypertension has been observed in a number of studies, although results have been somewhat inconsistent (Mottl et al. 2008). A possible explanation for the inconsistencies observed in these studies is the presence of unexamined interactive effects with environmental factors, such as those observed in the present analysis. Our observation that blood pressure and the identified polymorphism in AGTR1 appear to interact to produce susceptibility to the hypertrophic effects of traffic exposure is consistent with the well-known role of AGTR1 in the regulation of blood pressure and provides support to the hypothesis that the effect of air pollution is mediated by genes involved in blood pressure regulation. This observation also suggests an important role of both genetic and other environmental factors (in this case, factors that contribute to blood pressure) in modulating the effects of air pollution on CVD.
The protein encoded by ALOX15 is an oxidizing enzyme that can produce reactive lipid hydroperoxides within the vasculature. Experimental and human epidemiologic studies have demonstrated a range of contrasting effects of ALOX15 on CVD, and its role at present is not fully understood (Mochizuki and Kwon 2008; Wittwer and Hersberger 2007). Variants specifically at the rs2664593 locus in the 5′ promoter region of the gene have previously (but inconsistently) been associated with carotid atherosclerosis and have been shown to interact in the relationship between carotid wall thickness and insulin resistance (Bevan et al. 2009; McCaskie et al. 2008). In MESA, this specific SNP locus has not shown main effects on LVM (Bahrami H, unpublished observations).
Both ALOX15 and AGTR1 are likely to play important roles in inflammation, one of the primary putative mechanisms for the impact of air pollution on CVD and also an important mechanism involved in the development of left ventricular hypertrophy. These genes’ apparent interaction with air pollution to produce increases in LVM could suggest an important role of vascular inflammation in the pathogenesis of traffic-related air pollution–induced CVD more generally.
Linkage disequilibrium mapping of AGTR1 and ALOX15 in these analyses suggests that regions near to tagSNPs rs6801836 on AGTR1 and rs2664593 on ALOX15 are responsible for the interactions seen in this study. Finer mapping of ALOX15 and AGTR1 in these regions may help identify the specific polymorphism associated with these results.
Because the MESA cohort has four distinct racial groups, it represents a unique opportunity to examine gene–environment interactions in a multiethnic population. Analyses performed in this heterogeneous group must take into account the important issue of potential confounding by population stratification. To account for this issue of multiple ethnic groups that also vary in structure by site, we adjusted for both study site and the principal components of AIMs. We additionally examined three-way interactions with race in an analysis that included both site and AIMs and observed no evidence of substantial confounding or effect modification by race. Although we found no significant evidence overall of modification of the interactions by race, the finding that African Americans show apparently less pronounced interactions (Figure 2) bears further investigation. It is possible that additional relationships with other underlying genetic factors that predispose African Americans to known higher LVM (gene–gene interactions) may play a role in attenuating this particular interaction.
Few studies to date have examined gene–environment interactions in the cardiovascular health effects of air pollutants (Baccarelli et al. 2008; Chahine et al. 2007; Park et al. 2006; Schwartz et al. 2005). Of those studies, most have focused on individual functional polymorphisms or individual candidate genes, with few exceptions (Peters et al. 2007; Ren et al. 2010). This is the first study to explore multiple polymorphisms in pathways of interest with the goal of furthering understanding of how air pollution affects cardiac structure specifically.
This study has several limitations. We cannot rule out the possibility that a portion of the findings may have arisen by chance. We chose an FDR of 0.2 as a measure of statistical significance in this study, as have other studies (Smith et al. 2007). By definition, the choice of an FDR threshold to account for multiple testing controls for an expected proportion of incorrectly rejected null hypotheses. Future studies examining these relationships in additional cohorts will be necessary to replicate and confirm these findings. As have other recent studies of the health impacts of air pollution, we used a relatively coarse indicator of exposure to traffic-related air pollution, advantages and disadvantages of which have been described in more detail previously (Van Hee et al. 2009). The exposure misclassification produced by such methods may lead to bias in estimating the effects in subpopulations and overall and may adversely affect standard error estimates (Gryparis et al. 2009; Kim et al. 2009; Szpiro et al. 2009). Because of the chosen exposure metric, this study is unable to distinguish between interactions produced by specific components of traffic-related air pollutants or even non-air pollutants related to traffic (e.g., noise; for more detail, see Van Hee et al. 2009). Ongoing work will help disentangle these traffic-related influences on health.
Although the interactions observed in this study are relatively modest, in this same data set a 10-mmHg increase in systolic blood pressure (one of the factors known to be most important in the development of increased LVM) is associated with only an adjusted 3% higher LVM. Given that individuals homozygous for the deleterious alleles show a 7–8% difference in LVM associated with close proximity to a major roadway, and given the high minor allele frequencies and high prevalence of close residential proximity to a major roadway seen here, the public health impacts of these findings are potentially significant.
In our prior analysis of the relationship between proximity to roadways and VM (Van Hee et al. 2009), we reported results using an untransformed LVM index (rather than log-transformed LVM adjusted for height and weight) as the dependent variable in order to make the results more accessible to the primary audience. As we described in that report, the preferred statistical methods for regression analysis avoid the use of ratios such as the LVM index to prevent spurious correlation (Kronmal 1993). For this reason, and to meet the more stringent requirements of population association studies, which require that the outcome be approximately normally distributed rather than only the residuals (Balding 2006), we have used log LVM adjusted for height and weight in this report. For comparison purposes, we have reported the results of our prior study with the same parameterization used here in Table 4 of the Supplemental Material (doi:10.1289/ehp.0901535).
In addition to replication of these findings specifically, human studies such as pharmacologic intervention trials may further understanding of these potentially modifiable genetic pathways. Although this study was not powered to detect three-way interactions among genes, air pollution, and medication use, the observation that the presence of optimal blood pressure may attenuate the effect of genes and air pollution on cardiovascular outcomes is a provocative one.
As described above, the mechanisms responsible for the effects of air pollutants on CVD remain uncertain, although several studies have implicated inflammation and oxidative stress, as well as impaired vascular function. Modification of the effect of traffic exposures on LVM by genes involved in inflammatory response and vascular function suggests that individuals with genetically determined impaired handling of inflammation and altered vascular responses have greater susceptibility to these effects. This finding lends further support to pathways involving these mechanisms.
Conclusions
SNPs in genes responsible for vascular function, inflammation, and oxidative stress (AGTR1 and ALOX15) modify associations between proximity to major roadways and LVM. Further understanding of how genes modify effects of air pollution on CVD may help guide research efforts into specific mechanistic pathways.
Footnotes
This study was supported by National Heart, Lung, and Blood Institute contracts N01-HC-95159 through N01-HC-95165, N01-HC-95169, R01 HL077612, HL070151, HL071205, HL071251-52, HL071258-59, and T32HL07902; U.S. Environmental Protection Agency Science to Achieve Results grant RD831697; and National Institute of Environmental Health Sciences grants P50ES015915, P30ES07033, and K24ES013195.
Supplemental Material is available online (doi:10.1289/ehp.0901535 via http://dx.doi.org/).
References
- Baccarelli A, Cassano PA, Litonjua A, Park SK, Suh H, Sparrow D, et al. Cardiac autonomic dysfunction: effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation. 2008;117:1802–1809. doi: 10.1161/CIRCULATIONAHA.107.726067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami H, Fang B, Bluemke DA, Heckbert S, Kaufman J, Hunter Young J, et al. Association of polymorphisms in genes related to renin-angiotensin-aldosterone system with left ventricular structure and function and incident heart failure in four ethnic groups; the Multi-Ethnic Study of Atherosclerosis [Abstract] Circulation. 2008;118:S1102. [Google Scholar]
- Balding DJ. A tutorial on statistical methods for population association studies. Nat Rev Genet. 2006;7:781–791. doi: 10.1038/nrg1916. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bevan S, Lorenz MW, Sitzer M, Markus HS. Genetic variation in the leukotriene pathway and carotid intima-media thickness: a 2-stage replication study. Stroke. 2009;40:696–701. doi: 10.1161/STROKEAHA.108.525733. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD. Is air pollution a cause of cardiovascular disease? Updated review and controversies. Rev Environ Health. 2007;22:115–137. doi: 10.1515/reveh.2007.22.2.115. [DOI] [PubMed] [Google Scholar]
- Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, et al. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Choudhry S, Coyle NE, Tang H, Salari K, Lind D, Clark SL, et al. Population stratification confounds genetic association studies among Latinos. Genetics of Asthma in Latino Americans GALA Study. Hum Genet. 2006;118:652–664. doi: 10.1007/s00439-005-0071-3. [DOI] [PubMed] [Google Scholar]
- Collins-Schramm HE, Chima B, Morii T, Wah K, Figueroa Y, Criswell LA, et al. Mexican American ancestry-informative markers: examination of population structure and marker characteristics in European Americans, Mexican Americans, Amerindians and Asians. Hum Genet. 2004;114:263–271. doi: 10.1007/s00439-003-1058-6. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Dogné J, Hanson J, Pratico D. Thromboxane, prostacyclin and isoprostanes: therapeutic targets in atherogenesis. Trends Pharmacol Sci. 2005;26:639–644. doi: 10.1016/j.tips.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- Fan JB, Gunderson KL, Bibikova M, Yeakley JM, Chen J, Wickham Garcia E, et al. Illumina universal bead arrays. Methods Enzymol. 2006;410:57–73. doi: 10.1016/S0076-6879(06)10003-8. [DOI] [PubMed] [Google Scholar]
- Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2:637–650. doi: 10.1038/ncpneph0301. [DOI] [PubMed] [Google Scholar]
- Gradman EH. Evolving understanding of the renin-angiotensin-aldosterone system: pathophysiology and targets for therapeutic intervention. Am Heart J. 2009;157:S1–S6. doi: 10.1016/j.ahj.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- Gryparis A, Paciorek CJ, Zeka A, Schwartz J, Coull BA. Measurement error caused by spatial misalignment in environmental epidemiology. Biostatistics. 2009;10:258–274. doi: 10.1093/biostatistics/kxn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Hoek G, Meliefste K, Cyrys J, Lewné M, Bellander T, Brauer M, et al. Spatial variability of fine particle concentrations in three European areas. Atmos Environ. 2002;36:4077–4088. [Google Scholar]
- International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- Ivey ME, Osman N, Little PJ. Endothelin-1 signalling in vascular smooth muscle: pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis. 2008;199:237–247. doi: 10.1016/j.atherosclerosis.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kim SY, Sheppard L, Kim H. Health effects of long-term air pollution: influence of exposure prediction methods. Epidemiology. 2009;20:442–450. doi: 10.1097/EDE.0b013e31819e4331. [DOI] [PubMed] [Google Scholar]
- Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A. 1993;156:379–392. [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- McCaskie PA, Beilby JP, Hung J, Chapman CM, McQuillan BM, Powell BL, et al. 15-Lipoxygenase gene variants are associated with carotid plaque but not carotid intima-media thickness. Hum Genet. 2008;123:445–453. doi: 10.1007/s00439-008-0496-6. [DOI] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, et al. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med. 2009;6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Kwon YG. 15-lipoxygenase-1 in the vasculature: expanding roles in angiogenesis. Circ Res. 2008;102:143–145. doi: 10.1161/CIRCRESAHA.107.170191. [DOI] [PubMed] [Google Scholar]
- Mottl AK, Shoham DA, North KE. Angiotensin II type 1 receptor polymorphisms and susceptibility to hypertension: a HuGE review. Genet Med. 2008;10:560–574. doi: 10.1097/gim.0b013e3181809613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. Build 35. Database of Single Nucleotide Polymorphisms (dbSNP) Bethesda, MD: National Library of Medicine; 2004. [Google Scholar]
- National Center for Biotechnology Information. Release 124. Database of Single Nucleotide Polymorphisms (dbSNP) Bethesda, MD: National Library of Medicine; 2005. [Google Scholar]
- Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- Nickerson DA. SeattleSNPs. National Heart, Lung and Blood Institute Program for Genomic Applications. 2003. [[accessed 10 December 2009]]. Available: http://pga.gs.washington.edu.
- Park SK, O’Neill MS, Wright RO, Hu H, Vokonas PS, Sparrow D, et al. HFE genotype, particulate air pollution, and heart rate variability: a gene-environment interaction. Circulation. 2006;114:2798–2805. doi: 10.1161/CIRCULATIONAHA.106.643197. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G, Van Keulen JK, De Kleijn DP. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34:328–334. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- Peters A, Schneider A, Greven S, Bellander T, Forastiere F, Ibald-Mulli A, et al. Air pollution and inflammatory response in myocardial infarction survivors: gene-environment interactions in a high-risk group. Inhal Toxicol. 2007;19(suppl 1):161–175. doi: 10.1080/08958370701496129. [DOI] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Ren C, Baccarelli A, Wilker E, Suh H, Sparrow D, Vokonas P, et al. Lipid and endothelium-related genes, ambient particle matter, and heart rate variability—the VA Normative Aging Study. J Epidemiol Community Health. 2010;64:49–56. doi: 10.1136/jech.2008.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on interval censoring. Stata J. 2007;7:445. [Google Scholar]
- Schwartz J, Park SK, O’Neill MS, Vokonas PS, Sparrow D, Weiss S, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol. 2008;52:719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218:7–29. doi: 10.1002/path.2518. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Baldwin M, Pilger C, Dugandzic R, Brauer M. Small-scale spatial variability of particle concentrations and traffic levels in Montreal: a pilot study. Sci Total Environ. 2005;338:243–251. doi: 10.1016/j.scitotenv.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Smilde TD, Zuurman MW, Hillege HL, van Veldhuisen DJ, van Gilst WH, van der Steege G, et al. Renal function dependent association of AGTR1 polymorphism (A1166C) and electrocardiographic left-ventricular hypertrophy. Am J Hypertens. 2007;20:1097–1103. doi: 10.1016/j.amjhyper.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Smith NL, Hindorff LA, Heckbert SR, Lemaitre RN, Marciante KD, Rice K, et al. Association of genetic variations with nonfatal venous thrombosis in postmenopausal women. JAMA. 2007;297:489–498. doi: 10.1001/jama.297.5.489. [DOI] [PubMed] [Google Scholar]
- Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet. 2005;76:449–462. doi: 10.1086/428594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B. 2002;64:479–498. [Google Scholar]
- Suchankova P, Henningsson S, Olsson M, Baghaei F, Rosmond R, Holm G, et al. Association between the AGTR1 polymorphism +1166A > C and serum levels of high-sensitivity C-reactive protein. Regul Pept. 2009;152:28–32. doi: 10.1016/j.regpep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Szpiro AA, Sheppard L, Lumley T. UW Biostatistics Working Paper Series 350. Berkeley, CA: Berkeley Electronic Press; 2009. Efficient Measurement Error Correction with Spatially Misaligned Data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- van der Heijden GJ, Donders AR, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol. 2006;59:1102–1109. doi: 10.1016/j.jclinepi.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Van Hee VC, Adar SD, Szpiro AA, Barr RG, Bluemke DA, Diez Roux AV, et al. Exposure to traffic and left ventricular mass and function: the Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2009;179:827–834. doi: 10.1164/rccm.200808-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer J, Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]