Abstract
Objectives. To determine the effects of program policy changes, we examined service delivery benchmarks for breast cancer screening in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP).
Methods. We analyzed NBCCEDP data for women with abnormal mammogram or clinical breast examination (n = 382 416) from which 23 701 cancers were diagnosed. We examined time to diagnosis and treatment for 2 time periods: 1996 to 2000 and 2001 to 2005. We compared median time for diagnostic, treatment initiation, and total intervals with the Kruskal–Wallis test. We calculated adjusted proportions (predicted marginals) with logistic regression to examine diagnosis and treatment within program benchmarks (≤ 60 days) and time from screening to treatment (≤ 120 days).
Results. Median diagnostic intervals decreased by 2 days (25 vs 23; P < .001). Median treatment initiation intervals increased by 2 days (12 vs 14; P < .001). Total intervals decreased by 3 days (43 vs 40; P < .001). Women meeting the 60-day benchmark for diagnosis improved the most for women with normal mammograms and abnormal clinical breast examinations from 77% to 82%.
Conclusions. Women screened by the NBCCEDP received diagnostic follow-up and initiated treatment within preestablished program guidelines.
Screening for breast cancer reduces morbidity and mortality from breast cancer when women receive timely follow-up and appropriate treatment.1 There are few data to indicate what the optimal diagnostic and treatment intervals are that might ensure the best chances of survival from breast cancer detected by screening with mammography.2,3 Recent information from organized screening programs in Canada and the United Kingdom showed that women who waited longer than 6 to 12 months for diagnostic workup were more likely to have larger cancers and more positive lymph nodes, which might lead to poorer survival.2,3 In the case of symptomatic women, delays greater than 3 to 6 months to start therapy are associated with poorer survival.4
Recent modeling studies have shown that the declines in mortality are attributable to both early detection and subsequent treatment.1 Minority women, uninsured women, and women from lower socioeconomic backgrounds often do not have access to early detection.5–7 These women are less likely to participate in mammography screening,8 less likely to have timely and complete follow-up after an abnormal screening test result,9,10 more likely to be diagnosed with late-stage breast cancer,6,7,11 more likely to die from breast cancer once diagnosed,6,7 and might be more likely to receive suboptimal treatment.12–15
The National Breast and Cervical Cancer Early Detection Program (NBCCEDP) was authorized by Congress in 1990 to reach underserved women.16 Since the inception of the program, the NBCCEDP has established service delivery benchmarks to ensure timely and complete diagnostic follow-up and treatment initiation for underserved women screened through the program.17 Previous analysis of program benchmarks demonstrated that the national program was meeting its predefined quality standards of having a diagnosis within 60 days of an abnormal screening test result and initiation of treatment within 60 days of diagnosis.18 Legislation for program enhancements that added case management services, which was fully implemented in 2000, and a Medicaid waiver authorized by Congress in 2000 and fully implemented in 2003, should have improved the program's ability to meet these standards.19–22
Accordingly, we hypothesized that NBCCEDP service delivery benchmarks would improve over time with shortening of time intervals after an abnormal mammogram or clinical breast examination (CBE) finding to final diagnosis, as well as the interval to treatment initiation after diagnosis, and the interval to treatment initiation after abnormal screening test result. We investigated this by using 2 time periods, 1996 to 2000 and 2001 to 2005,20 to examine the effects of program policy changes on intervals in the 2001–2005 period.20–22
METHODS
The Centers for Disease Control and Prevention implemented cooperative agreements with states, American Indian/Alaska Native tribes, and territories to provide screening, referral, and follow-up services to women through the NBCCEDP and has been described in detail elsewhere.16,17,23
Since the program's inception in 1991, the Centers for Disease Control and Prevention has used a set of standardized data items to monitor screening, diagnostic follow-up, and treatment initiation activities. Women reported demographic characteristics, prior mammography history, and breast symptoms at enrollment. Providers reported dates and results of mammograms and CBEs. CBEs were completed by providers who evaluated women for screening. Providers also reported diagnostic procedures, outcomes, and the date of treatment initiation. For this study, data from 50 states, the District of Columbia, 13 tribes, and 4 territories were used for the study period of 1996–2005. Each woman's county of residence and a US Census data file were used to categorize residence at the time of screening as metropolitan, urban, or rural based on the Rural/Urban Continuum Code.24 This study was approved by the Centers for Disease Control and Prevention Human Subjects Committee.
Study Outcomes
We considered 3 time intervals for this study: (1) diagnostic interval: time from abnormal mammography or CBE (if mammogram was not done) to the date of definitive diagnosis; (2) treatment initiation interval: time from definitive diagnosis to treatment initiation; and (3) total interval: time from abnormal mammography or CBE to treatment initiation. The diagnostic interval examined all abnormal screening test results, defined as mammography Breast Imaging–Reporting and Data System categories25: 0 = assessment incomplete, 4 = suspicious abnormality, and 5 = highly suspicious of malignancy; and CBE results suspicious for cancer. Treatment and total intervals were examined in cancers only. We examined outcomes for 2 time periods. Time 1 encompassed 1996 –2000, and Time 2 encompassed 2001–2005. We chose these study periods to reflect several program enhancements that were fully implemented between 2000 and 2003, including case management in 200022 and the Medicaid waiver option for states, which was available in 48 of 50 states by the end of 2002.19
Study Population
During the study period from January 1, 1996, through December 31, 2005, 6 830 636 mammograms and CBEs were provided to 2 121 792 women aged 40 years and older. Among the 2 121 792 women screened, 420 494 women were found to have abnormal screening results. After all exclusions (see the figure available as a supplement to the online version of this article at http://www.ajph.org), our final study population consisted of 382 416 unique women with an abnormal mammogram or CBE with 23 701 cancers. Time 1 included 131 418 women with abnormal screening test results and 7838 cancers, and time 2 included 250 998 women with abnormal screening test results and 15 863 cancers.
Data Analysis
The diagnostic, treatment, and total interval distributions were highly skewed because of a few outliers. Therefore, we compared medians rather than means to provide a more accurate picture of the true distributions and used the Kruskal–Wallis test to assess statistical significance.26 We present median days and interquartile ranges (IQRs). We created multivariate logistic models stratified by time period because significant interactions were found between time periods and all covariates included in the final model. We used logistic regression analysis to produce adjusted proportions27 (predicted marginals) that controlled for screening test results, age, race/ethnicity, residence, whether women had symptoms or not, and whether cancer was diagnosed or not. All statistics were generated with SUDAAN version 9.0 (Research Triangle Institute, Research Triangle Park, NC) and SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Most women were White, aged 50 to 64 years, lived in a metropolitan area, and reported no breast symptoms (Table 1). Mammography reported as “assessment incomplete” increased from 60% in time 1 to 71.9% in time 2. Differences between the time periods included more women with an abnormal mammogram and normal CBE combination (54.7% vs 61.8%), and the proportion with only an abnormal CBE decreased from 6.1% to 3.8% (time 1 and time 2, respectively). More racial/ethnic minorities had abnormal screening test results in time 2 (the main increase came from the proportion of Hispanic women, which rose from 21.8% to 27.8% in time 2 compared with time 1). All differences by time period were significant at the P < .05 level. More than 80% of women were diagnosed within the 60-day standard, and 93% initiated treatment within 60 days (data not shown).
TABLE 1.
Characteristics of Women With Abnormal Mammograms or Clinical Breast Examinations (CBEs): National Breast and Cervical Cancer Early Detection Program, 1996–2005
| 1996–2000a (n = 131 418), No. (%) | 2001–2005a (n = 250 998), No. (%) | |
| Mammogram resultb/CBE resultc | ||
| Abnormal/normal | 71 843 (54.7) | 155 234 (61.8) |
| Abnormal/abnormal | 16 714 (12.7) | 32 058 (12.8) |
| Abnormal/not done | 9798 (7.5) | 19 366 (7.7) |
| Normal/abnormal | 25 106 (19.1) | 34 780 (13.9) |
| Not done/abnormal | 7957 (6.1) | 9560 (3.8) |
| Age, y | ||
| 40–49 | 54 326 (41.3) | 115 402 (46) |
| 50–64 | 69 154 (52.6) | 131 119 (52.2) |
| ≥ 65 | 7938 (6) | 4477 (1.8) |
| Race/ethnicity | ||
| White | 70 653 (53.8) | 118 910 (47.4) |
| Black | 20 091 (15.3) | 38 127 (15.2) |
| Asian | 4413 (3.4) | 11 567 (4.6) |
| AIAN | 5051 (3.8) | 6784 (2.7) |
| Hispanic | 28 594 (21.8) | 69 733 (27.8) |
| Unknown | 2616 (2) | 5877 (2.3) |
| Residence | ||
| Metropolitan | 93 856 (71.4) | 188 945 (75.3) |
| Urban | 31 775 (24.2) | 52 746 (21) |
| Rural | 5787 (4.4) | 9307 (3.7) |
| Breast symptoms | ||
| Yes | 31 315 (23.8) | 60 928 (24.3) |
| No | 89 358 (68) | 174 846 (69.7) |
| Unknown | 10 745 (8.2) | 15 224 (6.1) |
| Cancer diagnosed | ||
| Yes | 8100 (6.2) | 16 665 (6.6) |
| No | 123 318 (93.8) | 234 333 (93.4) |
Note. AIAN = American Indian/Alaska Native.
All variables were significantly different in 2001–2005 compared with 1996–2000 at the P < .05 level.
Abnormal mammography includes suspicious abnormality, highly suggestive for malignancy, or assessment incomplete.
Abnormal CBE is suspicious for cancer.
Table 2 displays median diagnostic, treatment, and total intervals with IQRs. For the diagnostic interval, the overall median time to diagnosis decreased by 2 days over the 2 time periods, 25 versus 23 days (time 1 to time 2, P < .001; Table 2). The biggest improvement in the median diagnostic interval occurred among women who had a normal mammogram and abnormal CBE (22 vs 12 days, time 1 to time 2). Women who reported breast symptoms and those eventually diagnosed with cancer also had decreased diagnostic intervals of 4 days (21 vs 17 days, time 1 to time 2) and 5 days (25 vs 20 days, time 1 to time 2), respectively. Within each time period, racial/ethnic minority women had longer median times to diagnosis than White women by 7 to 9 days in time 1 and by 7 to 16 days during time 2 (P < .001). Finally, women diagnosed with cancer had shorter diagnostic intervals in time 2 (20 days; IQR = 8–41 vs 23 days; IQR = 9–47) with larger improvements among those with cancer (5 days vs 2 days, respectively).
TABLE 2.
Distribution of Diagnostic, Treatment, and Total Intervals, by Screening Result, Demographics, Breast Symptoms, and Cancer Diagnosis: National Breast and Cervical Cancer Early Detection Program, 1996–2005
| Diagnostic Interval |
Treatment Interval |
Total Interval |
||||||||||||||||
| 1996–2000 |
2001–2005 |
1996–2000 |
2001–2005 |
1996–2000 |
2001–2005 |
|||||||||||||
| Characteristic | No. | Median Days (IQR) | P | No. | Median Days (IQR) | P | No. | Median Days (IQR) | P | No. | Median Days (IQR) | P | No. | Median Days (IQR) | P | No. | Median Days (IQR) | P |
| Mammogram resulta/CBE resultb | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||||||||||||
| Total combined | 131 418 | 25 (10–49) | 250 998 | 23 (9–47) | 7838 | 12 (0–28) | 15 863 | 14 (0–29) | 7838 | 43 (25–72) | 15 863 | 40 (22–67) | ||||||
| Abnormal/normal | 71 843 | 26 (13–49) | 155 234 | 26 (13–49) | 2834 | 12 (0–28) | 4981 | 14 (0–30) | 2834 | 52 (32–84) | 4981 | 54 (33–86) | ||||||
| Abnormal/abnormal | 16 714 | 23 (9–47) | 32 058 | 19 (6–41) | 3377 | 11 (0–27) | 7639 | 14 (0–28) | 3377 | 35 (20–58) | 7639 | 32 (18–53) | ||||||
| Abnormal/not done | 9798 | 26 (12–50) | 19 366 | 23 (10–45) | 718 | 11 (0–28) | 1455 | 17 (3–33) | 718 | 47 (26–77) | 1455 | 47 (27–77) | ||||||
| Normal/abnormal | 25 106 | 22 (4–54) | 34 780 | 12 (0–40) | 303 | 9 (0–24) | 427 | 13 (0–28) | 303 | 52 (28–93) | 427 | 50 (24–86) | ||||||
| Not done/abnormal | 7957 | 21 (8–49) | 9560 | 22 (8–47) | 606 | 14 (0–31) | 1361 | 15 (3–31) | 606 | 41 (23–72) | 1361 | 34 (19–60) | ||||||
| Age, y | <.001 | <.001 | <.001 | .151 | 0.27 | <.001 | ||||||||||||
| 40–49 | 54 326 | 23 (9–49) | 115 402 | 22 (8–46) | 2280 | 11 (0–28) | 5631 | 14 (0–29) | 2280 | 43 (23–70) | 5631 | 37 (21–63) | ||||||
| 50–64 | 69 154 | 25 (11–49) | 131 119 | 23 (10–47) | 4773 | 12 (0–28) | 9840 | 14 (0–29) | 4773 | 43 (25–73) | 9840 | 41 (22–69) | ||||||
| ≥ 65 | 7938 | 26 (12–50) | 4477 | 28 (13–54) | 785 | 9 (0–24) | 392 | 14 (0–31) | 785 | 44 (25–69) | 392 | 42 (22–72) | ||||||
| Race/ethnicity | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||||||||||||
| White | 70 653 | 21 (8–43) | 118 910 | 18 (7–36) | 4846 | 11 (0–26) | 8929 | 15 (2–28) | 4846 | 40 (23–64) | 8929 | 37 (21–60) | ||||||
| Black | 20 091 | 30 (14–59) | 38 127 | 27 (12–53) | 1329 | 12 (0–29) | 2617 | 18 (2–35) | 1329 | 50 (28–84) | 2617 | 48 (27–82) | ||||||
| Asian | 4413 | 28 (14–54) | 11 567 | 34 (15–61) | 258 | 10 (0–25) | 678 | 6 (0–28) | 258 | 47.5 (26–79) | 678 | 37 (18–70) | ||||||
| AIAN | 5051 | 29 (13–56) | 6784 | 25 (9–48.5) | 244 | 14 (2–30) | 382 | 14 (0–28) | 244 | 43 (26–73.5) | 382 | 46 (23–76) | ||||||
| Hispanic | 28 594 | 29 (13–56) | 69 733 | 29 (13–57) | 1003 | 15 (0–33) | 2867 | 12 (0–29) | 1003 | 54 (30–88) | 2867 | 43 (22–74) | ||||||
| Unknown | 2616 | 26.5 (11–53) | 5877 | 27 (12–54) | 158 | 7 (0–21) | 390 | 5.5 (0–25) | 158 | 37.5 (21–64) | 390 | 34 (13–63) | ||||||
| Residence | <.001 | <.001 | .684 | .525 | <.001 | <.001 | ||||||||||||
| Metropolitan | 93 856 | 26 (11–52) | 188 945 | 25 (10–49) | 5488 | 12 (0–28) | 11 578 | 14 (0–30) | 5488 | 45 (26–77) | 11 578 | 41 (22–69) | ||||||
| Urban | 31 775 | 21 (9–43) | 52 746 | 19 (8–37) | 2007 | 11 (0–25) | 3639 | 14 (3–27) | 2007 | 38 (22–63) | 3639 | 37 (21–61) | ||||||
| Rural | 5787 | 21 (9–41) | 9307 | 19 (8–36) | 343 | 12 (0–24) | 646 | 15 (5–27) | 343 | 39 (21–58) | 646 | 40 (22–61) | ||||||
| Breast symptoms | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||||||||||||
| Yes | 31 315 | 21 (7–47) | 60 928 | 17 (3–40) | 3337 | 12 (0–27) | 8123 | 13 (0–28) | 3337 | 36 (20–62) | 8123 | 33 (18–55) | ||||||
| No | 89 358 | 26 (12–50) | 174 846 | 26 (12–49) | 3796 | 11 (0–28) | 6675 | 16 (0–32) | 3796 | 49 (29–80) | 6675 | 50 (29–82) | ||||||
| Unknown | 10 745 | 23 (10–48) | 15 224 | 20 (8–41) | 705 | 14 (3–29) | 1065 | 16 (3–31) | 705 | 45 (26–71) | 1065 | 38 (22–65) | ||||||
| Cancer diagnosed | .019 | <.001 | NA | NA | NA | NA | ||||||||||||
| Yes | 8100 | 25 (12–47) | 16 665 | 20 (8–41) | 7838 | 12 (0–28) | 15 863 | 14 (0–29) | 7838 | 43 (25–72) | 15 863 | 40 (22–67) | ||||||
| No | 123 318 | 25 (10–49) | 234 333 | 23 (9–47) | 0 | NA | 0 | NA | 0 | NA | 0 | NA | ||||||
Notes. IQR = interquartile range; CBE = clinical breast examination; AIAN = American Indian/Alaska Native; NA = could not be calculated.
Abnormal mammography includes suspicious abnormality, highly suggestive for malignancy, or assessment incomplete.
Abnormal CBE is suspicious for cancer.
The opposite pattern was seen with treatment intervals (Table 2). For the overall study population, the median treatment interval increased 2 days (12 vs 14 days, time 1 to time 2, respectively; P < .001). Intervals increased for all subgroups except Asian women, Hispanic women, and women of unknown race. Only Hispanic women experienced a statistically significant decrease (15 vs 12 days, time 1 to time 2, respectively; P < .001). The overall proportion of women not meeting the program standard of initiating treatment within 60 days was 6.3% in time 2 (data not shown).
Table 2 describes the total time from abnormal screening test result to treatment initiation. Similar to the diagnostic interval, median times improved for most subgroups of women. The median time for the total interval decreased 3 days in the second time period (43 vs 40 days, time 1 to time 2, respectively; P < .001). Symptomatic women had the shortest median total interval in time 1 and time 2. All women with an abnormal CBE experienced shorter total intervals in time 2 regardless of mammography result. Again, racial/ethnic minority women had longer median total intervals compared with White women (P < .001). Women of unknown race/ethnicity had shorter total intervals compared with Whites. Women living in metropolitan areas had longer median total time intervals compared with women living in urban and rural areas.
Table 3 presents adjusted proportions for women being diagnosed and initiating treatment within the program standard of 60 days. Stratified models were constructed to examine differences within each time period because of significant interactions between all covariates and time period. When examined by time period, women with a normal mammogram and an abnormal CBE had the largest improvement in diagnostic interval from time 1 to time 2 of meeting the 60-day program benchmark from 77% (95% confidence interval [CI] = 76.0%, 78.0%) to 82% (95% CI = 82.0%, 82.0%), respectively. During the second time period only women with no mammogram and an abnormal CBE had slightly lower proportions meeting the 60-day standard (80%; 95% CI = 79.0%, 81.0%), compared with time 1 (81.0%; 95% CI = 80.0%, 82.0%). All groups of racial/ethnic minority women had lower proportions of women meeting the diagnostic standard of 60 days in each time period compared with White women. The proportions meeting program standards improved for women in the second time period by residence at screening, among symptomatic women and those with unknown symptoms, and for those who were eventually diagnosed with cancer (Table 3).
TABLE 3.
Adjusted Proportions (Predicted Marginals) Meeting Program Standards for Diagnostic and Treatment Initiation Intervals: National Breast and Cervical Cancer Early Detection Program, 1996–2005
| Diagnostic Interval |
Treatment Interval |
Total Interval |
||||||||||
| 1996–2000 |
2001–2005 |
1996–2000 |
2001–2005 |
1996–2000 |
2001–2005 |
|||||||
| Characteristic | Predicted Marginala (95% CI) | P | Predicted Marginala (95% CI) | P | Predicted Marginala (95% CI) | P | Predicted Marginala (95% CI) | P | Predicted Marginalb (95% CI) | P | Predicted Marginalb (95% CI) | P |
| Mammogram resultc/CBE resultd | <.001 | <.001 | <.001 | .027 | <.001 | <.001 | ||||||
| Abnormal/normal | 82 (81, 82) | 83 (82, 83) | 93 (92, 94) | 93 (93, 94) | 89 (87, 90) | 90 (89, 91) | ||||||
| Abnormal/abnormal | 82 (82, 83) | 84 (83, 84) | 95 (94, 96) | 94 (94, 95) | 94 (94, 95) | 96 (95, 96) | ||||||
| Abnormal/not done | 81 (80, 81) | 83 (82, 83) | 91 (89, 93) | 92 (91, 94) | 87 (85, 90) | 91 (89, 92) | ||||||
| Normal/abnormal | 77 (76, 78) | 82 (82, 82) | 95 (92, 97) | 92 (89, 95) | 84 (80, 88) | 86 (83, 89) | ||||||
| Not done/abnormal | 81 (80, 82) | 80 (79, 81) | 90 (87, 92) | 94 (92, 95) | 91 (89, 94) | 94 (93, 95) | ||||||
| Age, years | .126 | .629 | .118 | .113 | .368 | .340 | ||||||
| 40–49 | 81 (81, 81) | 83 (82, 83) | 94 (93, 95) | 93 (92, 94) | 91 (90, 92) | 92 (92, 93) | ||||||
| 50–64 | 81 (80, 81) | 83 (82, 83) | 93 (92, 94) | 94 (94, 94) | 91 (90, 92) | 93 (93, 94) | ||||||
| ≥ 65 | 80 (79, 81) | 82 (81, 83) | 95 (93, 96) | 93 (90, 95) | 92 (90, 94) | 92 (90, 95) | ||||||
| Race/ethnicity | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | ||||||
| White | 84 (83, 84) | 88 (88, 88) | 95 (94, 95) | 94 (94, 95) | 93 (92, 94) | 95 (94, 95) | ||||||
| Black | 76 (76, 77) | 80 (79, 80) | 91 (90, 93) | 91 (90, 92) | 87 (86, 89) | 89 (88, 90) | ||||||
| Asian | 79 (78, 80) | 76 (75, 77) | 95 (93, 98) | 95 (93, 97) | 88 (84, 92) | 92 (90, 94) | ||||||
| AIAN | 76 (75, 77) | 80 (79, 81) | 90 (86, 94) | 93 (91, 96) | 87 (83, 91) | 90 (87, 93) | ||||||
| Hispanic | 78 (77, 78) | 77 (77, 78) | 91 (89, 93) | 94 (93, 95) | 87 (85, 89) | 92 (91, 93) | ||||||
| Unknown | 79 (78, 81) | 79 (78, 80) | 96 (94, 99) | 96 (94, 98) | 89 (84, 93) | 92 (90, 95) | ||||||
| Residence | <.001 | <.001 | .003 | .016 | <.001 | <.001 | ||||||
| Urban | 83 (82, 83) | 85 (85, 86) | 95 (94, 96) | 95 (94, 95) | 93 (91, 94) | 94 (93, 95) | ||||||
| Metropolitan | 80 (80, 80) | 82 (82, 82) | 93 (92, 94) | 93 (93, 94) | 90 (89, 91) | 92 (92, 93) | ||||||
| Rural | 82 (81, 83) | 85 (85, 86) | 95 (93, 98) | 95 (94, 97) | 95 (92, 97) | 95 (93, 96) | ||||||
| Breast symptoms | <.001 | <.001 | <.359 | .051 | .235 | <.001 | ||||||
| Yes | 81 (81, 82) | 85 (84, 85) | 93 (92, 94) | 94 (94, 95) | 92 (91, 93) | 94 (94, 95) | ||||||
| No | 80 (80, 81) | 82 (82, 82) | 94 (93, 95) | 93 (92, 94) | 90 (89, 91) | 92 (91, 92) | ||||||
| Unknown | 81 (81, 82) | 84 (84, 85) | 93 (92, 95) | 94 (92, 95) | 91 (88, 93) | 94 (93, 96) | ||||||
| Cancer Diagnosed | 0.22 | <.001 | ||||||||||
| Yes | 82 (81, 83) | 85 (84, 85) | NA | NA | NA | NA | ||||||
| No | 81 (80, 81) | 82 (82, 83) | NA | NA | NA | NA | ||||||
Notes. CI = confidence interval; CBE = clinical breast examination; AIAN = American Indian/Alaska Native.
Percentage ≤ 60 days).
Percentage ≤120 days).
Abnormal mammography includes suspicious abnormality, highly suggestive for malignancy, or assessment incomplete.
Abnormal CBE is suspicious for cancer.
Regarding treatment initiation intervals (Table 3), more than 90% of women met the 60-day standard in both time periods, Of note, women with abnormal CBE but no mammogram had a 4% improvement in meeting the program standard of 60 days in time 2 compared with time 1 (94%; 95% CI = 92.0%, 95.0% vs 90%; 95% CI = 87.0%, 92.0%, respectively). The opposite pattern was noted for women with a normal mammogram and an abnormal CBE, of whom 3% fewer met the program standard. In contrast to the slightly longer median treatment initiation intervals for racial/ethnic minority women in time 2 (Table 2), minority women experienced improvements in the proportions of women meeting the standard with American Indian/Alaska Native and Hispanic women experiencing a 3% improvement. The relationships of more women with cancer initiating treatment within 120 days were similar to women initiating within 60 days, with most subgroups of women experiencing improvements (Table 3). Hispanic women had a 5% increase of women initiating treatment within 120 days (87% compared with 92%, time 1 to time 2).
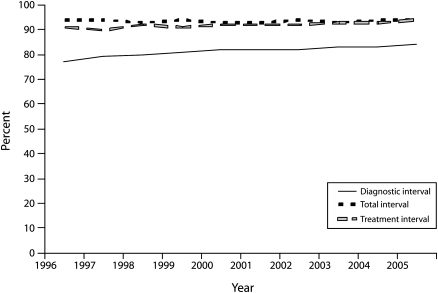
Figure 1 represents changes in diagnostic, treatment, and total intervals from 1996–2005. Women receiving a diagnosis within 60 days of an abnormal screening test result improved from 78% in 1996 to 84% in 2005 (P < .001). The treatment interval was stable at 94% (P = .782). The total interval from abnormal screening test result to treatment initiation also improved over time from 91% to 94% (P < .001). Most of this improvement occurred from 2000 to 2005 (P < .032; data not shown).
FIGURE 1.
Adjusted proportions (predicted marginals) for meeting diagnostic, treatment, and total intervals within program standards: National Breast and Cervical Cancer Early Detection Program, 1996–2005.
Note. Percentages (predicted marginals) are controlled for mammogram and CBE result, age, race/ethnicity, and residence. P values are for change in proportions meeting the program benchmarks during the study period. For the diagnostic interval, P < .001; for the treatment interval, P = .078; for the total interval, P < .001.
DISCUSSION
The NBCCEDP is the largest organized screening program in the United States.28 Our study showed that the state, tribal, and territorial programs funded by the NBCCEDP are meeting established program standards of timeliness and completeness, with 80% of women diagnosed within 60 days of abnormal screening test results and almost 94% of women initiating treatment within 60 days after diagnosis. The median diagnostic interval decreased from 25 days to 23 days in time 2, with a 2-day increase in treatment initiation interval. Using diagnostic intervals based on NBCCEDP program standards, researchers in New Jersey reported median diagnostic intervals of 36 days, which was almost 2 weeks longer than those we report, among a group of women of similar socioeconomic status.29 The NBCCEDP specifies that follow-up systems with appropriate diagnostic and treatment providers be in place to track and monitor care before implementing screening so as to ensure that programs meet standards for completeness and timeliness and are able to provide feedback to providers.20 We found that the proportion of women meeting program standards increased for diagnosis and remained stable for treatment initiation. Most of the improvements occurred in the second time period, when case management and states' Medicaid waivers were fully implemented.19,22
Symptomatic women with diagnostic delays longer than 3 months have shorter survival compared with women who are diagnosed more quickly.4 Until recently, the consequences of long diagnostic intervals in asymptomatic women screened for breast cancer were unknown. Researchers in Canada2 and France3 have reported that women with diagnostic intervals longer than 6 months were more likely to have larger tumors and more lymph nodes positive for cancer. Therefore, longer intervals may result in poorer long-term survival. In the NBCCEDP, only 3% of women diagnosed with cancer had diagnostic intervals longer than 6 months. Follow up of suspicious findings are based on the screening test results. Timely follow-up for those with abnormal mammography or CBE results did not differ by whether women eventually had noninvasive or invasive breast cancer as well (data not shown).
Caplan et al. were the first to report that screening test results affected the timeliness of follow-up.18 Subsequently, other researchers have shown similar results.2,3,30,31 We confirmed that timeliness and completeness of follow-up were dependent on mammogram or CBE results. Women with both an abnormal mammogram and CBE had the shortest median diagnostic intervals. The NBCCEDP has noted remarkable improvement in diagnostic follow-up of women with the combined screening test results of a normal mammogram and an abnormal CBE. The program designed quality improvement programs specifically targeted to this finding. Providers and women may experience false reassurance from the normal mammogram result, leading to less urgency to complete the workup of the abnormal CBE.32 Tracking and monitoring within the NBCCEDP has allowed funded programs to provide feedback to providers to improve follow-up after an abnormal CBE with or without an abnormal mammogram, improving program benchmarks.28 It is also possible that follow-up improved as Breast Imaging–Reporting and Data System reporting became more standardized with reports indicating the next step in the work-up of an abnormal mammogram.33
Although program standards for timeliness and completeness have been met for the majority of women since 1996 (Tables 2 and 3), differences in diagnostic intervals are noted for all subpopulations studied. The greatest variations have been observed among racial and ethnic minorities. Median diagnostic intervals for minority women are longer in both time periods. There may be multiple explanations for this finding, including individual characteristics,10,34 miscommunication of results between women and providers regarding the need for follow-up,35 provider characteristics,29,36,37 or the health care systems where minorities receive care.29,37–39 More research is needed within the program to ascertain what part of the diagnostic and treatment trajectory can be improved to ensure that racial and ethnic gaps in meeting program benchmarks are ameliorated.
Though all women enter the program underinsured or without health insurance, socioeconomic status variation by education and occupation might affect a woman's understanding of the need for follow-up of abnormal screening test results.35 We were unable to explore the impact of these factors because the program currently does not collect education or occupation information. Regarding communication of results, Jones et al. found that African American women were less likely to understand that additional testing was needed after an abnormal mammogram, although they were more likely to receive their results in person.35
New information is becoming available that indicates physician specialty may affect timeliness of follow-up. In an analysis by Ferrante et al., women cared for by family physicians were more likely to complete follow-up of abnormal breast cancer screening results compared with women who were cared for by internal medicine and obstetrics/gynecology specialists, regardless of race/ethnicity.29 Finally, reports are becoming more numerous indicating that neighborhood factors such as the number of persons living in poverty40,41 and resources available to referring physicians affect the quality of care received by patients.37,42,43 A study of NBCCEDP cervical cancer screening providers reported that providers were more likely to see clients in hospital-based outpatient clinics and community health centers, both of which have limited access to quality referral services.42–44 Bach et al. also reported that physicians who care for Black and Hispanic patients were more likely to feel that they did not have access to quality referral services and providers.37 Referral source, type of referral physician, and clinical tracking systems have also been reported to affect the length of time it takes to complete follow-up of abnormal breast cancer screening results.45
Treatment intervals increased slightly from 2001 through 2005 for women enrolled in the NBCCEDP. Though these increases may not be clinically significant, they may be signaling health system capacity challenges in the future. Researchers from Canada and the United Kingdom have reported similar findings.46,47 There are several possible explanations for this finding, including an aging population requiring more services, as more types of cancer are being treated than ever before (e.g., adjuvant therapy, operable lung cancer, and newer therapies for colon cancer), as well as multiple new agents, which might be overwhelming the oncology care system.48,49 The United Kingdom instituted a new “2-week wait” rule, which requires all women to be seen within 2 weeks of an abnormal finding on mammogram or CBE. Researchers feel that these requirements have led to longer waiting times to treatment because the system is now required to see women more quickly for diagnostic work-up, causing a backlog.47 In the United States, similar challenges may be on the horizon. The American Society of Clinical Oncology recently reported the demand for medical oncologists will far outstrip supply by 2020.48 Program data do not allow us to examine intervals with this level of detail, but given future workforce shortages in medical oncology, treatment intervals are not expected to improve.
Limitations and Strengths
Our study had several limitations. First, the program collects minimal data elements such as the demographics of patients, which does not allow us to determine the etiology of some of our findings. We currently do not collect information on location of care or the type of providers providing the care. This will require in-depth studies to probe reasons why differences in follow-up are occurring among women of similar insurance status. Secondly, we are not able to assess the women's preference for receiving services. We are not able to track the receipt of services outside the program. According to a report by Bobo et al. examining rescreening in the NBCCEDP, women were receiving mammograms outside the program, although we had no record of the additional screening.50 Therefore, we would anticipate that our results may underestimate patient follow-up. Given the specific nature of the eligible population (low-income and uninsured), our results may not be applicable to the general population. To our knowledge, no other screening programs have published service delivery benchmarks for mammography that are available to the public. The Cancer Collaborative sponsored by Health Resources and Services Administration has adopted some of the NBCCEDP follow-up standards for Federally Qualified Health Centers participating in that study.51 It is also possible that secular improvements in the timeliness and completeness of abnormal screening tests may explain our results as well.
The major strength of this analysis is that it provides data on follow-up of large numbers of screening examinations and breast cancers among low income, minority, and uninsured women. The systematic collection of program data allows for monitoring and quality improvement. Local programs are able to monitor the successes and challenges of their program and compare them with other programs because of this. Others have generated outcomes by using our program standards29 and have shown that even insured women with abnormalities on screening tests are not able to obtain timely follow-up.
Conclusion
The NBCCEDP has been delivering services to women who might not otherwise receive care since 1991. Results from our study indicate that a group of women who are potentially at risk for not receiving diagnostic and treatment services are receiving them in a timely fashion. Though our results still show disparities across racial and ethnic groups, our program has continued to improve within each racial and ethnic subpopulation.18 Diverse programs and providers have been able to deliver high-quality services to women in need.36 In addition, NBCCEDP policies are being adopted by other health care systems that provide care to at-risk populations.51
Our results suggest that the NBCCEDP is effective in delivering screening and diagnostic services to low-income, uninsured women. In addition, national standards for all breast cancer screening programs could be modeled on the program standards to ensure that all women receive adequate follow-up care. The NBCCEDP currently only reaches 13.2% of the eligible population.52 Effective screening and follow-up will allow breast cancer incidence rates and mortality to continue to decline in the long term for low-income women as well.1 Many NBCCEDP programs currently link with population-based registries to supplement their Minimal Data Elements data. Starting in 2009, the NBCCEDP will require that all programs perform linkages with their state-based registries. These new data will enhance our ability to monitor the impact of the program by tracking stage at diagnosis from standardized reporting systems.
Acknowledgments
The information presented in this article reflects the views of the authors and does not necessarily reflect the views of Centers for Disease Control and Prevention.
Human Participants Protection
The research protocol was approved by the Centers for Disease Control and Prevention Human Subjects Committee.
References
- 1.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792 [DOI] [PubMed] [Google Scholar]
- 2.Olivotto IA, Gomi A, Bancej C, et al. Influence of delay to diagnosis on prognostic indicators of screen-detected breast carcinoma. Cancer. 2002;94(8):2143–2150 [DOI] [PubMed] [Google Scholar]
- 3.Ganry O, Peng J, Dubreuil A. Influence of abnormal screens on delays and prognostic indicators of screen-detected breast carcinoma. J Med Screen. 2004;11(1):28–31 [DOI] [PubMed] [Google Scholar]
- 4.Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126 [DOI] [PubMed] [Google Scholar]
- 5.Yabroff KR, Washington KS, Leader A, et al. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60(3):294–331 [DOI] [PubMed] [Google Scholar]
- 6.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56(3):168–183 [DOI] [PubMed] [Google Scholar]
- 7.Li CI. Racial and ethnic disparities in breast cancer stage, treatment, and survival in the United States. Ethn Dis. 2005;15(Suppl 2):S5–S9 [PubMed] [Google Scholar]
- 8.Breen N, Cronin KA, Meissner HI, et al. Reported drop in mammography. Cancer. 2007;109(12):2405–2409 [DOI] [PubMed] [Google Scholar]
- 9.Chang SW, Kerlikowske K, Nápoles-Springer A, Posner SF, Sickles EA, Pérez-Stable EJ. Racial differences in timeliness of follow-up after abnormal screening mammography. Cancer. 1996;78(7):1395–1402 [DOI] [PubMed] [Google Scholar]
- 10.Yabroff KR, Breen N, Vernon SW, Meissner HI, Freedman AN, Ballard-Barbash R. What factors are associated with diagnostic follow-up after abnormal mammograms? Findings from a U.S. national survey. Cancer Epidemiol Biomarkers Prev. 2004;13(5):723–732 [PubMed] [Google Scholar]
- 11.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96(12):2173–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griggs JJ, Culakova E, Sorbero MES, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25(18):2522–2527 [DOI] [PubMed] [Google Scholar]
- 13.Griggs JJ, Sorbero ME, Stark AT, et al. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81(1):21–31 [DOI] [PubMed] [Google Scholar]
- 14.Richardson LC, Tian L, Voti L, et al. The roles of teaching hospitals, insurance status, and race/ethnicity in receipt of adjuvant therapy for regional-stage breast cancer in Florida. Am J Public Health. 2006;96(1):160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voti L, Richardson LC, Reis IM, et al. Treatment of local breast carcinoma in Florida. Cancer. 2006;106(1):201–207 [DOI] [PubMed] [Google Scholar]
- 16.Henson RM, Wyatt SW, Lee NC. The National Breast and Cervical Cancer Early Detection Program: a comprehensive public health response to two major health issues for women. J Public Health Manag Pract. 1996;2(2):36–47 [PubMed] [Google Scholar]
- 17.Ryerson AB, Benard VB, Major AC. The National Breast and Cervical Cancer Early Detection Report: 1991–2002 National Report. 2005. Available at: http://www.cdc.gov/cancer/nbccedp/pdf/national_report.pdf. Accessed November 8, 2009
- 18.Caplan LS, May DS, Richardson LC. Time to diagnosis and treatment of breast cancer: results from the National Breast and Cervical Cancer Early Detection Program, 1991–1995. Am J Public Health. 2000;90(1):130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French C, True S, McIntyre R, Sciulli M, Maloy KA. State implementation of the Breast and Cervical Cancer Prevention and Treatment Act of 2000: a collaborative effort among government agencies. Public Health Rep. 2004;119(3):279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lantz PM, Richardson LC, Sever LE, et al. Mass screening in low-income populations: the challenges of securing diagnostic and treatment services in a national cancer screening program. J Health Polit Policy Law. 2000;25(3):451–471 [DOI] [PubMed] [Google Scholar]
- 21.Lantz PM, Richardson LC, Macklem DJ, et al. Strategies for follow-up and treatment services in state breast and cervical cancer screening programs. Womens Health Issues. 1999;9(1):42–49 [DOI] [PubMed] [Google Scholar]
- 22.Lantz PM, Keeton K, Romano L, et al. Case management in public health screening programs: the experience of the national breast and cervical cancer early detection program. J Public Health Manag Pract. 2004;10(6):545–555 [DOI] [PubMed] [Google Scholar]
- 23.Eheman CR, Benard VB, Blackman D, et al. Breast cancer screening among low-income or uninsured women: results from the National Breast and Cervical Cancer Early Detection Program, July 1995 to March 2002 (United States). Cancer Causes Control. 2006;17(1):29–38 [DOI] [PubMed] [Google Scholar]
- 24.United States Department of Agriculture, Economic Research Service Measuring Rurality: Rural-Urban Continuum Codes [Web site]. Available at: http://www.ers.usda.gov/Briefing/Rurality/RuralUrbCon. Accessed December 22, 2007
- 25.Liberman L, Menell JH. Breast imaging reporting and data system (BI-RADS). Radiol Clin North Am. 2002;40(3):409–430 [DOI] [PubMed] [Google Scholar]
- 26.Conover WJ. Practical Nonparametric Statistics. 2nd ed New York, NY: John Wiley & Sons; 1980 [Google Scholar]
- 27.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659 [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention National Breast and Cervical Cancer Early Detection Program (NBCCEDP). NBCCEDP Program Manual [Web site]. Available at: http://www.cdc.gov/cancer/nbccedp/bccpdfs/policies_procedures.pdf. Accessed March 1, 2008
- 29.Ferrante JM, Rovi S, Das K, et al. Family physicians expedite diagnosis of breast disease in urban minority women. J Am Board Fam Med. 2007;20(1):52–59 [DOI] [PubMed] [Google Scholar]
- 30.Chiarelli AM, Mai V, Halapy EE, et al. Effect of screening result on waiting times to assessment and breast cancer diagnosis: results from the Ontario Breast Screening Program. Can J Public Health. 2005;96(4):259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decker KM, Harrison M, Chateau D. Influence of direct referrals on time to diagnosis after an abnormal breast screening result. Cancer Detect Prev. 2004;28(5):361–367 [DOI] [PubMed] [Google Scholar]
- 32.Cady B, Steele GD, Morrow M, et al. Evaluation of common breast problems: guidance for primary care providers. CA Cancer J Clin. 1998;48(1):49–63 [DOI] [PubMed] [Google Scholar]
- 33.Geller BM, Ichikawa LE, Buist DSM, et al. Improving the concordance of mammography assessment and management recommendations. Radiology. 2006;241(1):67–75 [DOI] [PubMed] [Google Scholar]
- 34.Elmore JG, Nakano CY, Linden HM, et al. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care. 2005;43(2):141–148 [DOI] [PubMed] [Google Scholar]
- 35.Jones BA, Reams K, Calvocoressi L, et al. Adequacy of communicating results from screening mammograms to African American and White women. Am J Public Health. 2007;97(3):531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saraiya M, Irwin KL, Carlin L, et al. Cervical cancer screening and management practices among providers in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP). Cancer. 2007;110(5):1024–1032 [DOI] [PubMed] [Google Scholar]
- 37.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351(6):575–584 [DOI] [PubMed] [Google Scholar]
- 38.Richardson LC, Tangka FK. A comparison of cancer patients treated in physician's offices with those treated in hospital outpatient departments. J Natl Med Assoc. 2007;99(12):1350–1358 [PMC free article] [PubMed] [Google Scholar]
- 39.Zuckerman S, Haley J, Roubideaux Y, et al. Health service access, use, and insurance coverage among American Indians/Alaska Natives and whites: what role does the Indian Health Service play? Am J Public Health. 2004;94(1):53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutchinson JR, Chagpar AB, Scoggins CR, et al. Surgeon and community factors affecting breast cancer sentinel lymph node biopsy. Am J Surg. 2005;190(6):903–906 [DOI] [PubMed] [Google Scholar]
- 41.Davidson PL, Bastani R, Nakazono TT, et al. Role of community risk factors and resources on breast carcinoma stage at diagnosis. Cancer. 2005;103(5):922–930 [DOI] [PubMed] [Google Scholar]
- 42.Cook NL, Hicks LS, O'Malley AJ, et al. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26(5):1459–1468 [DOI] [PubMed] [Google Scholar]
- 43.Gusmano MK, Fairbrother G, Park H. Exploring the limits of the safety net: community health centers and care for the uninsured. Health Aff (Millwood). 2002;21(6):188–194 [DOI] [PubMed] [Google Scholar]
- 44.Weissman JS, Moy E, Campbell EG, et al. Limits to the safety net: teaching hospital faculty report on their patients' access to care. Health Aff (Millwood). 2003;22(6):156–166 [DOI] [PubMed] [Google Scholar]
- 45.Mojica CM, Bastani R, Boscardin WJ, et al. Low-income women with breast abnormalities: system predictors of timely diagnostic resolution. Cancer Control. 2007;14(2):176–182 [DOI] [PubMed] [Google Scholar]
- 46.Rayson D, Saint-Jacques N, Younis T, et al. Comparison of elapsed times from breast cancer detection to first adjuvant therapy in Nova Scotia in 1999/2000 and 2003/04. CMAJ. 2007;176(3):327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson D, Bell CM, Møller H, Basnett I. Effect of the UK government's 2-week target on waiting times in women with breast cancer in southeast England. Br J Cancer. 2003;89(3):492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saint-Jacques N, Younis T, Dewar R, et al. Wait times for breast cancer care. Br J Cancer. 2007;96(1):162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bobo JK, Shapiro JA, Schulman J, et al. On-schedule mammography rescreening in the National Breast and Cervical Cancer Early Detection Program. Cancer Epidemiol Biomarkers Prev. 2004;13(4):620–630 [PubMed] [Google Scholar]
- 51.Health Disparities Collaborative Cancer [Web page]. Available at: http://www.healthdisparities.net/hdc/html/collaboratives.topics.cancer.aspx. Accessed July 16, 2008
- 52.Tangka FK, Dalaker J, Chattopadhyay SK, et al. Meeting the mammography needs of underserved women: the performance of the National Breast and Cervical Cancer Early Detection Program in 2002-2003 (United States). Cancer Causes Control. 2006;17(9):1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]