Abstract
Objective
Although B cells are implicated in the pathogenesis of systemic lupus erythematosus (SLE), the role of B cell depletion (BCD) as a treatment is controversial given the variable benefit in human disease. The development of a murine lupus model of BCD would be helpful to better understand mechanisms, heterogeneity, and effects on disease outcomes.
Methods
NZB/NZWF1 female mice of varying disease severity received anti-CD20 antibody (IgG2a), BR3-Fc, or control antibody (10 mg/kg). Tissues were harvested and analyzed by flow cytometry. Nephritis was monitored by proteinuria (Uristix) and kidney IHC. Serum immunoglobulin levels were measured by ELISA.
Results
After a single injection of anti-mCD20 B cell depletion was more efficient in peripheral blood, lymph node, and spleen compared to the bone marrow and peritoneum in normal mice as well as in young and diseased lupus mice. Since depletion of the marginal zone and peritoneal B cells was incomplete and variable particularly in older mice with established nephritis, a sequential weekly dosing strategy was subsequently used with improved depletion. BAFF blockade further enhanced depletion in spleen and lymph node. Early BCDT delayed disease onset, whereas BCDT in mice with advanced disease reduced the progression of nephritis. These effects were long-lasting even after B cell reconstitution occurred and associated with a reduction in T cell activation but no significant change in autoantibody production.
Conclusion
The lasting benefit of a short course of BCD in lupus prone mice with an intact immune system and established disease highlights the validity of this treatment approach.
Keywords: B cells, anti-CD20, autoimmunity, Systemic Lupus Erythematosus
Systemic lupus erythematosis (SLE) is a complex autoimmune disease involving multiple organ systems. The immunological events triggering the onset of clinical manifestations are not been fully defined, but a central role for B cells in the pathogenesis of this disease has been established by work performed in multiple laboratories using both mice and humans (1–3). Given the strong evidence supporting an abnormal B cell compartment in SLE, B cell depletion therapy (BCDT) is being investigated as a potential treatment strategy. Several open-label studies, including our own, demonstrate that B cell depletion provides significant clinical benefit in SLE (4, 5). Our studies show that clinical improvement precedes the decline of conventional serum autoantibody, strongly supporting the notion of antibody-independent pathogenic roles of B cells. However, there is heterogeneity in response to BCDT, as also highlighted by the recent failure of two large multi-center trials of rituximab in general lupus and lupus nephritis (6). The mechanism underlying the clinical benefit and the variation in response to BCDT are not clearly defined. Developing a murine lupus model to explore the effect of B cell depletion on the disease, variability in effects, mechanisms of benefit, and potential synergy between therapies is thus of interest.
In addition to antibody production, both protective and pathogenic roles of B cells are mediated by poorly understood antibody-independent mechanisms (7). The latter are highlighted by the abrogation of disease and reduction in activated T cells in B cell deficient MRL/lpr mice (2), yet the maintenance of T cell abnormalities in mice with B cells incapable of secreting antibody (8). However, these studies do not establish the benefit of B cell depletion as a treatment in mice with an intact immune system or established disease. Autoantibody-independent B cell functions include antigen-presentation, T cell activation and polarization, and dendritic cell (DC) modulation, that may be mediated at least in part by the ability of B cells to produce cytokines (9, 10). However, the relative importance of these distinct B cell functions to the autoimmune process in SLE and the impact of B cell depletion therapy are not yet defined.
Moreover, given the recent demonstration of regulatory B cell subsets that dampen harmful immune responses it is possible that B cell depletion may worsen certain autoimmune diseases depending on the relative balance of protective and pathogenic B cells (11, 12). Indeed, it has been suggested that the loss of negative regulatory B cells may contribute to exacerbation of disease in a number of murine models including collagen induced arthritis, inflammatory bowel disease, and experimental autoimmune encephalomyelitis (EAE) (13). Thus, in EAE B cell depletion therapy either exacerbated or suppressed disease depending on the timing (14).
Effective reagents for depleting B cells in mice have not been available until recently, necessitating the use of human CD20 transgenic mice and anti-human CD20 for mechanistic studies in the mouse (15, 16). However, antibodies against mouse CD20 have recently become available and cause significant depletion of B cells in normal mice (17) as well as a variety of autoimmune mouse models (18–21). In this study, anti-mouse CD20 was used to determine the role of B cells in the development and maintenance of murine lupus. We find that B cell depletion therapy in NZB/NZWF1 lupus prone mice mirrors many of the clinical and immunological effects of BCD in human SLE in a number of critical aspects including B cell depletion variability, a disconnect between clinical improvement and autoantibody titers, and B cell reconstitution with an immature transitional B cell pool. This study also provides unique mechanistic insights including the significant impact of B cell depletion on the T cell compartment with decreases in T cell activation and memory T cells and the profound delay in lupus disease onset and progression even after short-term depletion. These results suggest that B cells are critical for both the initiation and maintenance of autoimmunity in SLE, and B cell depletion may favorably shift the balance of protective versus pathogenic B cell functions.
MATERIALS AND METHODS
Mice and experimental design
NZB/NZWF1 mice, C57BL/6 mice and BALB/c mice were supplied by Jackson Laboratories. All mice were housed in the animal facility in the University of Rochester Medical center. All experiment protocols were reviewed and approved by the University of Rochester Committee on Animal Resources. Young NZB/NZWF1 mice (8–12 weeks), mice with early disease (18 weeks), and mice with established nephritis (24–30 weeks with durable proteinuria ≥2+ proteinuria or 100 mg/dl) (female) were treated with anti-mouse CD20 antibody IgG2a (18B12) or control anti-human CD20 mAb (an engineered IgG2a derivative of the 2B8 IgG1 mouse parent of rituximab that has no cross-reactivity with mouse CD20) at ~10 mg/kg (300 μg) intravenously weekly via a retro-orbital route. C57BL6 and BALB/c mice were utilized as controls. In a treatment group of anti-mouse CD20 combined with BR3-Fc, BR3-Fc (supplied by Genentech) was given at 300μg per week with retro-orbital i.v. injections for 4 weeks. During the treatment, mice were bled every 2 weeks and proteinuria was monitored with urine dipstick (Uristix by Bayer). Upon treatment completion, mice were sacrificed with CO2 euthanasia. Spleen, lymph node, peritoneum, peripheral blood and bone marrow lymphocytes were collected in 4° C FACS buffer (3% FBS in PBS) for flow cytometry analysis. Kidneys were collected and formalin fixed for histological analysis.
ELISA assay
Sera from anti-mCD20 treated mice blood samples were collected and frozen prior to quantifying anti-mCD20 serum levels. Quantification of circulating 18B12 was done by capture ELISA, using an anti-idiotype monoclonal (18C8) specific for 18B12. Detection of captured 18B12 was with anti-mIgG2a biotin (BD Biosciences 553502) followed by SA-HRP (BD Biosciences 554066). Absorbance (OD450-650) values were converted to μg using a standard curve of 18B12. Animal identification and treatment information were blinded for all ELISA samples. Serum was also used for total IgG and IgM measurements and anti-dsDNA analysis with mouse anti-dsDNA ELISA kit (alpha diagnostic international) by following the manufacturer’s instructions.
Detection of antibody-secreting cells by enzyme-linked immunosorbent spot assay
For quantification of antibodies to dsDNA–secreting cells, 96-well multiscreen plates (Millipore) were coated with poly–L-lysine (Sigma) and calf thymus DNA (Sigma) and then blocked with 2% fetal calf serum in PBS. Cell suspensions from spleens or bone marrows were incubated as serial dilutions starting with 5E5 cells/well overnight at 37 oC in a 5% CO2– containing incubator. After incubation, plates were washed and incubated with alkaline phosphatase–conjugated goat antibody to mouse IgG (Jackson) for 1 h at RT and detected with Vector Blue Alkaline Phosphatase Substrate Kit III (Burlingame, CA). For assessment of IgG-secreting cells, serial dilutions starting with 1E5 cells/well were incubated on goat antibody to mouse IgG–coated plates (Southern Biotech).
Flow cytometry analysis
FITC-CD21, biotin-CD23, PE-CD23, PE-CD1d, APC-B220, FITC-B220, APC-AA4.1, biotin-IgM, FITC-IgM, biotin-CD5 (BD Biosciences) and PE-CD11b (BD Biosciences) were used for B cell and B cell subset identification. APC-CD4 (BD Biosciences) and biotin-CD69 (BD Biosciences) were used for T cell analysis. PE-CD11b (BD Biosciences) and FITC-CD11c (BD Biosciences) were used as dendritic cell markers. Streptavidin-PerCP (BD Biosciences) was used as a secondary marker for biotinylated antibodies. Samples were run on the FACSCalibur. A multicolor flow panel to additionally characterize GC cells consisted of PE-CY7-23, PE-CY5.5-IgM, APC750-B220, APC-AA4.1, FITC-CD21 (BD Bioscience), PE-CD1d, Biotin-GL7 followed by SA-Pacblue (Invitrogen) and was run on a FACSCanto. All antibodies were purchased from eBioscience except where indicated. B cell subsets were defined as follows: T1 (B220+,CD23−,IgM+,AA4.1+), T2 (B220+,CD23+,IgM+,AA4.1+), T3 (B220+,CD23+,IgMlow,AA4.1+), FO (B220+,CD23+,IgMlow,AA4.1−), MZ (B220+,IgM+,CD1d+,CD21+), B1a (CD11b+,CD5+,IgM+,B220+/−), B1b (CD11b+,CD5−,IgM+,B220+/−), B2 (CD11b−,CD5−,IgM+,B220+), prepro (IgM−,B220low), immature (IgM+,B220low/med), and mature( IgM+,B220hi).
Histological analysis
Kidneys of NZB/NZWF1 mice from different treatment groups and C57BL/6 normal control mice were fixed in 10% formalin and paraffin embedded or frozen. Kidney sections (4 μm) were stained with hematoxylin and eosin. Pathology was analyzed and scored in a blinded fashion (B.I.G.) (1). Briefly, the severity of glomerular, interstitial, and vascular lesions was determined on a scale of 0 to 4+. Multiple sections at a minimum of two different levels were examined, with each section typically containing >50 glomeruli and >25 blood vessels as described. Spleen sections were frozen and cut into 4μm sections. Immunohistochemistry slides were stained for FDC-M1 (BD Bioscience), PE-B220, and Biotin-GL7 using a Dako LSAB2 kit. 3-color fluorescent slides were stained for FITC-MOMA (ABD serotec), AMCA-IgM (Vector labs), ALEXA647-IgD OR -FITC-B220, BIOTIN-GL7 followed by SA-PE, and APC-CD4 (BD Bioscience). Antibodies were purchased from eBioscience except where indicated. Sections were quantitated in blinded fashion using IPLab 4.0 software to discriminate and enumerate area occupied by positive staining after imaging. GC size was quantitated by morphometric analysis of GL7+ staining on IHC. GC size, expressed in an arbitrary unit for total area of positive pixels, was averaged for all GL7+ clusters in spleen sections from at least 4 anti-CD20 or control treated mice.
Statistical analysis
Student’s t-test was used for comparison between treatment groups. Chi-squared test was performed on protein survival data. Significance is based on a value of p<0.05.
RESULTS
B cells from NZB/NZWF1 mice are resistant to BCDT
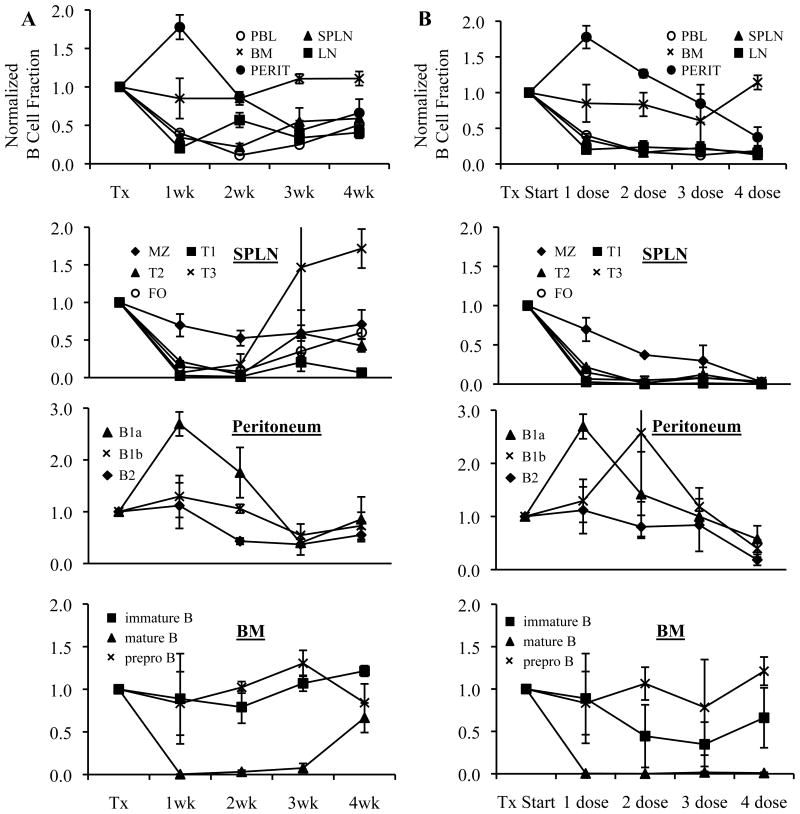
To evaluate the ability of anti-mCD20 antibody to deplete B cells in lupus prone mice, we treated 10 week old NZB/NZWF1 mice with one dose of anti-mCD20 or control antibody. Mice were sacrificed sequentially every week following the treatment to monitor the efficacy of anti-mCD20 mediated B cell depletion in peripheral blood, lymph node, bone marrow, spleen and peritoneal cavity. With one dose of anti-CD20, maximal depletion was observed 1 to 3 weeks after the injection depending on the B cell compartment (Fig. 1A). Enhanced depletion of the more resistant splenic marginal zone (MZ) and peritoneum was noted over time. Depletion was most efficient in the peripheral blood (PB) (89%), lymph node (LN) (80%), and spleen (78%) and least efficient in the peritoneum (36%) and bone marrow (BM). Of note, mature B cells in the BM were very efficiently depleted but immature B cells less so and precursor populations not at all (Fig. 1A), consistent with absent to low CD20 expression on the latter populations. B cells began to recover at 2 to 3 weeks following the one dose anti-mCD20 injection (Fig. 1A). By comparison, normal C57BL6 mice had more efficient depletion as follows: PB 96% >spleen 91% >LN 90% >peritoneum 69% >BM 56% (data not shown) and remained depleted for greater than 4–6 weeks.
Figure 1. B cell depletion in lupus prone mice improves with prolonged treatment.
10 wk old NZB/NZWF1 mice were treated with (A) a single dose of anti-mCD20 (300 μg) or control anti-human CD20 mAb intravenously and tissues were harvested at 1,2,3,4 weeks following treatment or (B) 1,2,3 or 4 weekly doses of anti-mCD20 and tissues were harvested one week after the last antibody injection. B cells were enumerated by flow cytometry, with subset definitions as defined in the Materials and Methods. B cell subsets are depicted as a normalized ratio (mean +/− SE) compared to control treated group with n=4 animals per group and time point. PBL, peripheral blood. SPLN, spleen. LN, lymph node. BM, bone marrow. PERIT, peritoneum. MZ, marginal zone. FO, follicular.
Given the incomplete depletion of some subsets (MZ) and in certain tissues (peritoneum), as well as the early reconstitution, we next administered anti-mCD20 at 10 mg/kg weekly for one, two, three or four weeks. Mice were sacrificed one week after the last injection of anti-mCD20. We found that B cells were progressively depleted with increased frequency of dosing (Fig. 1B), an effect particularly striking in the splenic MZ as reflected in the improved overall splenic depletion (from 78% after 1 dose to 87% after 4 doses) and peritoneum (from 36% after 1 dose to 62% after 4 doses) (Fig. 1B).
B cell depletion is variable in NZB/NZWF1 mice with more advanced disease
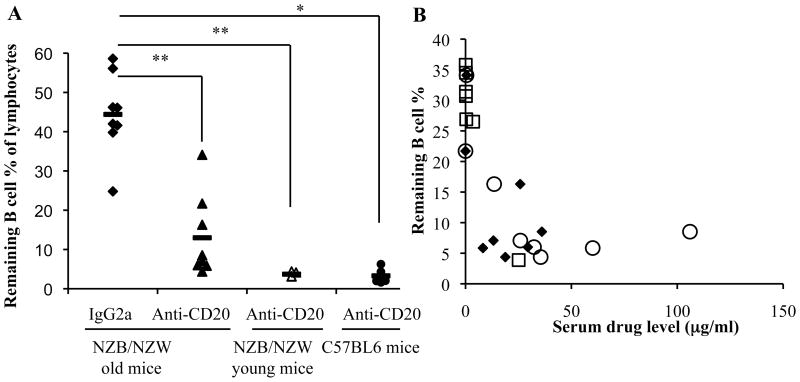
To determine whether older NZB/NZWF1 mice with more advanced disease display further resistance to B cell depletion, we compared the efficacy of depletion in young (pre-disease, 10 week) and old (post-nephritis onset with proteinuria ≥ 100 mg/dl and < 300 mg/dl, ~6 month) NZB/NZWF1 mice treated with four consecutive weekly injections of anti-CD20. Although significant depletion was achieved in older mice compared to disease and age matched mice treated with control antibody (Fig. 2A), depletion was not as complete as in young NZB/NZWF1 mice or in C57BL/6 mice. Moreover, there was wide variability in depletion ranging from 7% to 91% in the spleen. Similar variability in depletion was also seen in other tissues.
Figure 2. B cell depletion with anti-CD20 in older lupus mice is more variable.
Six month old NZB/NZWF1 mice were treated with anti-mCD20 (n=8) or control antibody (IgG2a) (n=8) (300 μg) once per week for 4 weeks. Mice were selected based on two consecutive measures of proteinuria ≥ 100 mg/dl (2+) and < 300 mg/dl (3+). Younger NZB/NZWF1 mice (10 wk) (n=4) and C57BL6 mice (n=6) were treated with anti-mCD20 for comparison. (A) Splenic B cells (B220+) were quantitated by flow cytometry 1 week after the fourth and final antibody injection and displayed as a % of the total lymphocyte gate. Astericks indicate significant differences: **, p<0.001 by student’s t-test. *, p<0.05 by student’s t-test. (B) Serum anti-mCD20 concentration was measured at day 14 (closed diamonds) and day 28 (open circles) during the course of weekly injections (x 4) of anti-mCD20 (300 μg). Mice that were able to maintain good serum drug levels had more effective B cell depletion (splenic depletion at day 28 shown). Mice with 3+ or greater proteinuria at the time of treatment are depicted with squares; the remaining mice began treatment at 2+ proteinuria.
We also found that resistance to depletion correlated with rapid clearance of anti-mCD20 from the serum (Fig 2B) and, conversely, that those mice achieving effective B cell depletion maintained serum levels of anti-mCD20 of at least 10 μg/ml. Importantly, mice with ≥ 3+ proteinuria were highly resistant to depletion (Fig 2B, squares) and had very low levels of circulating anti-CD20. Overall, these results indicate a close relationship between disease activity, serum drug levels, and depletion efficiency. To exclude clearance of antibody through the kidney, drug levels were measured in the urine but were undetectable. ,Thus, the mechanism of enhanced anti-CD20 antibody clearance in lupus prone mice is still unclear.
Residual B cells after B cell depletion
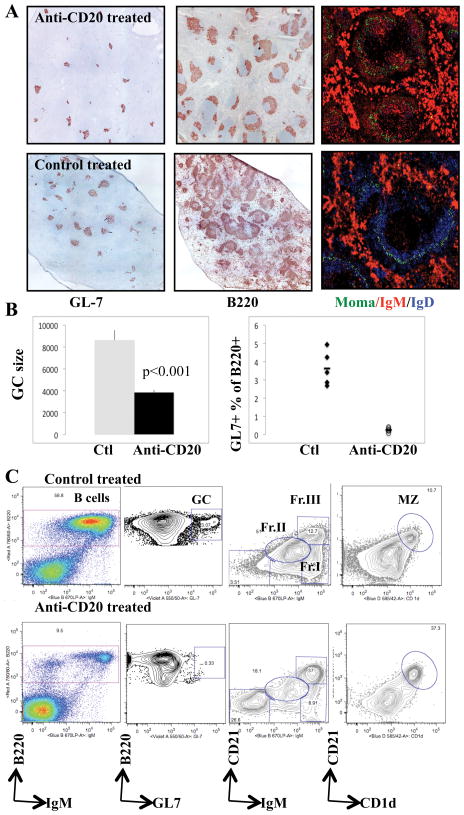
To determine where residual B cells after anti-mCD20 were localized in the spleen, we analyzed various B cell populations by histology. We found that residual B220+ B cells in the spleen largely consisted of the IgM+ MZ (around the MOMA staining marginal sinus) (Fig. 3A). This analysis also highlighted the sensitivity of mature IgD+ B cells to depletion and the lack of effect of short-term B cell depletion on the large numbers of IgM bright plasma cells (Fig. 3A). Given that lupus prone mice spontaneously form germinal centers (GC) with age, we also examined germinal center B cells in the spleen. Although the numbers of GCs was unchanged after BCD (Fig. 3A), in contrast to prior studies (15, 16) we found that GC B cells were sensitive to BCDT with a decrease in both the size of GCs and the number of GL7+ cells (Fig. 3B).
Figure 3. Residual B cells after anti-CD20 mediated BCD.
Six month old NZB/NZW F1 mice (≥ 100 mg/dl proteinuria) were treated as in Figure 2 with anti-mCD20 or control antibody (IgG2a) (300 μg) once per week for 4 weeks. (A) Immunohistochemistry of spleens stained with GL-7 (GC stain) and B220 antibody (B cell marker) or antibodies against MOMA (green), IgM (red), and IgD (blue) from NZB/NZWF1 mice treated with anti-CD20 (top panels) or control antibody (middle panels). Magnification, x10. (B) Enumeration of GCs, based on size as calculated from IHC staining for GL7+, and flow cytometry detection. (C) Depletion of B cells is depicted in spleen, with additional characterization of residual B cells via flow cytometry expression of the indicated markers. GC B cells are defined as GL7+ cells in the B220+ gate. Fractions I, II, and III are defined based on CD21 and IgM expression, gated through B220+. MZ B cells are defined here as CD21+CD1d+, gated through B220+. Representative dot plots are shown.
To more quantitatively assess the effects of BCDT on the frequencies of GC, MZ, and other B cell subsets in the spleen, we performed seven-color flow cytometry to analyze simultaneously multiple surface markers on residual splenic B cells (22). Consistent with the histologic analysis, we found that GL7+ GC B cells in the spleen were efficiently depleted (93% depletion) (Fig. 3C). In contrast, the MZ subset was relatively resistant to BCDT (Fig. 3C, within Fraction III or alternatively defined as CD21+CD1d+) and formed the largest fraction of the residual B220+ population after B cell depletion (22). Fractions I and II (containing follicular/T3 and T1/T2 subsets respectively) were relatively sensitive to depletion. Additional residual B cells included a CD21lowIgMlow population that likely represents a class-switched memory B cell population (Fig. 3C).
B cell survival factors contribute to B cell depletion resistance in lupus prone mice
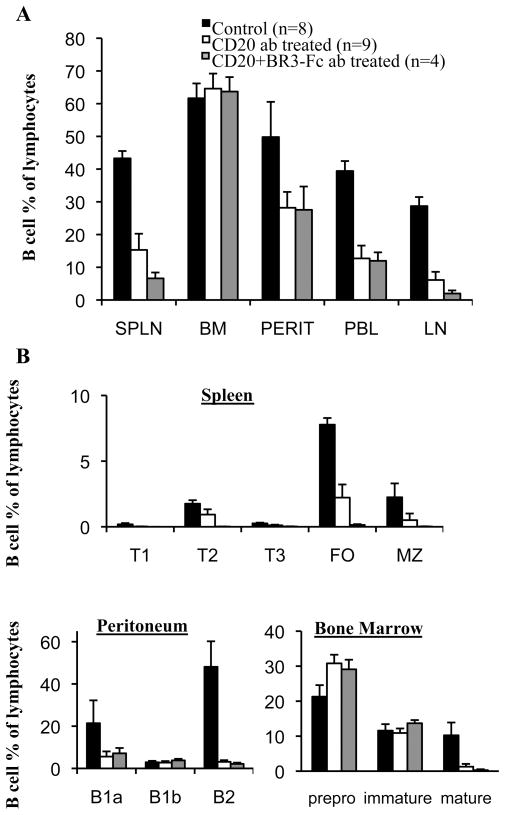
B cell activation factor belonging to the TNF family (BAFF) is a key cytokine for the survival and maturation of transitional, follicular, and MZ B cells. Mice over-expressing BAFF develop an SLE-like phenotype (23). Moreover, lupus-prone mice have elevated levels of circulating BAFF, and administration of soluble BAFF receptor (BAFFR/BR3-Fc fusion protein) ameliorates disease progression and improves survival (24). To explore the role of BAFF in mediating resistance of B cells to depletion in lupus mice, we quantified the extent of B cell depletion after combination anti-mCD20 and BAFF blockade with BR3-Fc. We found that the combination therapy enhanced B cell depletion in the lymph nodes and spleen, particularly in the relatively depletion-resistant MZ, but had no effect on depletion in the BM, peritoneum, or peripheral blood (Fig. 4). Thus, survival factors clearly play a role in susceptibility to anti-mCD20 mediated B cell depletion at least in some tissues.
Figure 4. B cell depletion is improved by combination BAFF blockade.
Six month old NZB/NZWF1 mice (≥ 100 mg/dl proteinuria) were treated with anti-mCD20 (n=9), anti-mCD20 with BR3-Fc (n=4) or control antibody (IgG2a) (n=8) (300 μg) once per week for 4 weeks. B220+ B cells were enumerated 1 week after the final antibody injection in multiple tissues by flow cytometry. (A) Depletion of B cells (B220+) in spleen (SPLN), bone marrow (BM), peritoneum (PERIT), peripheral blood (PBL), and lymph node (LN), reported as the B220+ B cell % of the lymphocyte gate (mean +/− SE). (B) Depletion in B cell subsets in spleen, peritoneum and bone marrow. B cell subsets were defined as in the Methods.
Effects of BCDT on progression of nephritis in NZB/NZWF1 mice
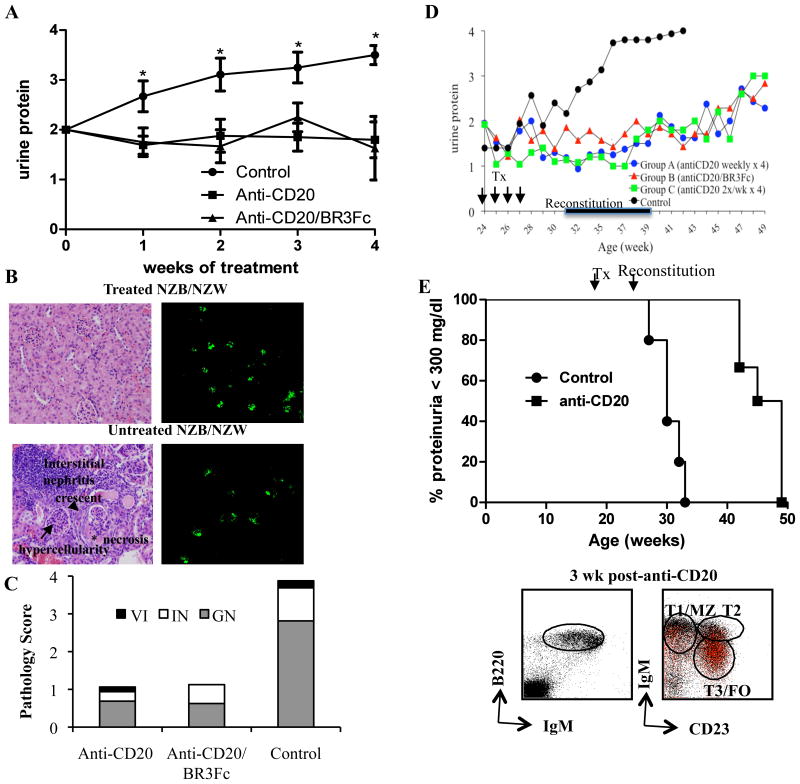
In order to define the clinical effects of BCDT, we treated six month old lupus mice with established nephritis (proteinuria between 100mg/dl (2+) and 300 mg/dl (3+)) with anti-mCD20 (weekly x 4) and monitored proteinuria weekly. We found that BCDT prevented nephritis progression relative to controls (Fig. 5A). Despite the additional B cell depletion that we observed in the spleen and lymph nodes using BR3-Fc, the combination of BCDT with BR3-Fc had no additional clinical benefit over BCDT alone (Fig 5A). To determine whether BCDT also had a measurable effect on kidney pathology, we looked for nephritis in treated and control mice. We found that NZB/NZWF1 mice that received BCDT or combination therapy had a significant reduction in interstitial nephritis and none to minimal glomerulonephritis. In contrast, the control group displayed severe glomerulonephritis with crescents, necrosis, and mesangial hypercellularity and massive interstitial nephritis (Fig 5B–C). Thus, BCDT clearly reduces both clinical markers of lupus and the histopathology associated with disease.
Figure 5. B cell depletion prevents the progression of nephritis.
(A) Six month old NZB/NZW F1 mice were treated with control antibody (IgG2a), anti-mCD20 alone (300 mg) or anti-mCD20 combined with BR3-Fc (8 mg/kg) once per week for 4 weeks. Mice were selected based on two consecutive measures of proteinuria ≥ 100 mg/dl and < 300 mg/dl. Proteinuria monitored with urine dipstick during the treatment. Mean +/−SE.*p<0.05. (B) Hematoxylin and eosin stained kidney sections anti-mCD20 treated and untreated NZB/NZWF1 mice (20x). Deposition of IgG in the glomeruli is unchanged with anti-CD20 (10x). (C) Kidney pathology was scored according to anatomic site as described in the Materials and Methods. GN, glomerulonephritis. IN, interstitial nephritis. VI, perivascular infiltration. (D) Prolonged follow-up. NZB/NZW F1 mice with 2+ protein (100 mg/dl) were treated at 24–28 weeks with control antibody (IgG2a,n=15), anti-mCD20 (300 mg, n=8) once per week for 4 weeks (Group A), anti-mCD20 combined with BR3-Fc (n=7) for 4 weeks (Group B), or anti-mCD20 (300 mg, n=5) 2x/week for 4 weeks (Group C). The difference in proteinuria free survival was significant between the groups (p=0.01). The timing of reconstitution is indicated by the bar. (E) Early B cell depletion delays lupus disease onset. 18 weeks old pre-diseased NZB/NZW F1 mice were treated with control antibody (IgG2a,n=5) or anti-mCD20 (300 mg, n=6) once per week for 4 weeks (start of treatment and beginning of reconstitution indicated by arrows). Proteinuria was monitored and is depicted here as the percentage of proteinuria <300 mg/dl in both treatment groups. The difference in proteinuria free survival was significant between the two groups (p=0.0007). The dot plots on the right indicate that immature transitional B cells predominate during early reconstitution after BCD. Analysis of splenocytes with total lymphocytes on the left and B220+ gated B cells on the right. In the CD23 v IgM dot plots red cells are AA4.1+ and indicative of ‘immature’ status, also seen on the IgM v B220 plots as a high IgM population.
To further define the duration of benefit of short-term BCDT we treated NZB/NZWF1 mice with either anti-CD20 (1x/week for 4 weeks), with anti-CD20 and BR3-Fc (1x/week for 4 weeks) or with anti-CD20 alone (2x/week for 4 weeks) and followed disease progression for 25 weeks. We found that all three groups receiving treatment had an improvement in nephritis, with progression delayed for over 5 months from initial treatment (Fig. 5D). The difference in proteinuria free survival (proteinuria <300 mg/dl) was significant between the treated and control groups (p=0.01), although a similar frequency of mice began to progress beyond 2+ protein early in treatment (data not shown). Interestingly, the effects on nephritis were not significantly different between groups that received BCDT alone or in combination with BR3-Fc, despite the more consistent B cell depletion achieved in the latter group. There was some suggestion that twice weekly anti-mCD20 had greater effects early in the course, with a lower frequency of mice progressing (data not shown). Overall, the effect of a short course of BCD was extended well beyond the timing of reconstitution, which occurred between 31 and 39 weeks.
To determine whether BCDT could prevent disease, we treated 18 week old NZB/NZWF1 mice pre-nephritis with four doses of anti-mCD20 or control antibody over four weeks. We found that without treatment, 100% of mice developed high levels of proteinuria (> 100 mg/dl) by 33 weeks of age. In contrast, mice that received BCDT had a significant delay (4 months) in the onset of proteinuria onset (Fig. 5E). The difference in proteinuria free survival between the two groups was highly significant (p=0.0007). Thus, BCDT can be prophylactic in addition to therapeutic in the context of lupus.
Autoantibody dependent and independent effects of B cell depletion
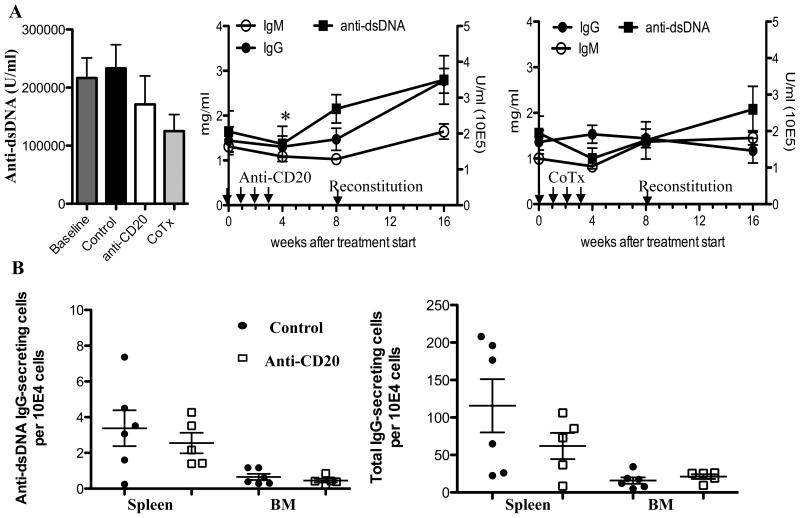
We next wanted to test whether the beneficial effects of BCDT correlated with alterations in immune effector functions, such as autoantibody production or T cell activation. We found that despite the clinical improvement of lupus after B cell depletion therapy, serum anti-dsDNA autoantibody level did not significantly decline compared to baseline or control treated animals at the end of 4 weeks of treatment (Fig. 6A). This is in accord with the persistence of immune deposits in the kidneys of treated animals (Fig. 5B). Co-treatment with anti-CD20 and BAFF blockade tended to have a greater effect on serum immunoglobulins with the difference in anti-dsDNA between the control treated and co-treatment group approaching significance at 4 weeks (p=0.06). This is in accord with longer term measurements of total IgG, total IgM, and IgG anti-dsDNA antibodies after 4 weeks of treatment (8 and 16 weeks after treatment initiation), where anti-CD20 alone failed to abrogate progressive increases in total IgG and anti-dsDNA with age but co-treatment stabilized this increase (Fig. 6A) However, the difference between anti-CD20 and co-treatment at 16 weeks was only significant for total IgG (p=0.003).
Figure 6. B cell depletion has minimal impact on autoantibody production.
(A) Effects on serum autoantibodies: NZB/NZWF1 mice with 2+ proteinuria were treated with anti-mCD20 (n=8), anti-mCD20 combined with BR3-Fc (CoTx) (n=7) or control antibody (IgG2a,n=6) (300 mg) once per week for 4 weeks. Immunoglobulin levels (mean +−SE) were assessed by ELISA before (baseline) and after treatment. The plot on the left shows anti-dsDNA levels before and 1 week after completion of treatment (4 wks after treatment start). There was no significant difference between baseline and any one of the treatment groups by student’s t-test. The middle graph depicts serum IgG, IgM, and anti-dsDNA in mice treated for 4 weeks with anti-CD20, or on the right anti-CD20 and BR3-Fc, and then followed as in Fig. 5D. Even with prolonged follow-up serum immunoglobulin levels do not decrease. The point of maximal B cell reconstitution rate is indicated by the arrow. (B) Effects on antibody secreting cells: ELISPOT results for IgG to dsDNA (anti-dsDNA)-secreting cells and total IgG-secreting cells in the spleens and bone marrows 1 week after 4 weekly treatments of anti-mCD20 (time point shown by asterick in A) (n=5) vs. control antibody (IgG2a,n=6) (time point shown by asterick in A). Each point represents an individual mouse with mean+/−SE also depicted.
Given that serum autoantibody concentrations reflect a complicated balance between production and consumption, we next directly evaluated the impact of BCD on numbers of IgG-secreting cells by enzyme linked immunosorbent spot (ELISPOT) analysis. Notably, there was no change in total IgG–secreting and dsDNA-specific IgG–secreting cells in the spleen or bone marrow of mice treated with BCDT for 4 weeks compared to control treated animals (Fig. 6B).
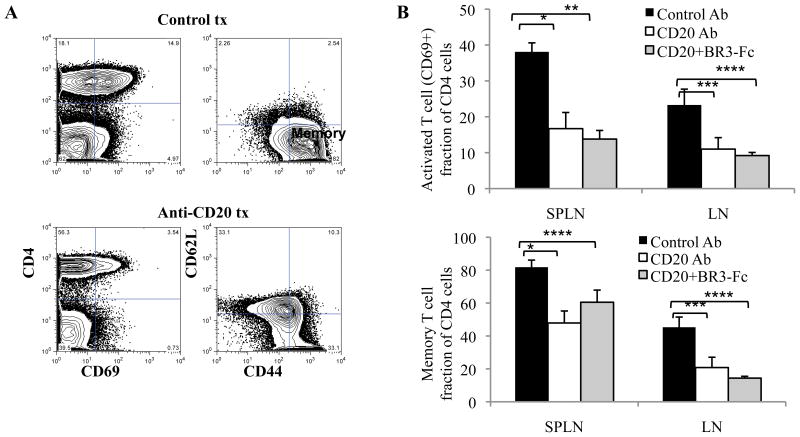
In contrast to the minimal effects on autoantibody production, there was a significant effect of BCD on the T cell compartment after 4 weeks of treatment. Untreated or control treated mice accumulated CD4+CD69+ activated T cells as well as CD44hiCD62Llow antigen-experienced T cells in the spleen (Fig. 7A). However, with BCD there was a significant reduction in the frequency of activated T cells and antigen-experienced T cells (Fig. 6B) as well as an increase in naïve T cells (both frequencies and absolute numbers). Similar effects were observed in the lymph nodes (Fig. 7B).
Figure 7. Autoantibody independent effects of B cell depletion.
Six month old NZB/NZWF1 mice were treated with anti-mCD20 (n=9), anti-mCD20 combined with BR3-Fc (n=4) or control antibody (IgG2a,n=8) (300 μg) once per week for 4 weeks as in Figure 5. (A) T cells were analyzed by FACS 1 week after the completion of 4 weeks of treatment. Activated (CD69+ of the CD4+ T cells) and memory (CD44hiCD62Llow) T cells in the spleen are reduced by B cell depletion therapy. Representative dot plots are shown. Data not shown for combination treatment. (B) The decrease in activated and memory T cells for each cohort in the spleen or LN. *, p=0.002, **, p=0.0002, ***, p=0.001, ****, p=0.03, *****, p=0.01 by student’s t-test
Immature transitional B cells predominate during B cell reconstitution
In addition to the quantity and quality of B cell depletion, a central question regarding BCD therapy is the nature of immunologic reconstitution and whether the B cell compartment is altered after therapy. To answer this question, we studied the phenotype of B cells emerging after treatment. When C57BL/6 mice were treated with a single injection of anti-mCD20, B cell reconstitution began to occur at around 6 weeks. In the peripheral blood and spleen, an immature B cell population with high IgM expression emerged, consisting of T1 (IgMhiCD23negAA4.1pos), T2 (IgMhiCD23posAA4.1pos) and T3 (IgMloCD23posAA4.1pos) B cells. By 9 weeks, these immature populations disappeared, and most B cells in the peripheral blood were mature B220hiIgMloCD23+AA4.1− (data not shown). In contrast, B cell reconstitution occurred earlier in NZB/NZWF1 lupus prone mice, with emerging AA4.1 positive transitional B cells in the peripheral blood and spleen by 3 weeks (Fig. 5E). The reconstitution in lupus prone mice was more heterogeneous with the presence of both newly emerging bone marrow B cells (AA4.1+) and residual B cells (AA4.1− MZ, follicular, and memory B cells) at depletion time points and early reconstitution (data not shown).
DISCUSSION
The physiological importance and complexity of B cell functions has been brought to the fore in recent years by the success of rituximab-based B cell depletion therapy (BCDT) in multiple autoimmune diseases including Rheumatoid Arthritis and Multiple Sclerosis, often conventionally viewed as T-cell mediated conditions. Given the exploration of BCDT in various autoimmune diseases and the key role of B cells in both protective immunity and pathogenic autoimmunity, it is important to better understand B cell functions in disease. This is particularly highlighted by the recent failure of two large clinical trials of BCDT in human SLE, raising doubts about the therapeutic efficacy of this approach. However, we find that B cell depletion has robust effects in a murine lupus model, resulting in both significant delays in disease onset when used in a prevention regimen and improvement in nephritis when used for therapeutic treatment of advanced disease. This indicates that B cells are critical for both the initiation and maintenance of autoimmunity in this spontaneous SLE model, and even short-term B cell depletion may have lasting benefit.
In light of data regarding the variability in effects of BCD in human SLE (5, 25), it is notable that lupus prone mice with more active nephritis display some resistance to B cell depletion with anti-CD20. Although this phenomenon has been demonstrated previously (16), our data extend these findings, particularly given that the prior study relied predominantly on human CD20 transgenic MRL-lpr mice that express lower levels of human CD20 as compared with endogenous CD20, utilization of unusually large quantities of anti-CD20 antibody (1–10 mg; 10–100-fold higher than here or the comparable dosing in humans) of a less efficient isotype (IgG1), and observed very inefficient B cell depletion (<50%). In our hands, resistance of B cells to anti-CD20 appears to be multi-factorial and at least in part related to more rapid drug clearance, suggesting that it could be overcome by more frequent or higher dosing. The mechanistic basis for more rapid clearance of drug may include accelerated clearance through the reticuloendothelial system and/or formation of an antibody response against the anti-mCD20. However, we were unable to detect the latter (data not shown). It is also likely that there are B cell intrinsic factors that contribute to the resistance to depletion in autoimmune mice. Although it remains controversial how anti-CD20 mediates B cell depletion, with evidence for complement-dependent cytotoxicity, antibody dependent cellular cytotoxicity, and induction of apoptosis, Fc receptor dependent mechanisms via the latter two pathways may predominate in vivo (15, 25, 26). Thus it is plausible that an imbalance of B cell survival/apoptotic pathways, a defect shared among genetically distinct mouse models (27), plays a significant role in depletion resistance, a hypothesis we are currently exploring. Notably, we find that combined treatment with anti-CD20 and BAFF blockade improves the extent of depletion suggesting that elevated levels of BAFF contribute to enhanced B cell survival. This has been suggested in normal mice (15) but not previously demonstrated in lupus, a disease where such mechanisms may be particularly relevant given the excess BAFF found in both mice (24) and man (28).
The availability of anti-mCD20 provides the opportunity to monitor tissue depletion of B cells in a systematic fashion that is not possible in human studies. It is interesting that there is a tissue and subset specific hierarchy of sensitivity of B cells to depletion, with peripheral blood B cells most sensitive, tissue B cells (spleen and LN) less so, and peritoneal and BM B cells most resistant- the latter likely explained by the low CD20 expression by the majority of BM B cells. The greater difficulty depleting tissue B cells is consistent with prior publications (15, 29) and emerging though more limited data in humans (30, 31). We find that in the spleen residual B cells are predominantly of a MZ and memory phenotype, with plasma cells also resistant due to lack of CD20 expression. The resistance of MZ B cells to depletion has been reported in human CD20 transgenic mice (15) as well as more recently in autoimmune models using some anti-mCD20 (21) but not others (29). This suggests that antibody specific, microenvironment, and mouse strain effects are likely to be important in resistance of B cells to anti-CD20 depletion. The increased resistance of the MZ to depletion in lupus mice may be due in part to excess BAFF and the dependence of MZ B cell survival on this cytokine given that MZ depletion is significantly improved with combination BAFF blockade. We also find residual memory B cells in the spleen although, in contrast to prior studies (15, 16), GC B cells appear to be quite sensitive to depletion. This suggests strain specific differences in the sensitivity of certain B cell subsets to depletion. Alternatively, the factors maintaining spontaneous GCs in lupus prone mice may be different from GCs in Peyer’s patches or splenic GCs arising after immunization (15). Of note, the disruption of spontaneous GCs and ectopic GCs may be an important mechanism of action of BCD in the treatment of lupus. The presence of residual memory B cells is notable given our prior findings in human studies of relative resistance of memory B cells to depletion in a subset of SLE patients (32). In accord with this data, it has recently been demonstrated that the human spleen is a reservoir for long-lived memory B cells that are resistant to B cell depletion (31). This may be beneficial for maintenance of protective immunity after BCDT but limit the deletion of autoreactive memory. It is interesting that a number of recent reports in mouse models of BCD find inhibition of both primary and secondary immune responses, suggesting that memory B cells are deleted (33). However, this may depend on the timing of anti-CD20 treatment relative to the establishment of a memory response, as well as the host microenvironment. It is also possible that autoreactive memory B cells are protected in inflammatory niches, either within target tissue, spleen, or peritoneum.
Importantly, we find that B cell depletion prevents the progression of lupus nephritis in both early and late disease despite the variability in extent of depletion. Similar to human SLE (34), there is a lack of a significant effect of BCD on serum autoantibodies. Our results significantly extend prior observations with the demonstration that autoreactive antibody secreting cells in spleen and bone marrow are not impacted by B cell depletion, at least at early time points. We are currently exploring whether B cell depletion longer term alters the pro-inflammatory milieu that contributes to the maintenance of long-lived autoreactive plasma cells. Different mechanisms of action have been invoked to explain the benefit of BCD in SLE and other autoimmune diseases including the elimination of autoantibodies, decreased T cell activation, the expansion of Treg cells, the disruption of ectopic lymphoid tissue, and the elimination of effector B and other cells from target organs (35–38). The lasting benefit seen here of a short course of BCDT in NZB/NZWF1 mice even after B cell reconstitution suggests either profound effects of BCD on other cell populations and/or the emergence of a B regulatory cell population. Regarding the former possibility, we do find a significant impact of B cell depletion on the T cell compartment with decreases in activated and memory T cells. Notably, BCD has been shown to inhibit antigen-specific CD4 T cell expansion in both collagen-induced arthritis and autoimmune diabetes mouse models (39). Whether this is mediated via direct B cell APC functions or indirectly through B cell cytokine secretion or other B cell functions remains an important area of future study.
It has been known for many years that B cells can produce cytokines with immunosuppressive, polarizing, inflammatory, and tissue-organizing properties, yet the potential biologic relevance of cytokine-producing B cells was largely unappreciated. However, recent findings have renewed interest in this area and have raised the intriguing possibility that cytokine-producing B cells actively modulate both humoral and cellular immune responses (40). From an autoimmunity standpoint, B cells may either stimulate or inhibit pathogenic responses. A pathogenic role for B cells in SLE is strongly supported by mouse models that are genetically B cell deficient (41), although these models have some limitations including the presence of immunologic and lymphoid organ structural defects engendered by the genetic absence of B cells and the inability to study effects on established disease. Our data firmly establish the pathogenic role of B cells in lupus disease development and progression, adding to results in human CD20 transgenic lupus prone mice (16) and B cell depletion with other approaches (42, 43). On the other hand, evidence is accumulating for regulatory B cells (11) capable of preventing or suppressing autoimmunity in different mouse models (13, 14). This protective role may be mediated by inducing T cell anergy during antigen presentation or inducing Treg expansion or activity (11). These activities are mediated, at least in part, by the B cell production of IL-10 or TGFβ and may control a variety of auto-inflammatory diseases including: inflammatory arthritis, inflammatory bowel disease, autoimmune diabetes, experimental autoimmune encephalitis, and lupus (12, 39, 44–48).
Given the hierarchy of sensitivity of distinct B cell subsets to anti-mCD20, it is important to consider the actual nature and mechanisms of action of Breg cells and the impact of BCD. Mouse Breg activity has been variously assigned to cells with a transitional (in particular, T2-MZ Precursors – T2/MZP), MZ, or B1 phenotype, and lupus resistance has been associated with expansion of MZ cells (13, 49). Our observation that expansion of transitional B cells correlates with long-term remission in SLE patients treated with BCDT is also consistent with a regulatory nature for certain B cell subsets in humans (32, 50). Thus, it is interesting that the B cell subsets most resistant to anti-mCD20 include the MZ and B1 cells, leading us to speculate that BCD shifts the balance of protective versus pathogenic B cell functions. Our results are also the first to carefully delineate the kinetics and phenotype of B cell reconstitution in autoimmune mice and impact on clinical and immunologic outcomes. The predominance of an immature transitional phenotype is in keeping with human B cell depletion therapy (50) and, in the context of persistent disease suppression, highlights the potential regulatory function of these cells. Further delineation of the cytokine secreting ability of these discrete B cell subsets in the context of the autoimmune process and B cell depletion is necessary.
In conclusion, our results indicate that B cells are critical for both the initiation and maintenance of autoimmunity in SLE. B cell depletion is associated with a reduction in T cell memory and activation but a lack of significant effect on autoantibodies and autoreactive B cell memory. The lasting benefit of a short course of BCDT in lupus prone mice suggests a favorable shift in the balance of protective versus pathogenic B cell functions and supports the validity of this approach in the treatment of lupus.
Acknowledgments
We thank Genentech for provision of the mouse BR3-Fc. We are grateful to Troy Randall for critical review of the manuscript.
This work was supported in part by the Lupus Research Institute and National Institutes of Health Grants R01AI077674-01A1to J.H.A.
Non-standard abbreviations
- BAFF
B cell activator of the TNF family
- BCDT
B cell depletion therapy
- BM
bone marrow
- GC
germinal center
- FO
follicular
- LN
lymph node
- MZ
marginal zone
- MOMA-1
metallophillic macrophage-1
- PB
peripheral blood
References
- 1.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180(4):1295–306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160(1):51–9. [PubMed] [Google Scholar]
- 3.Anolik JH. B cell biology and dysfunction in SLE. Bull NYU Hosp Jt Dis. 2007;65(3):182–6. [PubMed] [Google Scholar]
- 4.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 2002;46(10):2673–7. doi: 10.1002/art.10541. [DOI] [PubMed] [Google Scholar]
- 5.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50(8):2580–9. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 6.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase ii/iii systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62(1):222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin F, Chan AC. Pathogenic roles of B cells in human autoimmunity; insights from the clinic. Immunity. 2004;20(5):517–27. doi: 10.1016/s1074-7613(04)00112-8. [DOI] [PubMed] [Google Scholar]
- 8.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189(10):1639–48. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunology. 2000;1(6):475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 10.Anolik JH, Looney RJ, Lund FE, Randall TD, Sanz I. Insights into the heterogeneity of human B cells: diverse functions, roles in autoimmunity, and use as therapeutic targets. Immunol Res. 2009 doi: 10.1007/s12026-009-8096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8(5):391–397. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- 12.Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel Suppressive Function of Transitional 2 B Cells in Experimental Arthritis. J Immunol. 2007;178(12):7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118(10):3420–30. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174(2):817–26. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 16.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179(5):3351–61. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 17.Hamaguchi Y, Uchida J, Cain DW, Venturi GM, Poe JC, Haas KM, et al. The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol. 2005;174(7):4389–99. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, et al. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol. 2006;169(3):954–66. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179(2):1369–80. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa I, Tedder TF, Zhuang Y. B-lymphocyte depletion ameliorates Sjogren’s syndrome in Id3 knockout mice. Immunology. 2007;122(1):73–9. doi: 10.1111/j.1365-2567.2007.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu S, Dunn R, Kehry MR, Braley-Mullen H. B cell depletion inhibits spontaneous autoimmune thyroiditis in NOD. H-2h4 mice. J Immunol. 2008;180(11):7706–13. doi: 10.4049/jimmunol.180.11.7706. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202(9):1225–34. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackay F, Woodcock S, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross J, Johnston J, Mudri S. TACI and BCMA are receptors for a TNF homologue implicated in B cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 25.Anolik JH, Campbell D, Felgar RE, Young F, Sanz I, Rosenblatt J, et al. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 2003;48(2):455–9. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 26.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med. 2006;203(3):743–53. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu T, Qin X, Kurepa Z, Kumar KR, Liu K, Kanta H, et al. Shared signaling networks active in B cells isolated from genetically distinct mouse models of lupus. J Clin Invest. 2007;117(8):2186–96. doi: 10.1172/JCI30398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stohl W, Metyas S, Tan SM, Cheema GS, Oamar B, Xu D, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48(12):3475–86. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 29.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199(12):1659–69. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kneitz C, Wilhelm M, Tony HP. Effective B cell depletion with rituximab in the treatment of autoimmune diseases. Immunobiology. 2002;206(5):519–27. doi: 10.1078/0171-2985-00200. [DOI] [PubMed] [Google Scholar]
- 31.Mamani-Matsuda M, Cosma A, Weller S, Faili A, Staib C, Garcon L, et al. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood. 2008;111(9):4653–9. doi: 10.1182/blood-2007-11-123844. [DOI] [PubMed] [Google Scholar]
- 32.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56(9):3044–56. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 33.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008;105(12):4802–7. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isenberg DA. Treating patients with lupus with B-cell depletion. Lupus. 2008;17(5):400–404. doi: 10.1177/0961203308090024. [DOI] [PubMed] [Google Scholar]
- 35.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki V, Iniotaki A, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: An open-label trial. Arthritis & Rheumatism. 2005;52(2):501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 36.Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147–50. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 37.Sanz I, Anolik JH, Looney RJ. B cell depletion therapy in autoimmune diseases. Front Biosci. 2007;12:2546–67. doi: 10.2741/2254. [DOI] [PubMed] [Google Scholar]
- 38.Ng KP, Cambridge G, Leandro MJ, Edwards JC, Ehrenstein M, Isenberg DA. B cell depletion therapy in systemic lupus erythematosus: long-term follow-up and predictors of response. Ann Rheum Dis. 2007;66(9):1259–62. doi: 10.1136/ard.2006.067124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104(52):20878–83. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lund FE. Cytokine-producing B lymphocytes -- key regulators of immunity. Current Opinion in Immunology. 2008;20(3):332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunological Reviews. 1999;169:107–21. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Chen F, Putt M, Koo YK, Madaio M, Cambier JC, et al. B cell depletion with anti-CD79 mAbs ameliorates autoimmune disease in MRL/lpr mice. J Immunol. 2008;181(5):2961–72. doi: 10.4049/jimmunol.181.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116(3):724–34. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic Intestinal Inflammatory Condition Generates IL-10-Producing Regulatory B Cell Subset Characterized by CD1d Upregulation. Immunity. 2002;16(2):219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 45.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 46.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of Arthritis by Interleukin 10-producing B Cells. J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu C-y, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117(12):3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182(6):3492–502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 Activation of Marginal Zone B Cells in Lupus Mice Regulates Immunity Through Increased IL-10 Production. Journal of Clinical Immunology. 2005;25(1):29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 50.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182(10):5982–93. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]