Abstract
Although malaria parasites infecting non-human primates are important models for human malaria, little is known of the ecology of infection by these parasites in the wild. We extensively sequenced cytochrome b (cytb) of malaria parasites (Apicomplexa: Haemosporida) from free-living Southeast Asian monkeys Macaca nemestrina and M. fascicularis. The two most commonly observed taxa were P. inui and Hepatocystis sp., but certain other sequences did not cluster closely with any previously sequenced species. Most of the major clades of parasites were found in both Macaca species; and the two most commonly occurring parasite infected the two Macaca species at approximately equal levels. However, P. inui showed evidence of genetic differentiation between the populations infecting the two Macaca species, suggesting limited movement of this parasite among hosts. Moreover, coinfection with Plasmodium and Hepatocystis species occurred significantly less frequently than expected on the basis of the rates of infection with either taxon alone, suggesting the possibility of competitive exclusion. The results revealed unexpectedly complex communities of Plasmodium and Hepatocystis taxa infecting wild Southeast Asian monkeys. Parasite taxa differed with respect to both the frequency of between-host movement and their frequency of coinfection.
Introduction
Haemosporidians (Apicomplexa: Haemosporida), including the genus Plasmodium and related genera such as Hepatocystis, are protist parasites of vertebrates; in humans, Plasmodium infections cause devastating disease, resulting in approximately one million deaths per year (Greenwood et al. 2008). A number of recent studies have applied polymerase chain reaction (PCR) techniques to the mitochondrial cytochrome b (cytb) gene or other genes in order to survey the Haemosporidian parasites infecting natural populations of a number of vertebrate taxa, including lizards (Perkins 2001), bats (Olival et al. 2007), birds (Chasar et al. 2009; Ishtiaq et al. 2007; Loiseau et al. 2010), and African great apes (Ollomo et al. 2009; Prugnolle et al. 2010; Rich et al. 2009). These studies have revealed a remarkable degree of complexity in haemosporidian parasite communities, including evidence that coinfection of individual hosts by more than one parasite species may be widespread (Hellgren 2005; Križanauskiene 2010; Pérez-Tris and Bensch 2005).
Theoretical studies of coinfection by parasites have focused on competitive exclusion, particularly on the prediction that a more virulent parasite will out-compete a less virulent parasite when they infect the same host (Mideo 2009; Read and Taylor 2001; Richie 1988). Support for the latter prediction in experimental infections in the laboratory has been equivocal, perhaps because of factors such as genetic diversity in the susceptibility of the hosts (De Roode et al. 2004, 2005; Read and Taylor 2001). Less theoretical attention has been paid to the possibility that two parasites might be mutually beneficial, with infection by one species affecting the environment within the host in such a way that infection by a second parasite is facilitated (Richie 1988; Schall and Bromwich 1994).
Early evidence suggested that in human malaria, coinfection occurs less frequently than expected by chance (Cohen 1973). On the other hand, molecular studies have shown that coinfection with multiple Plasmodium species is more frequent in humans than previously supposed (Mayxay et al. 2004; McKenzie and Bossert 1999; Mueller et al. 2007, 2009; Snounou and White 2004). In recent field studies of human malaria infections in Papua New Guinea, Mehlotra and colleagues (2000, 2002) reported evidence that infections with each of the four species P. falciparum, P. vivax, P. ovale, and P. malariae occur at random with respect to infection by the other species, implying no competitive or facilitative interactions between them. Another recent study, also in Papua New Guinea, found positive associations in infection between all pairs of the same four species except P. falciparum and P. vivax, supporting the hypothesis of mutual facilitation in infection by certain species pairs (Mueller et al. 2009).
Three of the malaria species commonly infecting humans (P. knowlesi, P. malariae, and P. vivax) show evidence of a close relationship with nonhuman primate malaria parasites from Southeast Asia (Escalante et al. 1998, 2005; Hayakawa et al. 2008; Jongwutiwes et al. 2005; Mitsui et al. 2010; Mu et al. 2005). In addition, the discovery that the monkey malaria parasite Plasmodium knowlesi causes abundant natural human infections in Southeast Asia suggests a previously unappreciated extent of transmission across host species boundaries in that region (Jongwutiwes et al. 2004; Singh et al. 2004; Putaporntip et al. 2009). Over an evolutionary time frame, phylogenetic analyses suggest that the evolutionary history of malaria parasites of primates in Southeast Asia has been particularly complex, with multiple events of host switching in the past and many instances of extant species infecting more than one primate host species (Garamszegi 2009). These considerations suggest that an understanding of non-human primate malarias in Southeast Asia may yield insights into both the evolutionary origin and the present-day epidemiology of important human parasites; yet to date there have been relatively few studies of haemosporidian parasites in natural populations of Southeast Asian nonhuman primates (Seethamchai et al. 2008).
A previous study of free-living long-tailed or crab-eating macaque Macaca fascicularis from Thailand used small subunit ribosomal RNA sequency to survey haemosporidia from the genera Plasmodium and one Hepatocystis (Seethamchai et al. 2008). Here we report the results of a more extensive survey, using PCR amplification and sequencing of cytb sequences of haemosporidia from M. fascicularis and the pigtail macaque M. nemestrina. We examine the extent to which clades of malaria parasites show specificity to one or other of the two host species. In addition, because we obtained data from a substantial number (N = 171) of individuals of M. nemestrina, we test for evidence of competitive exclusion or mutual facilitation between Plasmodium and Hepatocystis taxa infecting the latter species.
Materials and Methods
Study population
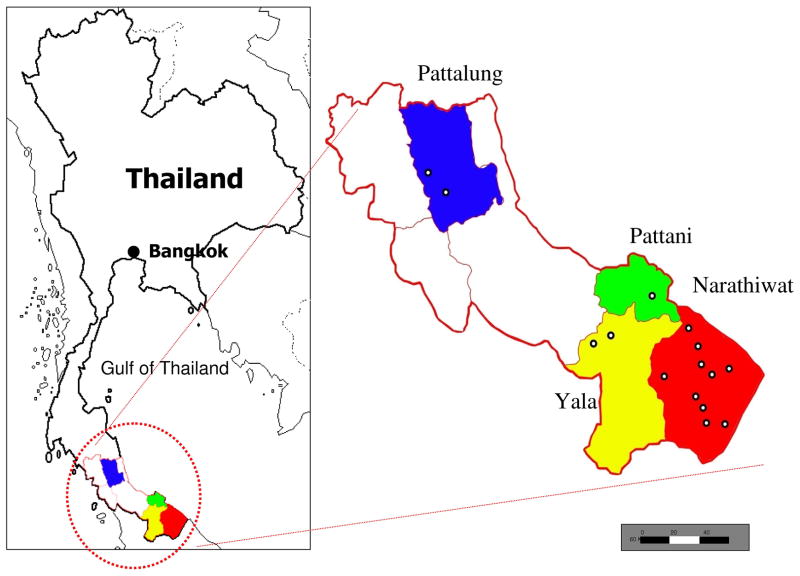
A prospective survey of malaria and Hepatocystis among wild monkey populations in southern Thailand was conducted during December 2008 to June 2009. The areas of capture were located in forest areas of 4 provinces (Pattalung, Pattani, Yala and Narathiwat) as indicated on the map in Figure 1. Venous blood (1–2 ml) was collected from each animal and preserved in EDTA. This study was reviewed and approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University.
Figure 1.
Map of Thailand showing monkey capture sites (open circles).
Microscopy detection
Aliquots of fresh blood samples were used for both thin and thick blood film preparations, followed by treatment with Giemsa stain. Malaria parasites were examined in at least 200 fields with an Olympus BX51 light microscope (Center Valley, PA) at a magnification of 1000.
Isolation of parasite DNA
DNA was extracted from 0.2 mL of EDTA-blood samples by using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The DNA purification procedure was essentially as described in the manufacturer’s instruction manual. Purified DNA was dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and stored at −20°C until used.
Polymerase chain reaction
The DNA fragments spanning the entire coding region of both Plasmodium and Hepatocystis cytb were amplified by a polymerase chain reaction (PCR) using primers, MtCybF0: 5′-GTAATGCCTAGACGTATTCCTG-3′ and MtCybR0: 5′-GCAAGACATGATAGGGAGT-3′. The thermal cycling profile contained 35 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 2 minutes. DNA amplification was performed by using a Gene-Amp 9700 PCR thermal cycler (Applied Biosystems, Foster City, CA). To minimize error introduced in the sequences during PCR amplification, we used ExTaq DNA polymerase (Takara, Shiga, Japan), which has efficient 5′ → 3′ exonuclease activity to increase fidelity and shows no strand displacement. Several studies that require high fidelity of sequence data have used this enzyme for PCR assays (e.g. Atamna-Ismaeel et al. 2008; Seyfang and Jin, 2004; Seyfang et al. 1997). The size of PCR products for Plasmodium cytb and Hepatocystis cyb were clearly distinguishable as the former had ~1490 bp and the latter ~1660 bp.
Subcloning
The PCR products were excised from agarose gel, purified by using a QIAquick PCR purification kit (Qiagen), and ligated into pGEM-T-Easy Vector (Promega, Madison WI). After incubation overnight at 4°C, the reaction mixture was precipitated, dissolved in 10 μL of double-distilled water, and transformed into Escherichia coli strain JM109. Recombinant DNA from positive clones was prepared by using the QIAGEN plasmid mini kit (Qiagen).
DNA sequencing
The DNA sequences were determined from at least 10 plasmid subclones for each isolate. Sequencing analysis was performed from both directions for each template using the BigDye Terminator version 3.1 Cycle Sequencing Kit on an ABI3100 Genetic Analyzer (Applied Biosystems). Overlapping sequences were obtained by using sequencing primers (available upon request). Each unique haplotype was verified by obtaining identical sequences from at least 2 different clones. Isolates containing more than 3 unique haplotypes were reaffirmed by sequencing of recombinant clones generated from two independent PCR amplifications from the same DNA samples.
Statistical methods
We analyzed a total of 365 cytb sequences assigned to the genera Plasmodium, Hepatocystis, and Haemoproteus, 334 from the present study and 31 from Genbank (Supplementary Table S1). Assignments to genus and species of previously published sequences were derived from Genbank annotations. Sequences were aligned using the CLUSTAL X program (Thompson et al. 1997) (Supplementary Figure S1). Phylogenetic trees were reconstructed by the neighbor-joining (NJ) method (Saitou and Nei 1987), using a variety of distances. The NJ method was used, rather than a model-based phylogenetic method, because the number of sites was small; and, when the number of sites is small, a simpler method is likely to be more accurate because it has a lower error of estimation (Nei and Kumar 2000). All distances used in NJ analyses yielded similar results; therefore we report only trees based on the Kimura 2-parameter method, which has a smaller variance than more complex distances (Nei and Kumar 2000). Reliability of clustering patterns was tested by bootstrapping (Felsenstein 1985); 1000 bootstrap samples were used.
We used Nei and Gojobori’s (1986) method to estimate dN and the number of synonymous substitutions per synonymous site (dS). We computed the mean of all pairwise dS values, designated the synonymous nucleotide diversity (πS); and the mean of all pairwise dN values, designated the nonsynonymous nucleotide diversity (πN). Pairwise FST between parasite populations infecting different hosts was estimated using the Arlequin 3.11 software (Excoffier et al. 2005). The hypothesis that two parasite taxa co-occurred in hosts at random was tested by the 2 × 2 χ2 test of independence.
Results
Monkeys and Plasmodium infections
In total, 655 monkeys were captured comprising Macaca fascicularis (n = 195), M. nemestrina (n = 449), Macaca arctoides (n = 4) and Semnopithecus obscurus (n = 7). Of these, 164 monkeys harbored Plasmodium and/or Hepatocystis sp. in their circulation above microscopic threshold (>50 parasites/μl) while 26 monkeys had submicroscopic infections as detected by the PCR method. Subcloning and sequencing of PCR-amplified products reveal that mixed infections between parasites carrying different cytb sequences occurred in 72 monkeys (37.9%), yielding 125 cytb sequences of Hepatocystis (GenBank accession numbers GU92944-GU930068) and 209 sequences of Plasmodium (GenBank accession numbers GU930069-GU930277). No Hepatocystis or Plasmodium infections were found in monkeys in the provinces of Pattalung or Pattani (Figure 1; Supplementary Table S2). Infected monkeys were found only in the two adjacent provinces of Yala (area = 4521 km2) and Narathiwat (area = 4475 km2; Figure 1); and the majority if infected monkeys (88.4%) were from Narathiwat (Supplementary Table S2).
Phylogenetic analysis
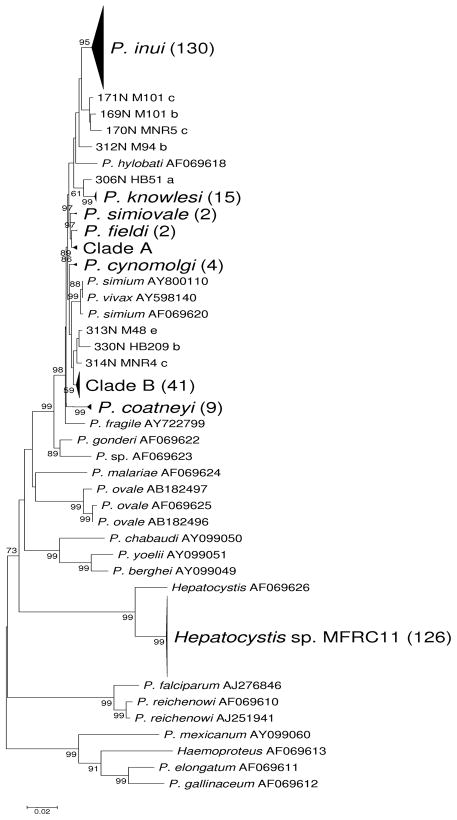
A phylogenetic tree was constructed of 365 cytb sequences from Plasmodium and related genera, including sequences isolated from Southeast Asian monkeys and related sequences from the Genbank database (Figure 2). The tree was rooted following Perkins and Schall (Perkins and Schall 2002). As in certain previous phylogenies (Perkins and Schall 2002; Seethamchai et al. 2008), the genus Plasmodium, as currently defined, was shown to be polyphyletic, with taxa assigned to the genera Haemoproteus and Hepatocystis clustering within Plasmodium (Figure 2). A phylogeny based on four genes from nuclear, mitochondrial and apicoplast genomes found that Hepatocystis clustered within Plasmodium but Haemoproteus did not (Martinsen et al. 2008).
Figure 2.
Neighbor-joining tree of cytb sequences from Plasmodium, Haemoproteus and Hepatocystis species. Large clades are condensed, with numbers of sequences indicated. Numbers on branches are percentages of 1000 boostrap samples supporting a given branch; only values ≥ 50% are shown.
Several clusters in the phylogenetic tree included Genbank sequences assigned to known species or genera, along with sequences from our field collections. The largest of these was a cluster receiving 95% bootstrap support that included a Genbank sequence assigned Plasmodium inui (AF069617) along with 129 sequences from our field collections (Figure 2). The next largest cluster, which received 99% bootstrap support, included a Genbank sequence assigned to Hepatocystis sp. MFRC11 (EU400408) and 125 field isolates (Figure 2). Other sequences formed clusters with Genbank sequences from the following species: P. knowlesi (13 field isolates along with two previously reported sequences, AF069621 and AY722797); P. coatneyi (8 field isolates along with one previously reported sequence, EU400407); P. cynomolgi (2 field isolates along with two previously reported sequences, AF069616 and AY800108); P. fieldi (1 field isolate along with 1 previously reported sequence, AF069615); and P. simiovale (1 field isolate along with 1 previously reported sequence, AY800109; Figure 2). Each of the latter five clusters received 86–99% bootstrap support (Figure 2).
There were two major clusters that included no previously reported sequences and thus could not be assigned to any known taxon; these are designated Clade A and Clade B (Figure 2). Clade A included 6 sequences and received 89% bootstrap support (Figure 1). Clade A clustered with P. fieldi (Figure 2), but this pattern received very low (29%) bootstrap support. Clade B included 41 sequences and received 59% bootstrap support (Figure 2). Clade B clustered close to P. vixax and P. simium (Figure 2), but grouping of this clade with the latter species received very low (8%) bootstrap support. Thus, the phylogeny suggested that Clade A and Clade B did not correspond to any previously sequenced species. In addition to these major groups, there were a total of eight other sequences that did not group with previously sequenced species (Figure 2).
Difference between host species
The single Semnopithecus obscurus in our survey was infected by a parasite that clustered with P. knowlesi. The parasites infecting the 171 Macaca nemestrina and 18 M. fascicularis are summarized in Table 1. There was not a significant difference between the two host species with regard to the incidence of infection by either of the two most commonly observed parasite taxa, P. inui and Hepatocystis (Table 1). On the other hand, M. fascicularis was significantly more likely than M. nemestrina to be infected by P. coatneyi (P = 0.007; Table 1). Similarly, when we pooled the data for all Plasmodium species except P. inui, M. fascicularis was over three times as likely to be infected by these taxa as was M. nemestrina; and the difference was statistically significant (P =0.01; Table 1).
Table 1.
Incidence of malaria taxa in infected Macaca nemestrina and M. fascicularis.
| Taxon | M. nemestrina (N = 171) | M. fascicularis (N = 18) | Fisher’s exact test of equality of proportions |
|---|---|---|---|
| Plasmodium inui | 36.3% | 38.9% | n.s. |
| P. coatneyi | 1.2% | 16.7% | 0.007 |
| P. knowlesi | 2.3% | 5.6% | n.s. |
| Clade A | 2.9% | 0.0% | n.s. |
| Clade B | 11.7% | 5.6% | n.s. |
| Plasmodium other than P. inui | 9.9% | 33.3% | 0.01 |
| Hepatocystis | 55.0% | 44.4% | n.s. |
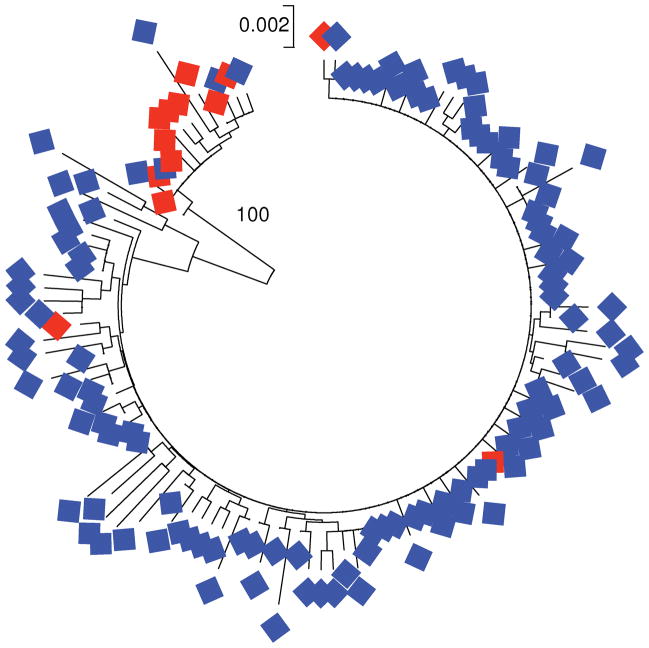
A phylogenetic tree of the 129 field collected species assigned to P. inui revealed two major clusters, separated by an internal branch that received 100% bootstrap support (Figure 3). The representation of these two clusters differed markedly between the two Macaca host species (Figure 3). In the smaller cluster, there were 10 sequences derived from M. fascicularis (66.6%) and 5 derived from M. nemestrina (33.3%; Figure 3). By contrast, the larger cluster included only 3 sequences derived from M. fascicularis (2.6%) but 111 (97.4%) derived from M. nemestrina (Figure 3). The difference between the two clusters with respect to the proportions of sequences derived from the two host species was highly significant (P < 0.001; Fisher’s exact test).
Figure 3.
Neighbor-joining tree of Plasmodium inui cytb field isolate sequences. Isolates from Macaca nemestrina are indicated by blue symbols, and those from P. fascicularis by red symbols. The bootstrap support (100%) for the branch separating the two major clades is shown.
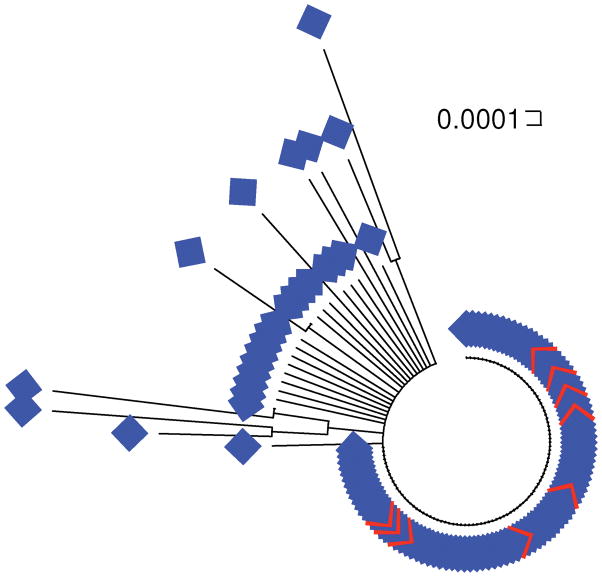
Unlike that of P. inui, the phylogeny of Hepatocystis sp. did not show any major subdivisions (Figure 4). The 9 sequences derived from M. fascicularis were identical to one another and to 89 of the 116 sequences derived from M. nemestrina (Figure 4). Table 2 shows synonymous nucleotide diversity (πS) and nonsynonymous nucleotide diversity (πN) for P. inui and Hepatocystis sp. derived from the two Macaca host species. Within both P. inui and Hepatocystis sp., πS was significantly greater than πN in every case, except for Hepatocystis sp. from M. fascicularis, which showed no synonymous or nonsynonymous differences (Table 2). This pattern is indicative of purifying selection on the cytb gene. Both πS and πN were significantly higher in P. inui than in Hepatocystis sp. both in M. nemestrina and in M. fascicularis (Table 2). Likewise, both πS and πN were significantly higher in P. inui than in Hepatocystis sp. when sequences derived from two Macaca host species were pooled. These results support the hypothesis that the population of P. inui infecting these monkeys is more ancient and diverse than that of Hepatocystis sp.
Figure 4.
Neighbor-joining tree of Hepatocystis sp. field isolate sequences. Isolates from Macaca nemestrina are indicated by blue symbols, and those from P. fascicularis by red symbols.
Table 2.
Synonymous (πS) and nonsynonymous (πN) nucleotide diversity in cytb sequences of Plasmodium inui and Hepatocystis sp. from Macaca nemestrina and M. fascicularis.
| Parasite and host species | πS ± S.E. | πN ± S.E. |
|---|---|---|
| P. inui | ||
| M. nemestrina (N = 116) | 0.0117 ± 0.0022*** | 0.0029± 0.0004*** |
| M. fascicularis (N =13) | 0.0251 ± 0.0080** | 0.0032 ± 0.0011** |
| All (N = 129) | 0.0181 ± 0.0045*** | 0.0033 ± 0.0006*** |
| Hepatocystis sp. | ||
| M. nemestrina (N = 116) | 0.0013 ± 0.0003 | 0.0004 ± 0.0001 |
| M. fascicularis (N = 9) | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| All (N = 125) | 0.0012 ± 0.0003 | 0.0004 ± 0.0001 |
Z-tests of the hypothesis that πS or πN in P. inui equals the corresponding value in Hepatocystis sp.:
P < 0.01;
P < 0.001.
The estimate of pairwise FST between Hepatocystis sp. populations infecting the two Macaca host species was −0.057, which was not significantly different from zero. On the other hand, FST between P. inui populations infection the two host species was 0.586, which was significantly different from zero (P < 0.05). In Sukhirin district, Narathiwat province, 50 isolates of Hepatocystis sp. were collected from M. nemestrina and 4 from M. fascicularis. FST between the Hepatocystis sp. populations infecting the two host species in this location (−0.142) was not significant. These results support the hypothesis of genetic differentiation between the P. inui populations infecting the two hosts but not between the Hepatocystis sp. populations infecting the same hosts. When we estimated FST values among collection locations for Hepatocystis sp. infecting M. nemestrina, there were significant FST values in several comparisons (Supplementary Table S3). By contrast, P. inui infecting M. nemestrina showed no evidence of population substructure (Supplementary Table S3).
Coinfection
Of the 171 infected M. nemestrina, 18 (10.5%) were infected by two different clades, and 3 (1.8%) were infected by three different clades (where clades are defined on the basis of the phylogenetic tree in Figure 2). Whereas 51 of the 171 M. nemestrina individuals (29.8%) were infected by P. inui alone and 83 (48.5%) by Hepatocystis alone, only 11 monkeys (6.4%) were infected by both P. inui and Hepatocystis sp. The proportion of coinfections with P. inui and Hepatocystis sp. was significantly less than the random expectation (χ2 = 54.6; 1 d.f.; P < 0.001). Similarly, although 17 of the 171 monkeys (9.9%) were infected by Plasmodium from clades other than P. inui, only one M. nemestrina individual (0.6%) was coinfected with both Hepatocystis sp. and Plasmodium from a clade other than P. inui. Again, the proportion of coinfection was significantly less than the random expectation (χ2 = 18.4; 1 d.f.; P < 0.001). On the other hand, coinfection with P. inui and with Plasmodium from a clade other than P. inui occurred in four M. nemestrina individuals (2.3%), not significantly different from the random expectation (χ2 = 1.3; 1 d.f.; n.s.).
One factor that might influence the probability of coinfection by two parasite taxa might be the absence of one of the taxa from a given site. We tested whether this factor influenced the results by analyzing data from seven collection sites where both Hepatocystis sp. and P. inui were found: one site in Yala (Kabang) and six sites in Narathiwat (Chanae, Ra-ngae, Sukhirin, Su-ngai Padi, Tak Bai, and Waeng). Of the 145 M. nemestrina from these seven sites, 50 (34.5%) were infected by P. inui alone and 74 (51.0%) by Hepatocystis alone. By contrast, only 11 monkeys (7.6%) from the seven sites were infected by both P. inui and Hepatocystis sp. The proportion of coinfections with P. inui and Hepatocystis sp. was significantly less than the random expectation (χ2 = 71.5; 1 d.f.; P < 0.001).
Discussion
Like previous studies of African apes (Ollomo et al. 2009; Prugnolle et al. 2010; Rich et al. 2009), extensive sequencing of cytb sequences of malaria parasites from free-living Southeast Asian monkeys Macaca nemestrina and M. fascicularis revealed unexpectedly complex communities of Plasmodium and Hepatocystis species. Phylogenetic analyses showed that certain sequences corresponded to taxa from which cytb sequences have already been reported, the most numerous of which were sequences that appeared to correspond to P. inui and to Hepatocystis sp. MFRC11. Previous sequencing of SSU rRNA from M. fascicularis in Thailand found the latter two taxa to be abundant (Seethamchai et al. 2008). On the other hand, certain cytb sequences from M. nemestrina and M. fascicularis did not cluster close to those from previously sequenced species, raising the possibility that some of these correspond to previously undescribed species.
Most of the major clades of parasites were found in both Macaca species; and the two most commonly occurring parasite taxa, P. inui and to Hepatocystis sp., infected the two Macaca species at approximately equal levels. However, these two parasite taxa showed very different patterns of genetic diversity across the two host species. Hepatocystis sp. showed little diversity, and no evidence of genetic differentiation between the isolates derived from M. nemestrina and those derived from M. fascicularis. By contrast, P. inui showed a much greater overall degree of genetic diversity and evidence of genetic differentiation between the isolates infecting M. nemestrina and those infecting M. fascicularis. On the other hand, Hepatocystis infecting M. nemestrina showed evidence of geographic differentiation among populations, even within the relatively restricted area of the province of Narathiwat, whereas P. inui showed no evidence of population subdivision across Narathiwat and Yala provinces (Supplementary Table S3). The evidence of genetic differentiation between the P. inui populations infecting the two macaque species suggests that movement of this particular parasite between these two host species is limited. By contrast, the evidence suggests that Hepatocystis sp. moves freely between host species but less freely between geographic localities. These differences may reflect differences in the dispersal and host-specificity of the respective vectors of the two parasite species; namely Culicoides midges (Ceratopogonidae) in the case of Hepatocystis sp. and anopheline mosquitos (Culicidae) in the case of P. inui.
Because the number of individuals from which parasite sequences were obtained was greatest in the case of M nemestrina (N = 171), we examined the community of malaria parasites inhabiting this species in detail. While coinfection with P. inui and Hepatocystis sp. occurred in M. nemestrina, both of these parasite taxa were found together in the same host significantly less frequently than expected on the basis of the overall occurrence in the host population. This result was found in the overall data set and at collection sites from which both Hepatocystis sp. and P. inui were collected. Similarly, coinfection with Hepatocystis sp. and Plasmodium taxa other than P. inui occurred significantly less frequently than expected by chance. On the other hand, coinfection with P. inui and other Plasmodium taxa occurred at a frequency no different from that expected. These results suggest that infection with Hepatocystis sp. reduces the likelihood of infection with Plasmodium species, and vice versa, implying that some sort of competitive exclusion occurs between these taxa.
There has been considerable discussion in the literature regarding potential artifacts of PCR-based methods for surveying haemosporidian diversity (Martinsen et al. 2006; Perkins et al. 1998; Szölli et al. 2008; Valkiúnas et al. 2006, 2009). One problem that has received considerable attention is that PCR may not amplify sequences from rarer parasites in the case of coinfection (Valkiúnas et al. 2006), for which one remedy is the use of haplotype-specific primers. In the present case, we did not use haplotype-specific primers; therefore our conclusion regarding competitive exclusion between P. inui and Hepatocystis sp. needs to be tested further with primers specific to these two taxa. Although we cannot rule out the possibility that certain haplotypes were missed in some hosts, we feel that our methods were likely to detect both taxa in most hosts for the reasons: (1) our PCR assay is sensitive enough to amplify a single copy of cytb from either Plasmodium or Hepatocystis; (2) the cytb PCR products of Plasmodium and Hepatocystis differ in size (the former ~1490 bp and the latter ~1660 bp) and are clearly discernible after agarose gel electrophoresis; and (3) all isolates that had 1660-bp PCR product contained clones whose sequences were cytb of Hepatocystis sp. However, it will be important to subject our methods to further empirical validation; for example, by attempting to PCR from experimentally generated mixed populations of the two haemosporidian taxa.
Another potential problem relates to artifacts due to PCR or cloning errors. We attempted to minimize the effect of such artifacts by verifying each unique haplotype sequences from at least 2 different clones. Evidence that the error rate was low included the observation that πS was significantly greater than πN in cytb of P. inui and Hepatocystis sp., as expected under purifying selection (Table 2). If a substantial portion of the observed polymorphism were artifactual, we would not expect to see this pattern, since artifacts would occur at random with respect to the reading frame. One source of sequence errors might be an elevated error rate in AT-rich sequences during recombinant plasmid amplification in bacteria hosts (Frangeul et al. 1999). If such errors contributed substantially to the observed polymorphism, the error rate of base substitutions should be similar for Plasmodium and Hepatocystis, since their AT content in the amplified region (74% and 72%, respectively) was almost equal. However, our data revealed a remarkable difference in the level of nucleotide diversity at the cytb locus between these two genera, which would be difficult to explain as an artifact of AT-richness (Table 2).
In summary, analysis of cytb sequences revealed a highly complex community of malaria parasites infecting Southeast Asian monkeys, including two numerically dominant species and numerous less common species, several of which were only infrequently recorded. The results were consistent with the hypothesis that competitive exclusion occurs between two most numerically dominant species, but there was no evidence of any mutually facilitative interaction among taxa. If further studies support the hypothesis of competitive exclusion between Hepatocystis and P. inui, the interaction between these two taxa may provide a useful model for advancing our understanding of the complex question of how different parasite species interact when infecting the same host (Mideo 2009; Read and Taylor 2001; Richie 1988). Moreover, our results imply that the three human malaria parasites of Southeast Asian origin (P. knowlesi, P. malariae, and P. vivax) arose in a complex haemosporidian parasite community. Thus, much of the biology of these parasites is likely to have been shaped by adaptation to coexistence with other haemosporidia. Understanding the immunological and biochemical mechanisms behind interactions between the members of such parasite communities will increase our knowledge of the basic biology of malaria infection and may suggest new avenues for prophylaxis and treatment.
Supplementary Material
Alignment of sequences used in analyses.
GenBank™ accession numbers of Plasmodium and Hepatocystis isolates and clones in this study.
Distribution of Plasmodium and Hepatocystis among macaques in southern Thailand.
FST Values for Hepatocystis and Plasmodium inui infecting Macaca nemestrina in different localities.
Acknowledgments
We thank Thongchai Hongsrimuang and Urassaya Pattanawong for technical assistance and staff of Hala-Bala Wildlife Research Station, National Park and Wildlife Research Division, Department of National Parks, Wildlife and Plant Conservation for assistance in field work. This research was supported by grants from the National Research Council of Thailand and the Thai Government Research Budget to S.J and C.P.; The Thailand Research Fund (RMU5080002) to C.P.; and grant from the National Institutes of Health GM43940 to A.L.H.
References
- Atamna-Ismaeel N, Sabehi G, Sharon I, Witzel K-P, Labrenz M, Jürgens K, Barkay T, Stomp M, Huisman J, Beja O. Widespread distribution of proteorhodopsins in fresh and brackish ecosystems. ISME Journal. 2008;2:656–662. doi: 10.1038/ismej.2008.27. [DOI] [PubMed] [Google Scholar]
- Chasar A, Loiseau C, Valkiúnas G, Iezhova T, Smith TB, Seghal RN. Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Molecular Ecology. 2009;18:4121–4133. doi: 10.1111/j.1365-294X.2009.04346.x. [DOI] [PubMed] [Google Scholar]
- Cohen JE. Heterologous immunity in human malaria. Quarterly Review of Biology. 1973;48:467–489. doi: 10.1086/407705. [DOI] [PubMed] [Google Scholar]
- De Roode JC, Culleton R, Cheesman SJ, Carter R, Read AF. Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proceedings of the Royal Society of London B. 2004;271:1073–1080. doi: 10.1098/rspb.2004.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roode JC, Pansini R, Cheesman SJ, Helinski ME, Huijben S, Wargo AR, Bell AS, Chan BH, Walliker D, Read AF. Virulence and competitive ability in genetically diverse malaria infections. Proceedings of the National Academy of Science USA. 2005;102:7624–7628. doi: 10.1073/pnas.0500078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante A, Freeland DE, Collins WE, Lal AA. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proceedings of the National Academy of Science USA. 1998;95:8124–8129. doi: 10.1073/pnas.95.14.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante AA, Cornejo OE, Freeland DE, Poe AC, Durrego E, Collins WE, Lal AA. A monkey’s tale: the origin of Plasmodium vivax as a human malaria parasite. Proc Natl Acad Sci USA, Proceedings of the National Academy of Science USA. 2005;102:1980–1985. doi: 10.1073/pnas.0409652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin version 3.0: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Frangeul L, Nelson KE, Buchrieser C, Danchin A, Glaser P, Kunst F. Cloning and assembly strategies in microbial genome projects. Microbiology. 1999;145:2625–2634. doi: 10.1099/00221287-145-10-2625. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ. Patterns of co-speciation and host switching in primate malaria parasites. Malaria Journal. 2009;8:110. doi: 10.1186/1475-2875-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alsonso PL, Collins FH, Duffy PE. Malaria: progress, perils, and prospects for eradication. Journal of Clinical Investigation. 2008;118:1266–1276. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Culleton R, Otani H, Horii T, Tanabe K. Big bang in the evolution of extant malaria parasites. Molecular Biology and Evolution. 2008;25:2233–2239. doi: 10.1093/molbev/msn171. [DOI] [PubMed] [Google Scholar]
- Hellgren O. The occurrence of haemosporidian parasites in the Fennoscandian bluethroat (Lucinia svecica) population. Journal of Ornithology. 2005;146:55–60. [Google Scholar]
- Ishtiaq F, Gering E, Rappole JH, Rahmani AR, Jhala YV, Dove CJ, Milensky C, Olson SL, Pierce PA, Fleischer RC. Prevalence and diversity of avian hematozoan parasites in Asia: a regional survey. Journal of Wildlife Diseases. 2007;43:382–398. doi: 10.7589/0090-3558-43.3.382. [DOI] [PubMed] [Google Scholar]
- Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerging Infectious Diseases. 2004;10:2211–2213. doi: 10.3201/eid1012.040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongwutiwes S, Putaporntip C, Iwasaki T, Ferreira MU, Kanbara H, Hughes AL. Mitochondrial genome sequences support ancient population expansion in Plasmodium vivax. Molecular Biology and Evolution. 2005;22:1733–1739. doi: 10.1093/molbev/msi168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križanauskiene A, Pérez-Tris J, Palinauskas V, Hellgren O, Bensch S, Valkiúnas G. Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporidia) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp. nov. Parasitology. 2010;137:217–227. doi: 10.1017/S0031182009991235. [DOI] [PubMed] [Google Scholar]
- Loiseau C, Iezhova TA, Valkiúnas G, Chasar A, Hutchinson A, Buerman W, Smith TB, Seghal RN. Spatial variation of haemosporidian parasite infection in African rainforest bird species. Journal of Parasitology. 2010;96:21–29. doi: 10.1645/GE-2123.1. [DOI] [PubMed] [Google Scholar]
- Martinsen ES, Paperna I, Schall JJ. Morphological versus molecular identification of avian Haemosporidia: an exploration of three species concepts. Parasitology. 2006;133:279–288. doi: 10.1017/S0031182006000424. [DOI] [PubMed] [Google Scholar]
- Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Molecular Phylogenetics and Evolution. 2008;47:261–273. doi: 10.1016/j.ympev.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends in Parasitology. 2004;20:233–240. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. Multispecies Plasmodium infections of humans. Journal of Parasitology. 1999;85:12–18. [PMC free article] [PubMed] [Google Scholar]
- Mehlotra RK, Lorry K, Kastens W, Miller SM, Alpers MP, Bockarie M, Kazura JW, Zimmerman PA. Random distribution of mixed species malaria infections in Papua New Guinea. American Journal of Tropical Medicine and Hygiene. 2000;62:225–231. doi: 10.4269/ajtmh.2000.62.225. [DOI] [PubMed] [Google Scholar]
- Mehlotra RK, Kasehagen LJ, Baisor M, Lorry K, Kazura JW, Bockarie MJ, Zimmerman PA. Malaria infections are randomly distributed in diverse holoendemic areas of Papua New Guinea. Am J Trop Med Hyg American Journal of Tropical Medicine and Hygiene. 2002;67:555–562. doi: 10.4269/ajtmh.2002.67.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mideo N. Parasite adaptations to within-host competition. Trends in Parasitology. 2009;25:261–268. doi: 10.1016/j.pt.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Mitsui H, Arisue N, Sakihama N, Inagaki Y, Horii T, Hasegawa M, Tanabe K, Hashimoto T. Phylogeny of Asian primate malarias inferred from apicoplast genome-encoded genes with special emphasis on the positions of Plasmodium vivax and P. fragile. Gene. 2010;450:32–38. doi: 10.1016/j.gene.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Mu J, Joy DA, Duan J, Huang Y, Carlton J, Walker J, Barnwell J, Beerli P, Charleston MA, Pybus OG, Su X. Host switch leads to emergence of Plasmodium vivax malaria in humans. Molecular Biology and Evolution. 2005;22:1686–1693. doi: 10.1093/molbev/msi160. [DOI] [PubMed] [Google Scholar]
- Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale – the “bashful” malaria parasites. Trends in Parasitology. 2007;23:278–283. doi: 10.1016/j.pt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller I, Widmer S, Michel D, Maraga S, McNamara DT, Kiniboro B, Sie A, Smith TA, Zimmerman PA. High sensitivity detection of Plasmodium species reveal positive correlations between infections of different species, shits in age distribution and reduced local variation in Papua New Guinea. Malaria Journal. 2009;8:41. doi: 10.1186/1475-2875-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Molecular Biology and Evolution. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Nei M, Kumar S. Molecular evolution and phylogenetics. Oxford University Press; New York: 2000. [Google Scholar]
- Olival KJ, Stiner EO, Perkins SL. Detection of Hepatocystis sp. in southeast Asian flying foxes (Pteropidae) using microscopic and molecular methods. Journal of Parasitology. 2007;93:1538–1540. doi: 10.1645/GE-1208.1. [DOI] [PubMed] [Google Scholar]
- Ollomo B, Durand P, Prugnolle F, Douzery E, Arnathau C, Nkoghe D, Leory E, Renaud F. A new malaria agent in African hominids. PLoS Pathogens. 2009;5(5):e1000446. doi: 10.1371/journal.ppat.1000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Tris J, Bensch S. Diagnosing genetically diverse avian malaria infections using mixed-sequence analysis and TA-cloning. Parasitology. 2005;131:1–9. doi: 10.1017/s003118200500733x. [DOI] [PubMed] [Google Scholar]
- Perkins SL. Phylogeography of Caribbean lizard malaria: tracing the history of vector-borne parasites. Journal of Evolutionary Biology. 2001;14:34–45. doi: 10.1046/j.1420-9101.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- Perkins SL, Schall JJ. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. Journal of Parasitology. 2002;88:972–978. doi: 10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Perkins SL, Osgood SM, Schall JJ. Use of PCR for detection of subpatent infections of lizard malaria: implications for epizootiology. Molecular Ecology. 1998;7:1587–1590. [Google Scholar]
- Prugnolle F, Durand P, Neel C, Ayala FJ, Arnathau C, Etienne C, Mpoudi-Ngole E, Nkoghe D, Leroy E, Delaporte E, Peeters M, Renaud F. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proceedings of the National Academy of Science USA. 2010;107:1458–1463. doi: 10.1073/pnas.0914440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaporntip C, Hongsrimuang T, Seethamchai S, Kobasa T, Limkittikul K, Cui L, Jongwutiwes S. Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. Journal of Infectious Diseases. 2009;199:1143–1150. doi: 10.1086/597414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- Rich SM, Leendertz FH, Xu G, LeBreton M, Djoko CF, Aminake MN, Takang EE, Diffo JL, Pike BL, Rosenthal BM, Formenty P, Boesch C, Ayala FJ, Wolfe ND. The origin of malignant malaria. Proceedings of the National Academy of Science USA. 2009;196:14902–14907. doi: 10.1073/pnas.0907740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie TL. Interactions between malaria parasites infecting the same vertebrate host. Parasitology. 1988;96:607–639. doi: 10.1017/s0031182000080227. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schall JJ, Browmwich CR. Interspecific interactions tested: two species of malarial parasite in a West African lizard. Oecologia. 1994;97:326–332. doi: 10.1007/BF00317322. [DOI] [PubMed] [Google Scholar]
- Seethamchai S, Putaporntip C, Malaivijitnond S, Cui L, Jongwutiwes S. Malaria and Hepatocystis species in wild macaques, southern Thailand. American Journal of Tropical Medicine and Hygiene. 2008;78:646–653. [PubMed] [Google Scholar]
- Seyfang A, Jin JH. Multiple site-directed mutagenesis of more than 10 site simultaneously and in a single round. Analytical Biochemistry. 2004;324:285–291. doi: 10.1016/j.ab.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Seyfang A, Kavanaugh MP, Landfear SM. Aspartate 19 and glutamate 121 are critical for transport function of the myo-inositol/H+ symporter from Leishmania donovani. Journal of Biological Chemistry. 1997;272:24210–24215. doi: 10.1074/jbc.272.39.24210. [DOI] [PubMed] [Google Scholar]
- Singh B, Sung LK, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway D. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. The Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- Snounou G, White NJ. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends in Parasitology. 2004;20:333–339. doi: 10.1016/j.pt.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Szöllsi E, Hellgren O, Hasselquist D. A cautionary note on the use of nested PCR for parasite screening – an example from avian blood parasites. Journal of Parasitology. 2008;94:562–564. doi: 10.1645/GE-1286.1. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiúnas G, Bensch S, Iezhova TA, Križéanauskiene A, Hellgren O, Bolshakov CV. Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. Journal of Parasitology. 2006;92:418–422. doi: 10.1645/GE-3547RN.1. [DOI] [PubMed] [Google Scholar]
- Valkiúnas G, Iezhova TA, Loiseau C, Sehgal RN. Nested cytochrome b polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of naturally infected birds. Journal of Parasitology. 2009;95:1512–1515. doi: 10.1645/GE-2105.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of sequences used in analyses.
GenBank™ accession numbers of Plasmodium and Hepatocystis isolates and clones in this study.
Distribution of Plasmodium and Hepatocystis among macaques in southern Thailand.
FST Values for Hepatocystis and Plasmodium inui infecting Macaca nemestrina in different localities.