Abstract
We report the results of a Phase I dose escalation trial of the multikinase inhibitor sorafenib in relapsed/refractory acute leukemias using an intermittent dosing regimen. Fifteen patients with advanced leukemia (Acute myeloid leukemia(AML), 2=Acute lymphoblastic leukemia(ALL), 1 Biphenotypic) and a median age of 63 (range 37–85) years were enrolled and treated on a dose escalation trial. Toxicities ≥grade 3 were present in 55% of cycles and the maximum tolerated dose (MTD) was determined to be 400mg BID × 21days in a 28 day cycle. Plasma inhibitory assays of kinase targets ERK and FLT3-ITD demonstrated excellent target inhibition, with FLT3-ITD silencing occurring below the MTD. The N-oxide metabolite of sorafenib appeared to be a more potent inhibitor of FLT3-ITD than the parent compound. Despite marked ex vivo FLT-3 ITD inhibition, no patients met criteria for complete or partial response in this monotherapy study. Eleven of fifteen patients experienced stable disease as best response. Although sorafenib demonstrated only modest clinical activity as a single agent in this heavily treated population, robust inhibition of FLT3 and ERK suggest there may be a potential important role in combination therapies.
Keywords: Acute Myeloid Leukemia, FLT3, Tyrosine Kinase inhibitors, Clinical trial
INTRODUCTION
Acute leukemia in adults remains difficult to cure with conventional cytotoxic chemotherapy with approximately 70% of adult patients diagnosed with AML and ALL dying of the disease.(1–2) One recent strategy is based on targeting cellular signal transduction pathways found to be mutated or overactive in the malignant clone. Acute leukemia has several potential targetable pathways and one pathway of great interest is the Ras/Raf/MEK/Erk pathway due to its role in cellular division, differentiation, and apoptosis. This pathway is constitutively activated in more than 50% of primary acute myeloid leukemia samples;(3) and constitutive activation is associated with an inferior clinical outcome.(4) This pathway can be activated by mutations in receptor tyrosine kinases such as FLT3, c-KIT and VEGF.(5)
Sorafenib is a multi-targeted tyrosine kinase inhibitor, with activity against RAF kinase, VEGF receptors, both wild type and ITD(Internal Tandem Duplication)-mutated FLT3, PDGF receptors, c-KIT, and RET kinase.(6) Sorafenib was recently approved by the U.S. Food and Drug Administration for the treatment of renal cell cancer(7) and hepatocellular carcinoma.(8) Preclinical studies of sorafenib in acute leukemia have demonstrated down-regulation of the MAPK pathway, sensitization to receptor-mediated apoptosis by down-regulation of Mcl-1(Myeloid cell leukemia-1),(9–10) and growth inhibition of AML cells with FLT3-ITD mutations.(11)
Early published clinical studies of sorafenib in AML suggest continuous dosing at a dose approved for solid tumors (400mg twice daily) is not tolerated well in patients with AML/MDS.(12) Sorafenib has been found to occasionally induce hematologic responses and complete remissions in select patients with FLT3-ITD AML.(13–14) Based on these data and earlier work that suggested improved tolerance in intermittently dosed schedules,(15) we undertook a phase I dose escalation trial to determine the dose limiting toxicities (DLT) and maximum tolerated dose (MTD) of sorafenib given orally, twice-daily (BID), for either 14 days or 21 days of a 28-day treatment cycle in patients with refractory acute leukemia. We also examined pharmacokinetics, pharmacodynamics, and tumor response. Correlative studies included the assessment of target modulation via plasma inhibitory assay (PIA) of phosphorylated-ERK (p-ERK) and phosphorylated-FLT3-ITD (p-FLT3) using methods previously developed for FLT3 targeted therapies.(16–17) Additionally, we investigated the metabolism of sorafenib during intermittent dosing to assess residual active compounds found after discontinuation of sorafenib. This examination focused on one metabolite, sorafenib N-oxide, which was thought to have biologic properties similar to the parent compound(18) and has been reported to represent the most common metabolite representing ~17% of circulating total drug.(19) Prior investigations of this metabolite have not studied its activity against mutated FLT3.
PATIENTS / METHODS
Patient selection
Patients over the age of 18 years with pathologic confirmation of relapsed or refractory AML, or ALL, were considered eligible. Standard end organ function and performance status criteria for Phase I investigations were used, including bilirubin <2.0 mg/dL, AST/ALT <5× upper limit of normal, and creatinine clearance >60mL/min/1.73m2. Patients were required to have a peripheral blast count <30,000/µL and no greater than a 50% increase in absolute blast count within the preceding week. Hydroxyurea was allowed up to 48 hours after starting sorafenib. Protocol and consent form were approved by the Johns Hopkins School of Medicine Institutional Review Board. All patients gave informed consent according to the Declaration of Helsinki.
Treatment plan
Patients were initiated on twice daily oral tablet dosing of sorafenib for either 14 or 21 consecutive days of treatment in each 28-day cycle and were managed in the outpatient clinic. Table 1 lists the planned dosing levels. All patients were evaluated for DLT for the purpose of determining the MTD.
Table 1.
Dose Escalation Schema
| Dose Escalation Schedule | ||
|---|---|---|
| Dose Level | Dose of Sorafenib | Pts |
| Level 1 | 400 mg PO BID × 14 days out of 28 days | 3 |
| Level 2 | 400 mg PO BID × 21 days out of 28 days | 9 |
| Level 3 | 600 mg PO BID × 14 days out of 28 days | 3 |
| Level 4 | 600 mg PO BID × 21 days out of 28 days | 0 |
Evaluation of response
Baseline evaluations, including an on-study bone marrow aspirate and biopsy, were conducted within 1 week prior to entry into the study. Bone marrow assessments were performed on or about cycle 1 day 8 (early treatment assessment), prior to initiation of cycle #2 and every two cycles thereafter. Clinical responses for AML and ALL were measured according to International Working Group definitions.(20)
Determination of DLT, MTD and Stopping Rules
All patients filled out medication and side effect/toxicity diaries that were reviewed weekly. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria, Version 3.0. Dose escalation continued until a DLT occurred in 2 patients out of the 3 patients in a cohort. When 1 DLT was observed in the first 3 patients during the first treatment cycle at a given dose level, 3 additional patients (up to 6 patients in total) were treated at that level. When DLTs occurred in the first 2 or 3 patients treated at a given dose level, no further dose escalation occurred. The dose immediately below the dose level that produced 2 DLTs was considered the maximum tolerated dose (MTD). Patients without evidence of disease progression or DLT secondary to therapy were allowed to continue on that dose for a total of 6 cycles. No intrapatient dose escalation was permitted.
Pharmacokinetic studies
Serial blood samples were collected in lithium heparin-containing tubes prior to and at 0.25, 0.5, 1, 2, 4, 6, and 8 hours after the administration of the first dose of sorafenib. Additional blood samples were collected prior to administration (Cmin) on day 2, 3, 8, 15 of continuous dosing and prior to the start of cycle 2. Samples were processed within 30 minutes of collection by centrifugation for 10 minutes at 1,500× g under refrigeration (~4°C). The resultant plasma was stored at −70°C until subsequent analysis for sorafenib and sorafenib N-oxide concentrations using a validated liquid chromatography/tandem mass spectrometry (LC/MS/MS) method.(21) Briefly, sorafenib and sorafenib N-oxide were extracted from plasma using acetonitrile precipitation. Separation of sorafenib, sorafenib N-oxide, and the internal standard, [2H3 15N] sorafenib, was achieved on a Waters X-Terra™ C18 (150 mm × 2.1 mm i.d., 3.5 µm) analytical column using a mobile phase consisting of acetonitrile/10 mM ammonium acetate (65:35, v/v) containing 0.1% formic acid and isocratic flow at 0.2 mL/min for 6 minutes. The analytes were monitored by tandem mass spectrometry with electrospray positive ionization. Linear calibration curves were generated over the range of 0.007–7.26 µg/mL(0.02–15.6µM) for sorafenib and 0.01–2.5 µg/mL(0.2–5.2µM) sorafenib N-oxide. Plasma samples that were diluted 1:10 (v/v) with pooled plasma were accurately quantitated. The accuracy and within- and between-day precisions were within the acceptance criteria for bioanalytical assays.(22)
Pharmacokinetic variables were calculated by standard noncompartmental methods using WinNonlin professional (version 5.2).(23) Cmax was the observed maximum concentration. Tmax was the time point at which the Cmax was observed. Cmin was the observed minimum concentration or pre-dose sampling. Cmin was determined to be valid if the interval between evening dose and the sample was within an allowed range of 12 ± 2 hours and obtained pre-dose. AUC(0–8h) was the observed AUC, calculated using a combination of linear and log trapezoidal rules. Pharmacokinetic parameters were summarized using descriptive statistics.
Correlative studies
Inhibitors
Sorafenib was provided by Bayer Pharmaceuticals through the Investigational Drug Branch, Cancer Treatment Evaluation Program, National Cancer Institute. Sorafenib N-oxide was obtained from Toronto Research Chemicals. Sorafenib and sorafenib N-oxide were dissolved in DMSO and stored at −80°C as 10 mM stock solutions. All samples in any given experiment contained identical concentrations of DMSO.
Cell Culture
All cell lines were cultured in RPMI/10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. The TF/ITD cell line was derived by transfecting TF-1 cells (growth factor dependent) with an expression vector containing the FLT3 coding sequence containing an ITD mutation from an AML patient, as described.(16) The resultant TF/ITD line is growth factor independent and expresses constitutively phosphorylated FLT3 and ERK 1/2.
Plasma Inhibitory Activity (PIA) Assay
The PIA assay was performed as described previously using the TF/ITD cell line as target cell line.(17) Phosphorylated ERK1/2(Cell Signaling, Danvers, MA) was assessed in whole cell lysates.
RESULTS
Sorafenib phase I patient characteristics
The patient characteristics are listed in Table 2 and were typical of patients in early phase leukemia trials with a median of 3 (range 1–6) prior therapies.
Table 2.
Patient Characteristics
| Characteristic n=15 | n (%) |
|---|---|
| Age, years: | |
| Median | 63 |
| Range | 37–85 |
| Male sex: | 8 (53) |
| ECOG performance status: | |
| 0 | 4 (27) |
| 1 | 9(60) |
| 2 | 2(13) |
| Disease type: | |
| AML | 12 (80) |
| AML/MDS | 2 |
| AML/CMML | 2 |
| ALL | 2 (13) |
| Bilineage | 1 (7) |
| Cytogenetics | |
| Normal | 6 (40) |
| complex | 6 (40) |
| trisomy 21 | 1 (7) |
| trisomy 13 | 1 (7) |
| 11q23 | 1 (7) |
| FLT3 | |
| Mut(ITD) | 2 (13) |
| Risk markers: | |
| refractory | 2 (13) |
| relapsed | 5 (33) |
| relapsed & refractory | 6 (40) |
| treatment-related | 2 (13) |
| No. of prior therapies | |
| Median | 3 |
| range | 1–6 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group; Mut(ITD), mutation(internal tandem duplication)
Sorafenib associated toxicities and Maximum Tolerated Dose
Toxicities (any grade) that were potentially related to therapy were seen in 23 out of 31 cycles (74%) (Table 3). Grade ≥3 toxicities were experienced in 17 (55%) cycles. Dose limiting toxicities at 600mg BID × 14 days were demonstrated in 2/3 patients and included elevated transaminases (n=1), and musculoskeletal back pain unimproved with standard measures (1). Based on the dose limiting toxicities described at 600mg BID ×14d, the dose of 400mg BID × 21d was determined to be the MTD and further dose escalation did not occur.
Table 3.
Grade 3 and 4 Toxicities
| Category | Grade | 400 mg bid × 14d (cycles=6) |
400 mg bid × 21d (cycles=21) |
600 mg bid × 14d (cycles=4) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Any | 3 | 3 (50) | 10 (48) | 4 (100) |
| 4 | 0 (0) | 3 (14) | 1 (25) | |
| Constitutional | ||||
| Edema (limb) | 3 | 0 (0) | 2 (10) | 0 (0) |
| Fatigue | 3 | 1 (17) | 4 (19) | 0 (0) |
| Muscle weakness | 3 | 0 (0) | 2 (10) | 0 (0) |
| Hepatic | ||||
| Alkaline phosphatase | 3 | 0 (0) | 2 (10) | 0 (0) |
| ALT/SGPT | 3 | 0 (0) | 2 (10) | 0 (0) |
| ALT/SGPT | 4 | 0 (0) | 0 (0) | 1 (25) |
| AST/SGOT | 4 | 0 (0) | 0 (0) | 1 (25) |
| Bilirubin | 4 | 0 (0) | 1 (5) | 1 (25) |
| Infectious | ||||
| Febrile neutropenia | 3 | 2 (33) | 3 (14) | 2 (50) |
| Fever (without neutropenia) | 3 | 0 (0) | 1 (5) | 0 (0) |
| Dermatologic | ||||
| Hand-foot skin reaction | 3 | 0 (0) | 1 (5) | 0 (0) |
| Metabolic | ||||
| Hypokalemia | 3 | 1 (17) | 2 (10) | 0 (0) |
| Hypophosphatemia | 3 | 0 (0) | 1 (5) | 0 (0) |
| Hypophosphatemia | 4 | 0 (0) | 1 (5) | 0 (0) |
| Pain | ||||
| Abdomen NOS | 3 | 0 (0) | 1 (5) | 0 (0) |
| Back | 3 | 0 (0) | 0 (0) | 1 (25) |
| Joint | 3 | 0 (0) | 1 (5) | 0 (0) |
n = number of events
Sorafenib and sorafenib N-oxide pharmacokinetics
All patients were evaluable for pharmacokinetic analysis (Table 4). Sorafenib exhibited a variable plasma concentration-time profile with a slow absorption phase followed by a long terminal elimination phase thus resulting in a relatively flat concentration-time profile as previously described.(24–27) Sorafenib N-oxide exhibited a similar profile with the maximum concentration occurring at the same time of after the Tmax for sorafenib.(19) Moderate inter-individual variability in pharmacokinetic parameters was noted, with a coefficient of variation for the sorafenib AUC(0–8h) and Cmax of up to 95% and 116 %, respectively. The variability was higher for the sorafenib N-oxide metabolite with the coefficient of variation for AUC(0–8h) and Cmax of up to 129% and 124 %, respectively. Sorafenib concentrations were detectable in 33% (1/3) of patients after 14 day break in treatment and in 80% (4/5) of patients after a 7 day break. Sorafenib N-oxide was only detectable in 40% (2/5) of patients after a 7 day break.
Table 4.
Summary of sorafenib and sorafenib N-oxide pharmacokinetic parameters (Supplementary Figure)
| Pharmacokinetic Parametersa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose Level |
Dose (mg) |
Tmax (h) |
Cmax (µg/mL) |
AUC0–8h (µg*h/mL) |
Day 2 Cmin (µg/mL) |
Day 3 Cmin (µg/mL) |
Day 8 Cmin (µg/mL) |
Day 15 Cmin (µg/mL) |
Css,min (µg/mL) |
Day 29 C (µg/mL) |
| Sorafenib | ||||||||||
| 1 | 400 | 2.3 (2.1–8.0, 3) |
1.93±2.25 (3) |
9.1±8.6 (3) |
0.49, 1.17 (2) |
1.79±1.60 (3) |
3.07±1.79 (3) |
2.06±1.50 (3) |
2.57±1.57 (3) |
0.19 (1) |
| 2 | 400 | 4.0 (2.0–4.1, 9) |
4.37±2.64 (9) |
20.8±12.9 (9) |
3.26±2.75 (5) |
5.08±3.22 (6) |
4.75±4.82 (4) |
7.13±6.88 (3) |
4.79±4. 90 (5) |
0.27±0.30 (4) |
| 1 and 2 | 400 | 3.1 (2.0–8.0, 12) |
3.76±2.68 (12) |
17.9±12.8 (12) |
2.57±2.55 (7) |
3.98±3.14 (9) |
4.03±3.68 (7) |
4.60±5.25 (6) |
3.95±3.97 (8) |
N.C. |
| 3 | 600 | 8.0 (4.0–8.0, 3) |
3.10±1.07 (3) |
12.6±4.1 (3) |
2.49 (1) |
N.R. | 7.79 (1) |
3.65 (1) |
5.72 (1) |
N.R. |
| Sorafenib N-oxide | ||||||||||
| 1 | 400 | 8.0 (1) |
0.04 (1) |
0.2 (1) |
0.01 (1) |
0.17 (1) |
0.18±0.17 (3) |
0.09±0.11 (3) |
0.14±0.14 (3) |
N.R. |
| 2 | 400 | 4.0 (1.1–8.0, 8) |
0.26±0.19 (8) |
1.3±1.0 (8) |
0.37±0.43 (4) |
0.560.58 (6) |
0.51±0.71 (4) |
0.54±0.69 (3) |
0.43±0.57 (5) |
0.09, 0.20 (2) |
| 1 and 2 | 400 | 4.0 (1.1–8.0, 9) |
0.23±0.19 (9) |
1.2±1.0 (9) |
0.30±0.41 (5) |
0.50±0.55 (7) |
0.37±0.54 (7) |
0.32±0.50 (6) |
0.32±0.46 (8) |
N.C. |
| 3 | 600 | 8.0 (4.0–8.0, 3) |
0.21±0.26 (3) |
0.8±1.0 (3) |
0.23 (1) |
N.R. | 0.53 (1) |
0.47 (1) |
0.50 (1) |
N.R. |
Values are reported as the mean ± standard deviation (n). For Tmax, values are reported as median (range, n). When n≤2, individual values are reported. One µg/mL of sorafenib or sorafenib N-oxide is equivalent to 2.15 and 2.08 µM, respectively.
Css,min is an average of day 8 and 15 Cmin (or either day if a sample was not obtained in a patient)
For Day 29 concentrations, the average for dose level 1 and 2 is not calculated (N.C.) since the dosing schedule is different
Abbreviations: AUC, area under the concentration-time curve; Cmax, maximal plasma concentration; Css,min, minimal plasma concentration; Css,min, minimal plasma concentration at steady-state (average of day 8 and 15 Cmin); N.R., not reported; Tmax, time of the maximal plasma concentration
FLT3 and ERK inhibition
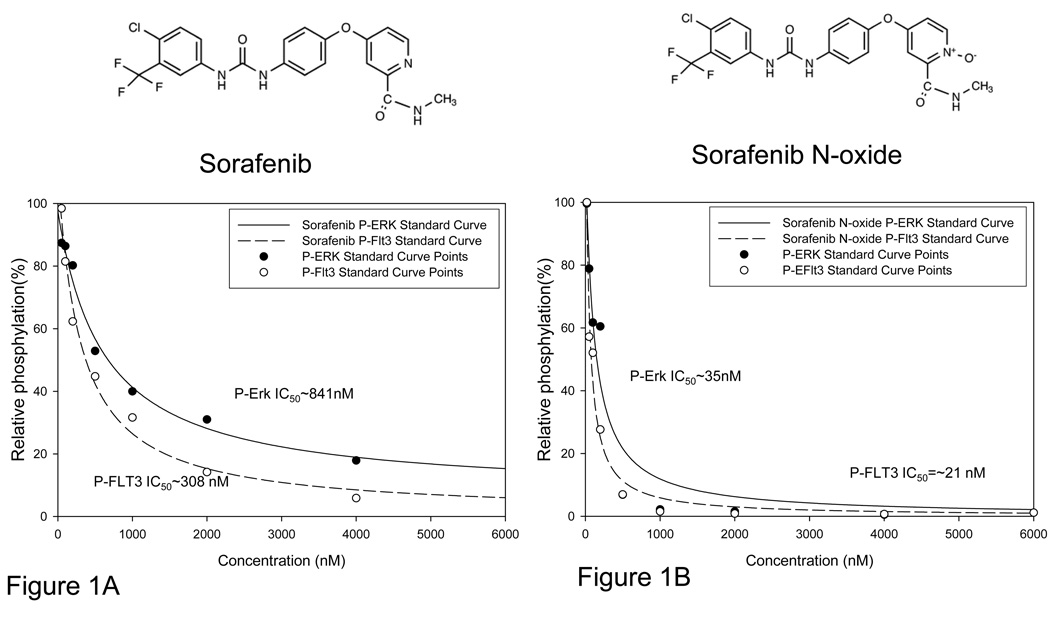
We prepared dose response curves assessing inhibitory potency of sorafenib on FLT-3 ITD autophosphorylation and ERK phosphorylation using TF-ITD cells in RPMI with 10% FBS. Immunoblot analysis revealed an IC50 of 1.2 nM for inhibition of phosphorylated FLT3 in media (data not shown). The IC50 for inhibition of phosphorylated ERK was similar (0.91nM, data not shown). Previous work with tyrosine kinase inhibitors has shown most inhibitors in development are highly protein bound.(16–17, 28) We therefore determined the IC50 values of sorafenib for inhibition of phosphorylation of FLT3 and ERK using TF-ITD cells, substituting normal human plasma for culture medium. In plasma, the IC50 of sorafenib for P-FLT3 inhibition shifts to approximately 308nM (Figure 1A). ERK signaling in plasma was more resilient with an IC50 rising to 841nM (Figure 1A).
Figure 1. Sorafenib N-oxide is a potent FLT3 inhibitor.
A. Standard curve generated as described previously(17), from western blot of TF-ITD cells in plasma exposed for one hour to increasing concentrations of sorafenib. B. Standard curve generated with sorafenib N-oxide in plasma.
FLT3 inhibition at trough time points
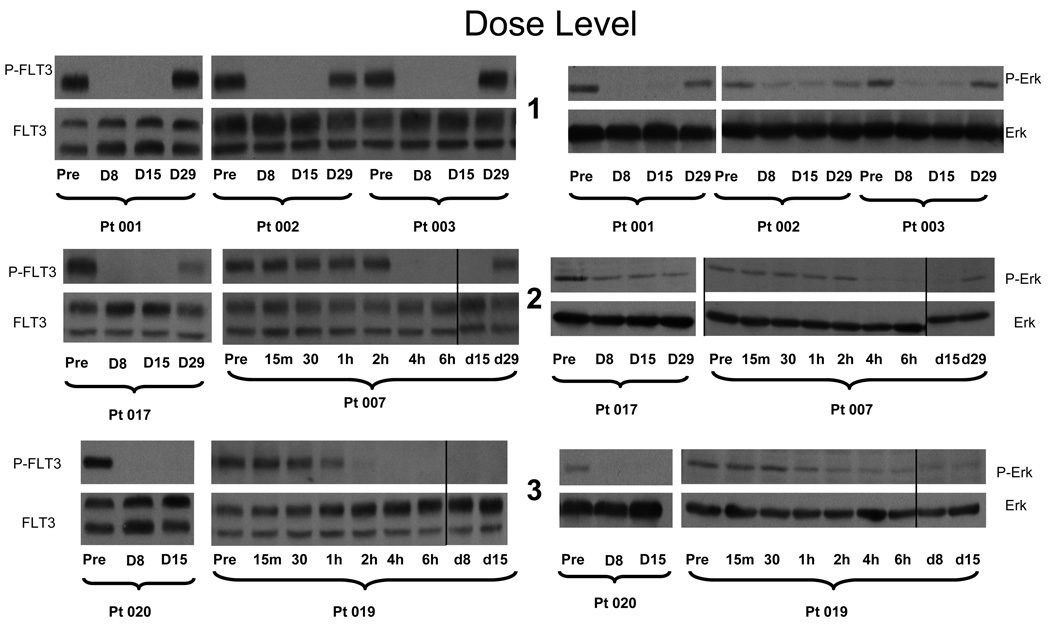
Pharmacodynamic analysis of FLT3 and ERK targeting was performed on patients completing one cycle of therapy (n=9). Direct analysis of leukemic phosphoprotein status was difficult as sufficient circulating leukemia cells were not available from most patients at each correlative time point due to low white blood count. We examined serial plasma specimens collected pretreatment on day 1, day 8, day 15, and day 29 in an inhibitory assay (PIA) using a FLT-3 mutant cell line(TF-ITD) to assess target inhibition potential in an ex vivo setting. PIA data takes into account protein binding, active metabolite levels, and cytokine levels which may influence target sensitivity to inhibition. Each of the patients studied (n=9) achieved complete inhibition of FLT3-ITD phosphorylation in the PIA on trough samples drawn while on therapy (Figure 2) suggesting inhibition of FLT3-ITD occurs below 400mg BID.
Figure 2. PIA results for patients receiving sorafenib.
Plasma was isolated from whole blood samples obtained from patients receiving increasing doses of sorafenib on the clinical trial. Samples were obtained immediately prior to dosing on Days 1, 8, and 15 and 29 of each cycle. Dose levels 1, 2, and 3 correspond to total daily doses of 800, 800, and 1200 mg, respectively (see Table 1). Shown are the results from representative patients on successively higher dose levels using the PIA assay on TF-ITD cells for phosphorylated FLT3 (left) and ERK (right). For dose level 2 and 3, extra time points on Day 1 show complete silencing of FLT3 activity within 2 hours of the first dose with maintenance of this inhibition throughout the treatment cycle. Vertical lines have been inserted to indicate a repositioned gel lane.
Sorafenib inhibits FLT3 partially through a metabolite
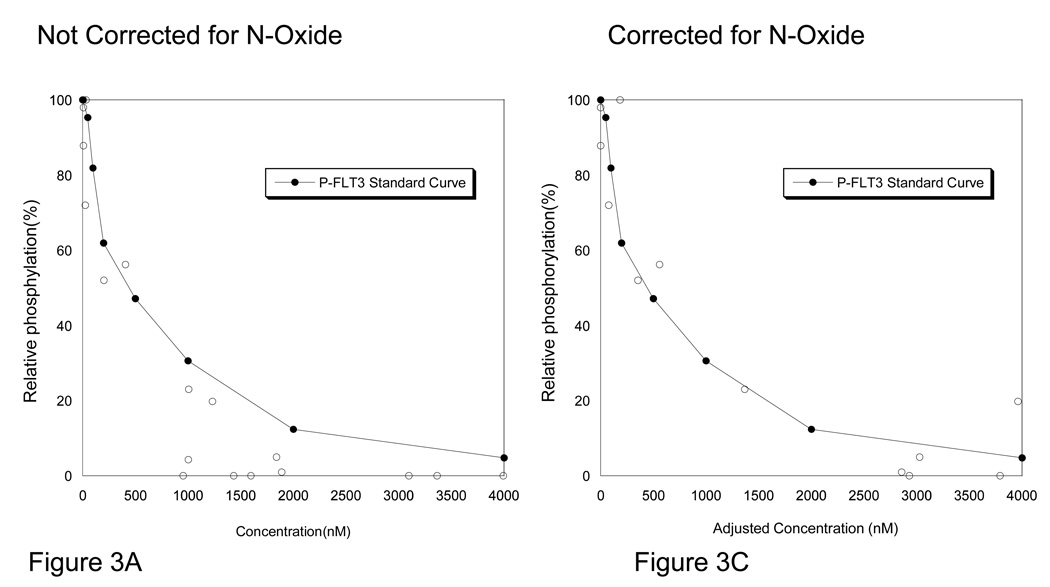
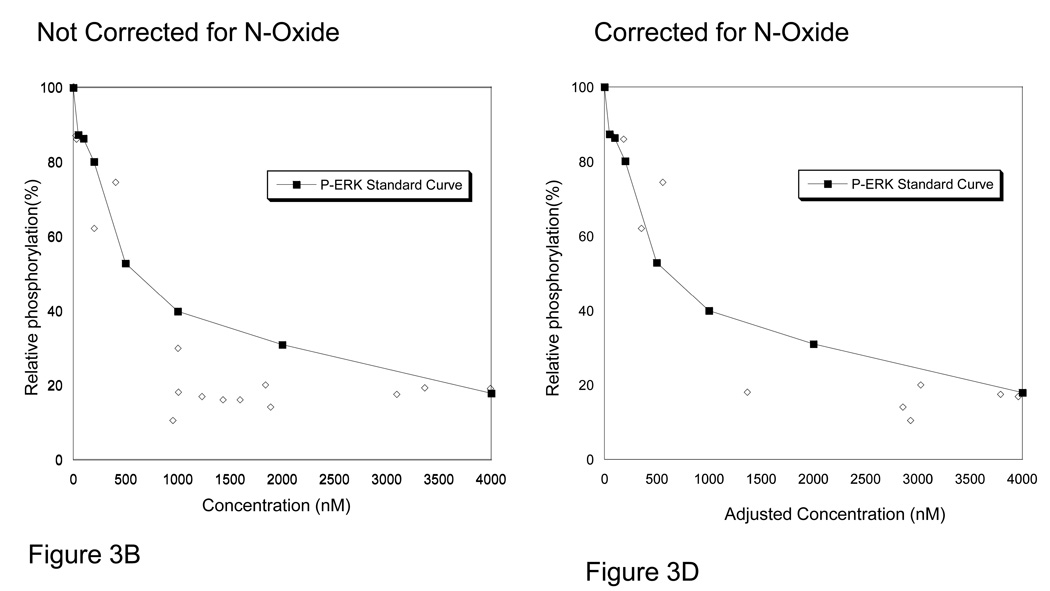
To better understand the activity of sorafenib against FLT3 and ERK, we plotted the results of the PIA assays with the pharmacokinetic values obtained at those time points (Figure 3A & 3B). We then overlaid the standard curves for sorafenib suppression of FLT3-ITD in normal human plasma. Interestingly, we found that nearly all PIA assessments appeared to have greater inhibitory activity than predicted by the standard curves with sorafenib alone. With the possibility of a drug metabolite contributing to the biologic activity of sorafenib on FLT3, we assayed serial plasma samples for sorafenib’s major metabolite, sorafenib N-oxide. Sorafenib N-oxide on average was found at levels approximately 12% of sorafenib levels, (median=8%, range 2%–44%, standard deviation 12%) with marked inter-patient variability. We found the N-oxide metabolite to be more potent than sorafenib(~14.59 fold) at inhibiting autophosphorylation of FLT3-ITD and ERK in human plasma when assessed by western blotting in cell based assay using TF/ITD cells (Figure 1B). To examine the additive effect of the presence of the N-oxide metabolite we then replotted the PIA/PK data to account for the activity of the N-oxide metabolite of sorafenib by including the sum of the parent and metabolite PK values corrected for the increased potency (14.59 fold) of sorafenib N-oxide. This created an “adjusted sorafenib concentration” value which more closely approximated the sorafenib standard curve for P-FLT3 inhibition and P-ERK inhibition (Figure 3C & 3D) for samples in the inhibitory range.
Figure 3. PIA results compared with standard curve for Sorafenib.
A. Plasma was collected from patients receiving sorafenib prior to dosing on day 1, 8, 15, and 29. The plasma samples underwent conventional pharmacokinetic analysis of concentrations of sorafenib and sorafenib N-oxide. In parallel, plasma from the same time points were examined in PIA assays for assessment of FLT3 and ERK inhibition potential. On the × axis the results of the pharmacokinetics are plotted for sorafenib. On the y axis, the degree of FLT3 inhibition, as assessed by PIA, is plotted as a percent of control. This data is overlaid by the standard curve for sorafenib in plasma as generated in TF-ITD cells(solid line, see Figure 1A). B. PIA results, as described in panel A, of P-ERK compared to standard curve for sorafenib inhibition of P-ERK(solid line). C. The PIA assay values for FLT3 inhibition were replotted after adjusting the “effective” sorafenib concentrations by adding the amount sorafenib N-oxide multiplied by its potency factor using the equation: Adjusted sorafenib concentration=Sorafenib + (Sorafenib N-Oxide*14.59). D. The same experimental data generated from analysis of P-Erk and corrected for sorafenib N-oxide as described in panel C.
FLT3 inhibitory activity significant and extends up to 7 days post dosing
Plasma samples at trough obtained during the trial resulted in complete FLT3 silencing (Figure 2) and the average drug concentrations (Table 4) were well above the FLT3 predicted inhibitory range (Figure 1). Pharmacodynamic examination of FLT3 inhibitory activity in the PIA also revealed 4/5 samples with inhibitory activity at day 29, seven days after the last dose (Figure 2) and all four patients received more than one cycle of therapy. In two samples the primary FLT3 inhibitory compound at Day 29 when adjusted for potency was sorafenib N-oxide (pt016 and pt 17) with levels of sorafenib N-oxide of 0.20 and 0.09 µg/mL (0.42 and 0.19µM) respectively.
Clinical activity of sorafenib
The best response demonstrated in 11/15 of patients on this trial was stable disease. The longest duration of SD was 3 months, experienced by 2 of the patients (13%). While no patients met the criteria for complete or partial response, bone marrow blast counts decreased in 6/15 (40%) patients after one cycle by an average of 18%. Table 5 represents pre and post treatment for all patients on trial. Five of the 9 patients treated on a three week schedule of drug showed a decrease in their marrow blasts while only 1 of five evaluable patients treated on either of the 2 week schedules showed a decrease. Interestingly, this patient was the only one on the 2 week dosing schedules with a FLT3-ITD mutation.
Table 5.
Treatment Effect and Duration (Supplementary Figure)
| Pt# | Disease | FLT3 | Marrow pre-tx % blasts |
Best marrow post–tx % blasts |
Peripheral blood blast % Day 1 |
Peripheral blood blast % Day 15 |
Treatment duration |
Indication for discontinuation |
|---|---|---|---|---|---|---|---|---|
| 400 mg bid × 14d | ||||||||
| 001 | AML | WT | 20 | 20 | 9 | 21 | 2 Cycles | PD |
| 002 | AML | WT | 6 | 12 | 0 | 0 | 2 Cycles | PD |
| 003 | AML | WT | 40 | 81 | 4 | 25 | 1+Cycle | PD |
| 600 mg bid × 14d | ||||||||
| 018 | ALL | WT | 100 | NA | 36 | NA | 3 Days | Neutropenic Sepsis |
| 019 | AML | WT | 44 | 91 | 0 | 0 | 1+Cycle | DLT(Hepatic toxicity) |
| 020 | AML | Mut (ITD) | 10(50% cellular) | 7(60–70% cellular) | 0 | 0 | 1 Cycle | DLT(back pain) |
| 400 mg bid × 21d | ||||||||
| 004 | Biphenotypic | WT | 92 | 93 | 81 | 36(day 8) | 8 Days | DLT Hepatic toxicity |
| 007 | AML | NA | 30 | 11 | 8 | 6 | 4 Cycles | PD |
| 008 | AML | WT | 97 | NA | 62 | NA | 1 Day | Head trauma/ICH |
| 009 | AML | WT | 13 | 3 | 0 | 0 | 4 Cycles | PD |
| 011 | AML | WT | 91 | 70 | 0 | 0 | 3 Cycles | DLT(Fatigue) |
| 013 | AML | Mut (ITD) | 35 | 13 | 33 | 5 | 2 Cycles | PD |
| 014 | AML | WT | 57 | NA | 26 | 88 | 7 Days | PD |
| 016 | AML | WT | 5 | 0 | 0 | 0 | 2 Cycles | Withdrew Consent |
| 017 | ALL | NA(t4;11) | 89 | 45 | 13 | 0 | 3 Cycles | Withdrew Consent |
Abbreviations: pt, patient; WT, wild-type; Mut (ITD), internal tandem duplication; PD, Progressive disease; DLT, Dose Limiting Toxicity, NA, Not Available.
One of the two ALL patients cleared their peripheral blood blasts while on sorafenib and demonstrated progressive improvement in bone marrow blasts from 89% pretreatement to 47% after cycle 1 and 45% after cycle 2. Interestingly, the patient had a MLL rearranged ALL with translocation (4;11). This translocation has been associated with over expression of WT FLT3 and in vitro sensitivity to FLT3 inhibitors.(29–30)
DISCUSSION
Sorafenib can inhibit numerous potential pathways in acute leukemia, but despite such broad biologic activity, we found relatively limited single-agent clinical activity. The clinical activity in our study population was limited to the observation of reduced bone marrow blasts in 56% (5/9) of patients treated at 400mg BID × 21 days and 1 patient with FLT3-ITD AML treated on the 14 day schedule. Dose escalation beyond 400mg BID was limited due to grade 3 and 4 toxicities. Similar clinical activity and toxicity has been reported with single agent sorafenib given continuously where 11 out of 20 patients were found to have a >50% reduction in peripheral blood blasts and 9 of 11 patients with FLT3-ITD AML had measured responses, including one morphologic marrow CR.(31) One potential explanation for the lack of improved tolerance to our intermittent dosing was the demonstration of prolonged biologic activity after therapy completion.
Interestingly in AML, one target of great importance is FLT3-ITD, and in our study, sorafenib demonstrated suppression of FLT3-ITD at dosing below the MTD. Prior studies targeting FLT3-ITD with lestaurtinib documented the association of sustained complete or near complete inhibition of FLT3-ITD with clinical response;(32) but unlike the use of lestaurtinib, sorafenib uniformly suppressed FLT3-ITD in all samples assessed by PIA. This finding was somewhat surprising based on sorafenib pharmacokinetics and preclinical studies of sorafenib alone; however our correlative studies suggest the sorafenib N-oxide metabolite contributes significantly to in vivo FLT3-ITD inhibition. This inhibitory activity persisted up to seven days after the completion of drug dosing in several patients. This observation is clinically important with preclinical modeling of FLT3 inhibitors in combination with cytarabine and daunorubicin demonstrating antagonism when the FLT3 inhibitor was used prior to the conventional therapy.(33) There may be a need for a wash out period prior to the use of cell cycle dependent salvage or even consolidative treatments with the concomitant use of sorafenib.
The targeting of signal transduction pathways therapeutically has yet to be broadly successful. Even attempts to target a pathway thought to be as tissue specific as mutated FLT3 in AML, has proven to be more complicated than many first appreciated. For example, the individual type of mutation is certainly critical as preclinical studies suggest that patients with a D835Y mutation in FLT3 are unlikely to be sensitive to some FLT3 inhibitors such as sorafenib.(11) Also, there is evidence that allelic burden of FLT3-ITD is important for ex vivo sensitivity of primary leukemia blasts to FLT3 inhibition, and perhaps those with high allelic ratio may be a subset that benefits the most from FLT3 targeted therapy.(34–35) Additionally, the clinical activity of targeted agents can be influenced by protein binding and drug-drug interactions.(32–33) Our study, like others has demonstrated the activity of metabolites of the primary agent, may in fact, play a major role in an agent’s biologic activity.(16) Finally, the disease state must also be factored into the equation as targeting mutated pathways at the time of minimal residual disease such as post induction, or following a stem cell transplant might have the best opportunity to suppress the leukemic clone long term.(13) Taken together, future clinical studies of targeted agents must include biologic correlatives if we hope to fulfill the hope that the new agents can impact clinical outcomes in a more discriminate way.
Acknowledgments
Carol Hartke, Ping He, Aleksandr Mnatsakanyan, Yelena Zabelina, and Linping Xu for their technical assistance; and Susan Davidson for quality assurance of the pharmacokinetic data.
This work was supported by National Institutes of Health grants P30CA006973, U01CA70095, UL1 RR025005, NCI Leukemia SPORE P50 CA100632-06, R01 CA128864 and the American Society of Clinical Oncology (ML). ML is a Clinical Scholar of the Leukemia and Lymphoma Society.
Footnotes
Study registered at ClinicalTrial.gov as NCT00131989
Contribution: K.W.P. designed and performed correlative assays, analyzed correlative assays, analyzed clinical trial results and wrote the manuscript. E.C. helped in analyzing correlative assays and writing the manuscript. M. L. helped to design and interpret correlative studies and contributed patients to the study J.E.K., S.D.G., M.M., each contributed to the study design, contributed patients to the study, and helped edit the manuscript. M.A.C. and J.J.W. contributed to the study design and helped edit the manuscript. A.S. processed clinical trial specimens and helped to conduct laboratory experiments. M.A.R., M.Z., and S.D.B. designed conducted and interpreted the pharmacokinetic studies. B.D.S. developed the study design and wrote the protocol, contributed patients to the study, served as the protocol PI, analyzed the clinical trials results, contributed patients to the study, and helped edit the manuscript.
Conflict of interests: There were no conflicts of interest to report.
References
- 1.Larson S, Stock W. Progress in the treatment of adults with acute lymphoblastic leukemia. Curr Opin Hematol. 2008 Jul;15(4):400–407. doi: 10.1097/MOH.0b013e3283034697. [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005 Aug 15;106(4):1154–1163. doi: 10.1182/blood-2005-01-0178. 2005. [DOI] [PubMed] [Google Scholar]
- 3.Ricciardi MR, McQueen T, Chism D, Milella M, Estey E, Kaldjian E, et al. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia. 2005 Sep;19(9):1543–1549. doi: 10.1038/sj.leu.2403859. [DOI] [PubMed] [Google Scholar]
- 4.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006 Oct 1;108(7):2358–2365. doi: 10.1182/blood-2006-02-003475. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilger RA, Scheulen ME, Strumberg D. The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie. 2002 Dec;25(6):511–518. doi: 10.1159/000068621. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004 Oct 1;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 7.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006 Dec 15;12(24):7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006 Sep 10;24(26):4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005 Oct 20;24(46):6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 10.Meng XW, Lee SH, Dai H, Loegering D, Yu C, Flatten K, et al. Mcl-1 as a buffer for proapoptotic Bcl-2 family members during TRAIL-induced apoptosis: a mechanistic basis for sorafenib (Bay 43-9006)-induced TRAIL sensitization. J Biol Chem. 2007 Oct 12;282(41):29831–29846. doi: 10.1074/jbc.M706110200. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008 Feb 6;100(3):184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 12.Tong FK, Chow S, Hedley D. Pharmacodynamic monitoring of BAY 43-9006 (Sorafenib) in phase I clinical trials involving solid tumor and AML/MDS patients, using flow cytometry to monitor activation of the ERK pathway in peripheral blood cells. Cytometry B Clin Cytom. 2006 May;70(3):107–114. doi: 10.1002/cyto.b.20092. [DOI] [PubMed] [Google Scholar]
- 13.Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009 Jun 25;113(26):6567–6571. doi: 10.1182/blood-2009-03-208298. 2009. [DOI] [PubMed] [Google Scholar]
- 14.Safaian NN, Czibere A, Bruns I, Fenk R, Reinecke P, Dienst A, et al. Sorafenib (Nexavar®) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD+ acute myeloid leukemia. Leukemia Research. 2009;33(2):348–350. doi: 10.1016/j.leukres.2008.04.017. 2009/2. [DOI] [PubMed] [Google Scholar]
- 15.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007 Apr;12(4):426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 16.Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006 Nov 15;108(10):3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratz KW, Cortes J, Roboz GJ, Rao N, Arowojolu O, Stine A, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009 Apr 23;113(17):3938–3946. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rini BI. Sorafenib. Expert Opinion on Pharmacotherapy. 2006;7(4):453–461. doi: 10.1517/14656566.7.4.453. [DOI] [PubMed] [Google Scholar]
- 19.Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006 May;57(5):685–692. doi: 10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003 Dec 15;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Rudek MA, He P, Hafner FT, Radtke M, Wright JJ, et al. A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Feb 1;846(1–2):1–7. doi: 10.1016/j.jchromb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, et al. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J Drug Metab Pharmacokinet. 1991 Oct-Dec;16(4):249–255. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- 23.Gibaldi M, Perrier D. Drug elimination and apparent volume of distribution in multicompartment systems. J Pharm Sci. 1972 Jun;61(6):952–954. doi: 10.1002/jps.2600610630. [DOI] [PubMed] [Google Scholar]
- 24.Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005 May 23;92(10):1855–1861. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005 Aug 1;11(15):5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 26.Moore M, Hirte HW, Siu L, Oza A, Hotte SJ, Petrenciuc O, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005 Oct;16(10):1688–1694. doi: 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 27.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005 Feb 10;23(5):965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 28.Kretz O, Weiss HM, Schumacher MM, Gross G. In vitro blood distribution and plasma protein binding of the tyrosine kinase inhibitor imatinib and its active metabolite, CGP74588, in rat, mouse, dog, monkey, healthy humans and patients with acute lymphatic leukaemia. Br J Clin Pharmacol. 2004 Aug;58(2):212–216. doi: 10.1111/j.1365-2125.2004.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown P, Levis M, Shurtleff S, Campana D, Downing J, Small D. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood. 2005 Jan 15;105(2):812–820. doi: 10.1182/blood-2004-06-2498. [DOI] [PubMed] [Google Scholar]
- 30.Stam RW, den Boer ML, Schneider P, Nollau P, Horstmann M, Beverloo HB, et al. Targeting FLT3 in primary MLL-gene-rearranged infant acute lymphoblastic leukemia. Blood. 2005 Oct 1;106(7):2484–2490. doi: 10.1182/blood-2004-09-3667. 2005. [DOI] [PubMed] [Google Scholar]
- 31.Delmonte J, Jr, Kantarjian HM, Andreeff M, Faderl S, Wright JJ, Zhang W, et al. Update of a Phase I Study of Sorafenib in Patients with Refractory/Relapsed Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndrome. ASH Annual Meeting Abstracts. 2007 Nov 16;110(11):893. 2007. [Google Scholar]
- 32.Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004 May 15;103(10):3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 33.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004 Aug 15;104(4):1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 34.Brown P, Meshinchi S, Levis M, Alonzo TA, Gerbing R, Lange B, et al. Pediatric AML primary samples with FLT3/ITD mutations are preferentially killed by FLT3 inhibition. Blood. 2004 Sep 15;104(6):1841–1849. doi: 10.1182/blood-2004-03-1034. [DOI] [PubMed] [Google Scholar]
- 35.Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115(7):1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]