Abstract
Single agent bortezomib results in response rates of 51% in patients with newly diagnosed multiple myeloma (MM) and is touted to be especially effective in high-risk disease. We are the first to prospectively explore single agent bortezomib as primary therapy (response rate, maintenance and reinduction) without consolidative autologous stem cell transplant in a cohort selected to have high-risk MM. Patients received 8-cycles of induction followed by maintenance bortezomib every other week indefinitely. Patients relapsing on maintenance had full induction schedule resumed. On an intention to treat basis the response rate (>=PR) was 48%. Among 7 patients, who progressed on maintenance bortezomib and received reinduction, two responded. With a median follow-up of 48.2 months, 1- and 2-year OS probabilities were 88% (95%CI, 74–95%) and 76% (95%CI, 60–86%), respectively. Median PFS was 7.9 months (95%CI, 5.8–12.0). Twenty-three and 34 patients had >=grade 3 hematologic and non-hematologic toxicity, respectively with treatment emergent neuropathy in: 7%, motor grade 1–2; 56%, sensory grade 1–2 and 2%, grade 3; and 14%, neuropathic pain grade 1–2 in and 2%, grade 3. In high-risk patients, upfront bortezomib results in response rates comparable to those reported for unselected cohorts, but single agent bortezomib is not sufficient as primary therapy.
Keywords: Therapy, Prognosis, Clinical trial, myeloma, proteasome inhibitors
Introduction
Multiple myeloma is a malignant plasma cell disorder that can result in highly disparate outcomes based on heterogeneous biology.1 The introduction of high dose chemotherapy with peripheral blood stem cell transplant and of novel agents such as thalidomide, bortezomib, and lenalidomide has impacted the natural history of the disease substantially over the past decade.2, 3 However, it is well accepted that patients with high-risk disease with high B2M, high proliferative rates and cytogenetic abnormalities of their plasma cells fare poorly with alkylator based therapy, be it standard dose or dose intensive with hematopoietic stem cell support.4
It is unclear whether the negative prognostic impact will be abrogated by the application of novel therapies. Preliminary data generated by post-hoc analyses would suggest that the negative impact of deletion 13 is abrogated by the proteosome inhibitor, bortezomib.5, 6 With this in mind, we designed a trial to prospectively evaluate the effect upfront bortezomib has upon response rate, 1- and 2-year progression free survival and overall survival among patients with high-risk multiple myeloma.
Methods
Eligibility
Patients were eligible to enter onto the study if they had previously untreated symptomatic multiple myeloma that was ‘high-risk,’ as defined as any of the following: B2M greater than or equal to 5.5 mcg/ml; a bone marrow PCLI of 1% or greater; or deletion 13q by metaphase cytogenetics. Patients were required to have measurable disease defined as serum monoclonal protein more than 1.0 g/dL and/or urine monoclonal protein more than 200 mg/24 h. Patients also needed to have hemoglobin more than 7 g/dL, platelet count more than 50,000 cells/uL, absolute neutrophil count more than 1,000 cells/mcL, creatinine less than 3 mg/dL, bilirubin less than or equal to 1.5 mg/dL, and ALT and AST less than or equal to 2.5 times the upper limit of normal. No prior systemic therapy, with the exception of bisphosphonates, was permitted. Also excluded were patients with grade 2 or higher peripheral neuropathy, active infection, current or prior deep vein thrombosis, and Eastern Cooperative Oncology Group (ECOG) performance score of 3 or 4. The study was approved by the National Institutes of Health central institutional review board as well as by institutional review boards in the participating institutions.
The trial was activated in January 2004 and 44 patients enrolled from March 2004–March 2005. The final analysis was performed July 2, 2009. Accrual was scattered over 22 ECOG institutions. There was one patient ineligible due to missing baseline labs. One patient expired before starting treatment. The analysis dataset includes 43 patients for the toxicity evaluation and 42 eligible and treated patients for all other analyses. Two patients were unevaluable for response for the following reasons: 1) participant 20001 was treated with simultaneous dexamethasone (protocol violation); and 2) the disease status of patient 20042 was assessable by urinary M-protein only, but urinary M-protein was not measured after baseline measurement. Although a third patient (participant 20035) died before disease measurement could be done, he was included in the analysis dataset since his death may have been related to treatment rather than to protocol violation.
Treatment schedule
The treatment schema is as shown in Figure 1. Eligible patient were treated for 8 cycles of bortezomib 1.3 mg/m2 days 1, 4, 8, and 11 every 21 days until toxicity or progression as induction. Patients were required to receive a minimum of 2 cycles before removal from study for progressive disease. Patients had the option to collect stem cells after cycle 4. The number of cycles was to be reduced if the patient achieved complete response before cycle 6, i.e. patients were to receive 2 cycles beyond CR and then proceed to maintenance.
Figure 1.
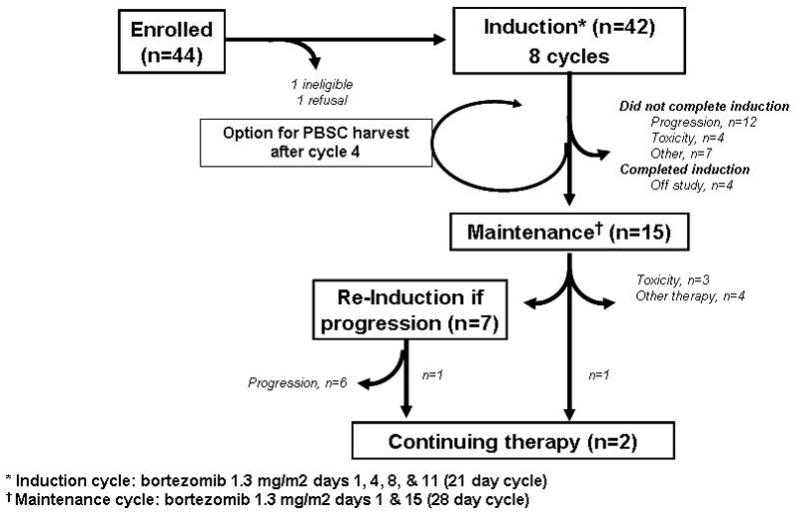
Treatment schema.
Italicized numbers represent actual numbers of patients progressing through each step.
* Induction cycle: bortezomib 1.3 mg/m2 days 1, 4, 8, & 11 (21 day cycle)
† Maintenance cycle: bortezomib 1.3 mg/m2 days 1 & 15 (28 day cycle)
Maintenance therapy was for patients who completed induction without progression. The maintenance treatment schedule was 1.3 mg/m2 days 1 and 15 every 28 days until progression or excessive toxicity.
Response and Toxicity Criteria
The Blade response criteria7 were used with the additional of the very good partial response category, which included a greater than or equal to 90 percent reduction of myeloma protein from the serum, a urinary M-spike of less than or equal to 100 mg/day.8 To satisfy the primary endpoint of this study, a partial response or better was required. All responses needed to be confirmed at least 6 weeks apart by two consecutive determinations. The National Cancer Institute Common Toxicity Criteria, version 2, was used to grade adverse effects.
Laboratory Correlates
The 24 patients who supplied research specimens had FISH performed to discover the following baseline abnormalities: t(4;14)(p16.3;q32), t(14;16)(q32;q23), or deletion 17p13. The PCLI and the FISH studies were done centrally as previously described.9, 10
Statistical Design and Analysis
The primary endpoint of this study was induction response rate. A response rate to bortezomib of 50% was considered to be promising in this population. We assumed an ineligibility rate of about 10%, requiring 44 patients to be accrued to the trial in order to have 39 eligible patients. This design had 90% power to detect a response rate of 50% with a type I error rate of 9% given a 30% response rate. Our hypothesis was that if we observed fewer than 16 responses among the 39 eligible patients, we would declare this treatment ineffective. Secondary endpoints of this trial included evaluation of progression-free survival (PFS), overall survival (OS) and response rates on maintenance as well as reinduction. Further, we set out to explore a possible differential outcome by defining risk characteristic. PFS and OS were estimated using the method of Kaplan and Meier.
For the PFS estimates, patients were counted as an event if they progressed or died without documented progression within 3 months of their last disease evaluation. Patients were censored at the date of their last disease evaluation if they were alive without progression. Patients were censored at the start of non-protocol therapy if that occurred before disease progression or the last disease evaluation. Patients without disease assessments were censored at the time of registration. PFS was estimated from the date of registration and from the start of maintenance and reinduction. OS estimates did not censor for non-protocol therapy.
Results
Baseline characteristics
The baseline characteristics of the 42 eligible and treated patients are shown in Table 1. Fifty percent were male, and the median age was 63 years, range 41–81. All patients had poor prognosis disease with at least one of the negative factors: B2M greater than or equal to 5.5 ug/mL, 76% (32/42); PCLI of 1% or higher, 40% (17/42); and deletion 13q 14% (6/42). Additional risk factors were examined in 24 patients: t(4:14) was present in 4 and deletion 17p in 2. None of the 24 patients tested harbored t(14;16). Since not every patient had PCLI and cytogenetics performed, these are likely underestimates.
Table 1.
Patient Characteristics
| Characteristic | Number evaluated or evaluable | Median (range) |
|---|---|---|
| Age, years | 42 | 63 (44–81) |
| Gender, male (%) | 42 | 21 (50) |
| Durie Salmon II/III (%) | 37 | 16 (39)/21 (51) |
| Durie Salmon A/B (%) | 41 | 34 (83)/7 (17) |
| Isotype: IgG/IgA/Light chain only (%) | 38 | 25 (60)/7 (17)/6 (14) |
| Hemoglobin, g/dL | 42 | 9.7 (7.6–15.3) |
| Creatinine, mg/dL | 42 | 1.2 (0.6–3.8) |
| Calcium, mg/dL | 42 | 8.8 (6.63–15.5) |
| Serum M-spike, g/dL | 37 | 4.4 (1.4–8.8) |
| Urine M-spike, g/day | 20 | 389 (10–4713) |
| Bone marrow plasmacytosis | 50 (1,100) | |
| Beta-2 microglobulin, mg/mL (range) | 42 | 7.7 (2.5–31.9) |
| B2M ≥ 5.5 | 32 (76) | |
| PCLI, % (range)§ | 33 | 1.6 (0 – 5) |
| PCLI ≥ 1 | 17 (40) | |
| Deletion 13q-*(%) | 41 | 6 (14) |
| Translocation (4;14)(%) | 24 | 4 (10) |
| Translocation (14;16)(%) | 24 | 0 (0) |
| Deletion 17p (%) | 24 | 2 (5) |
By metaphase cytogenetics
Response
The overall induction response rate to single agent bortezomib in previously untreated high-risk myeloma patients was 48% partial response or better (Table 2). An additional 5% of patients achieved a minimal response. The median time to response was 1.3 months. If unevaluable patients are excluded (n=3), the respective induction response rates were 51% and 5%. For those who responded during induction, response rates were held during maintenance and there were no up-grades. The two patients that started maintenance in NR/SD experienced progression on maintenance therapy. Response rates did not differ with the various risk factors (data not shown). Among the 4 patients recognized as having t(4;14), 1 was unevaluable, 1 experienced PD and 2 responded (1 VGPR and 1 PR).
Table 2.
Response Rates by Phase
| Induction N=42 | Maintenance N=15 | Reinduction N=7 | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Complete response | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Very good partial response | 4 (9.5) | 3 (20.0) | 0 (0.0) |
| Partial response | 16 (38.1) | 7 (46.7) | 2 (28.6) |
| Minimum response | 2 (4.8) | 1 (6.7) | 0 (0.0) |
| No response/stable disease | 8 (19.1) | 0 (0.0) | 3 (42.9) |
| Progressive disease | 9 (21.4) | 3 (20.0) | 1 (14.3) |
| Unevaluable/missing | 3 (7.1) | 1 (6.7) | 1 (14.3) |
Among the 7 patients, who progressed on maintenance bortezomib and went on to reinduction, two responded. One patient experienced progression 6.6 months from their second confirmed PR date (time to response was 0.7 months after starting reinduction). The other patient is still on treatment with PR status held for 17.6 months (time to response was 3.4 months after starting reinduction). The distribution of risk factors among this group of 7 was del 13q in 1, high PCLI in 4, and high B2M in 3.
Survival/Retention
With a median follow-up of 48.2 months, there have been 19 deaths (Figure 2A), including 1 possible treatment related death (heart block). The median OS has not been reached. 1- and 2-year OS probabilities based on Kaplan-Meier estimates were 88% (95%CI, 74 – 95%) and 76% (95%CI, 60–86%). Among the 42 eligible and treated patients, 28 (67%) had events. The median, 1- and 2-year PFS probabilities were 7.9 months (5.8–12.0 months), 36% (95%CI 19–54%) and 16% (5–33%), respectively (Figure 2B). There were overlapping confidence intervals for outcomes among the difference risk factors.
Figure 2.
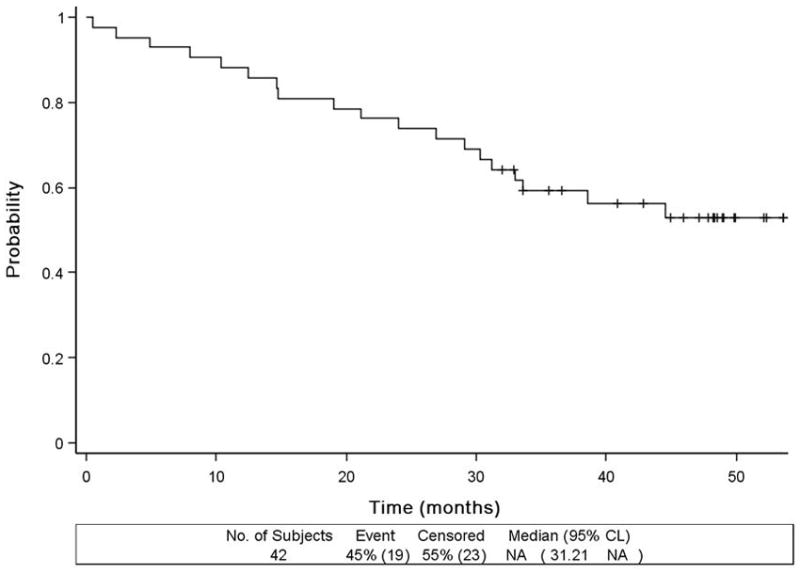
Overall survival
The PFS from initiation of maintenance for the 15 patients that were treated on maintenance therapy was estimated. There was one patient who progressed before start of maintenance that was dropped from this analysis. Median PFS from the start of maintenance only was 7.8 months (95%CI 2.8 to 10.7 months). 1- and 2-year PFS probabilities were 25% (95%CI 6 to 51%) and 8.3% (95%CI 0.5 to 31%), respectively. Median PFS on reinduction only was 7.3 months (95%CI4.1 to 36.9 months). 1- and 2- year PFS probabilities were the same: 22% (95%CI 1 to 62%).
Overall, the most common reason for patients to discontinue therapy was progressive disease (n=18, 45%) followed by toxicity (n=7, 18%), Figure 1. The median time from start of treatment to end of treatment was 4.2 months. The number of total treatment cycles ranged from 1–55. There are two patients still on-treatment: cycle 45 of on maintenance (53 total cycles); and cycle 23 treatment data on reinduction (55 total cycles). Figure 1 shows the reason off treatment by phase of-treatment. The median number of induction cycles administered was 6, with 19 patients receiving all 8 induction cycles. Fifteen patients continued onto the maintenance phase of treatment, with a median number of maintenance cycles being 9 (range: 1–45 cycles). For the 7 patients entering re-induction for progression while on maintenance, the median number of reinduction cycles was 3 (range 1–23).
Toxicity
All 43 patients that received treatment—including the one ineligible patient—are included in the toxicity analysis. Only those adverse events adjudicated to being at least be possibly related to treatment are reported. Table 3 summarizes adverse events reported by organ system and maximum grade. The maximum grade consolidates the reports of a given type of toxicity for a patient over time by taking the maximum across time (i.e., a patient appears only once for a given type of toxicity). Twenty-three patients had grade 3 hematologic toxicity. Thirty-four patients (79%; 95%CI 64–90%) had grade 3 or higher non-hematologic toxicity, including one patient with lethal heart block. The most common non-hematologic grade 1–4 and grade 3–4 toxicities included electrolyte disturbances, fatigue, neuropathy, and gastrointestinal disturbances. Six patients (14%) had grade 4 non-hematologic adverse events, predominantly electrolyte disturbances, but also one case each of sinus bradycardia and of weight loss.
Table 3.
Number of Patients with Toxicity at least Possibly Related to Treatment by System* (n=43)
| Type (Worst Grade) | Grade 1–2 | Grade 3 | Grade 4 | Overall |
|---|---|---|---|---|
| Blood/Bone Marrow | 20 | 14 | 9 | 43 |
| Metabolic/Laboratory | 22 | 14 | 5 | 41 |
| Constitutional Symptoms | 26 | 6 | 1 | 33 |
| Neurological† | 25 | 6 | - | 31 |
| Gastrointestinal | 23 | 6 | - | 29 |
| Pain | 19 | 5 | - | 24 |
| Dermatology/Skin | 14 | - | - | 14 |
| Pulmonary/Upper Respiratory | 9 | 2 | - | 11 |
| Cardiac General | 7 | 2 | - | 9 |
| Infection | 6 | 2 | - | 8 |
| Lymphatics | 7 | 1 | - | 8 |
| Ocular/Visual | 4 | - | - | 4 |
| Hemorrhage/Bleeding | 3 | - | - | 3 |
| Cardiac Arrhymia | - | 1 | - | 2 |
| Musculoskelatal/Soft Tissue | 2 | - | - | 2 |
| Hepatobiliary/Pancreas | - | 1 | - | 1 |
The maximum grade consolidates the reports of a given type of toxicity for a patient over time by taking the maximum across time (i.e., a patient appears only once for a given type of toxicity). Organ systems not included because only 1 patient with grade 1–2 toxicity include Auditory/Ear, Endocrine, Renal/Genitourinary, and Vascular
The breakdown of Neuropathy is as follows: motor grade 1–2 in 3 patients; sensory grade 1–2 in 24 patients and grade 3 in 1 patients; and neuropathic pain grade 1–2 in 6 patients and grade 3 in 1 patients
Twenty-five patients developed treatment emergent neuropathy. Considering all 43 patients evaluable for toxicity, the breakdown of treatment emergent neuropathy was as follows: motor grade 1–2 in 7%; sensory grade 1–2 in 56% and grade 3 in 2%; and neuropathic pain grade 1–2 in 14% and grade 3 in 2%.
Discussion
Although the current study is not the first report of the use of bortezomib as front line therapy for symptomatic myeloma, it is unique. It is the only up front bortezomib clinical trial that prospectively targeted high-risk patients, that tested maintenance bortezomib, and that tested reinduction after relapse despite maintenance bortezomib. Unlike the 4 other publications describing the front line use of single agent bortezomib,11–14 our patients were not slated for a set number of cycles of therapy to be followed by high dose chemotherapy with peripheral blood stem cell transplant. In fact, the most common reason for coming off active treatment was progression/relapse (45%), followed by death or adverse events (20%) and other complications of disease (7%). Only 10% of patients withdrew for the specific reason of seeking alternative therapy.
We demonstrated that among high risk patients, single agent bortezomib induced overall response rates of approximately 51%, which is comparable to those reported by others in a general myeloma population (Table 4). 11–13, 15 Moreover, the observed 2-year overall survival probability of 76% (95%CI 60–86%) was higher than expected for published rates for patients with ISS 3 (58%) or high risk cytogenetics (65%).16, 17
Table 4.
| Regimen | Evaluable patients | CR, % | VGPR, % | PR, % | OR, % | PFS | OS | |
|---|---|---|---|---|---|---|---|---|
| Current study | Bortez | 39 | 0 | 10 | 41 | 51 | 1 yr 36% 2 yr 16% |
1 yr 88% 2 yr 76% 3 yr 60% |
| Richardson 200914 | Bortez | 64 | 3 | 14 | 23 | 51 | 1 yr ~60% 2 yr ~25% |
1 yr ~94% 2 yr ~89% |
| Jagannath 200511 | Bortez+/-dex | 32 | 3*/6† | 9*/19† | 28*/63† | 40*/88† | NR | 1 yr 87% |
| Harousseau 200612 | Bortez-Dex | 48 | 21 | 10 | 35 | 66 | NR | NR |
| Rosinol 200713 | Bortez alt Dex |
40 | 12 | 7.5 | 40 | 60 | NR | NR |
| Orlowski 200618 | Doxil/bortez | 57 | 16 | NR | 42 | 58 | NR | NR |
| Popat ASH 200519 | LD-PAD | 20 | 11 | 31 | 47 | 89 | 2 yr | 1 yr 73% |
| Oakavee 200519, 20 | PAD | 21 | 24 | 38 | 33 | 95 | 29 mo | 1 yr 95% |
| Barlogie 200721 | VDT-PACE | 303 | 52 | 24 | 13 | 89 | 2 yr EFS 84% |
2 yr 87% |
| San Miguel 200822, 26 | MP-bortez | 344 | 33 | 8 | 33 | 74 | TTP 24 mo | 1 yr ~90% 3-yr OS 68% |
Bortez, bortezomib; dex, dexamethasone; LD-PAD, low dose bortezomib, doxorubicin, and dexamethasone; MP, melphalan and prednisone; VDT-PACE, bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide, NR, not reported.
2 cycles of single agent bortezomib
Overall response with dexamethasone added either after 2 cycles or after 4 cycles in 22 of the 32 patients.
In the present study, our 1- and 2-year PFS probabilities were 36% (95%CI 19–54%) and 16% (95%CI 5–33%), respectively. In comparison, Richardson et al reported on 64 patients treated with single agent bortezomib with an overall response rate of 41% (CR 3%, PR 38%).14 During their follow-up period of 29 months, 11 progressed, and 32 went on to peripheral blood stem cell transplantation. Their 1- and 2-year PFS was 60% and 25%, respectively. Although Richardson et al report that response rates did not differ based on the presence or absence of adverse cytogenetics, only deletion 13q by FISH was evaluated.
Jagannath et al treated 32 newly diagnosed myeloma patients with bortezomib plus or minus dexamethasone. Two thirds of patients received dexamethasone. The overall response rate was 88%. Since most patients went on to high dose chemotherapy with peripheral blood stem cell transplantation, PFS is not cited. The 1 year OS was 87%. Harousseau et al.12 treated 48 patients with first line bortezomib and dexamethasone combination therapy achieving an overall response rate of 66%. All patients went on to high dose PBSCT so no information available regarding PFS or OS.
Finally, Rosinol et al13 treated 40 patients newly diagnosed symptomatic myeloma patients with 6 alternating cycles of bortezomib and dexamethasone. Sixty-five percent of patients achieved a partial response or better, challenging the notion that there is synergy with dexamethasone. FISH abnormalities did not impact response rate. Since more than 90% of patients underwent planned autologous stem cell transplantation, the authors could offer no information about durability of response or PFS.
Other more complex induction combinations have also been described including bortezomib, doxorubicin, dexamethasone (PAD), pegylated doxorubicin/bortezomib, bortezomib, thalidomide, dexamethasone (VTD), and melphalan, prednisone, bortezomib (VMP). 18–22 These combinations have response rates approaching 90 to 100%, but are associated with more side effects and their long-term effect on high risk disease is not yet well understood. The VMP study offers some insight into bortezomib’s performance among high-risk patients. These authors found that the 26 patients with high-risk cytogenetics – including the a t(4;14),t(14;16) translocations or deletion 17p—had the same rate of complete response and with a median follow-up of 16 months similar times to progression and overall survival.22
Extended use of bortezomib in the upfront setting has not been clarified based on the studies listed above with the possible exception of the Richardson study, in which half of their patients did not proceed immediately to HSCT: 14 The estimated 30 month OS probability was 82% (95%CI, 66% to 98%) for those patients who underwent HSCT and 78% (95%CI, 63% to 92%) for those who did not undergo HSCT.
There are no prospective manuscripts on retreatment with bortezomib in the upfront setting, but there are two retrospective reports. In the first, among those patients who had achieved a PR or better to their original bortezomib therapy, 31% (12 of 39) had a PR or better to retreatment, which is similar to our rate of 29%.23 In the second, 63% of patients who had previously responded to bortezomib responded to retreatment with bortezomib (with concomitant dexamethasone in 2/3 of cases).24 Finally, the preliminary from the RETRIEVE study suggests that 40% response rates can be achieved in patients who re-treated with bortezomib.25
Finally, we offer further insight about the rates of bortezomib peripheral neuropathy. Fifty-eight percent developed grade 1–2 neuropathy and 16% developed painful peripheral neuropathy. Grade 3 neuropathy was observed in 4% of patients. Richardson et al, had similar findings: any peripheral neuropathy in 55% (36/65): grade 1, in 23; grade 2, 1in 2, and grade 3, in 1.14 Rates of peripheral neuropathy were comparable in the up front study of Jagannath et al, with grade 2–3 peripheral neuropathy in 26% of participants. 11 Rosinol found that only 22% of patients developed grade 1 PN and 2.5% of patients developed grade 2 PN. 13 Harousseau et al also reported grade 2–3 peripheral neuropathy in 14% of cases. 12
This study is relevant because if provides important information about single agent bortezomib for patients with newly diagnosed high-risk myeloma. A limitation of this study is that patients with t(4:14) are under-represented partially because it was designed in 2002, a time before it was recognized that bortezomib may abrogate the risk of t(4;14). Although 51% of patients achieved a response, the 1 and 2 year PFS rates were poor. It is worth knowing that even in high-risk patients, re-induction can be successful in 29% of patients failing maintenance. Although bortezomib appears to be equally efficacious in both high-risk and standard risk patients, single agent bortezomib is not sufficient. Multidrug combinations are preferred in this high-risk population.
Figure 3.
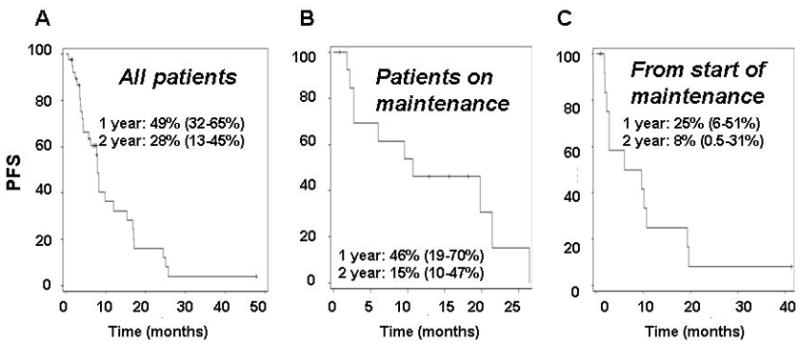
Progression free survival
A. All patients
B. Patients who did not progress and who began maintenance
C. From initiation of maintenance phase
References
- 1.Rajkumar SV, Buadi F. Multiple myeloma: new staging systems for diagnosis, prognosis and response evaluation. Best Pract Res Clin Haematol. 2007 Dec;20(4):665–680. doi: 10.1016/j.beha.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008 Mar 1;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kristinsson SY, Landgren O, Dickman PW, Derolf AR, Bjorkholm M. Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007 May 20;25(15):1993–1999. doi: 10.1200/JCO.2006.09.0100. [DOI] [PubMed] [Google Scholar]
- 4.Dispenzieri A, Rajkumar SV, Gertz MA, Fonseca R, Lacy MQ, Bergsagel PL, et al. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART): consensus statement. Mayo Clin Proc. 2007 Mar;82(3):323–341. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- 5.Jagannath S, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21:151–157. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- 6.San-Miguel JF, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008 Apr;22(4):842–849. doi: 10.1038/sj.leu.2405087. [DOI] [PubMed] [Google Scholar]
- 7.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. British journal of haematology. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 8.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006 Sep;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 9.Greipp PR, Lust JA, O’Fallon WM, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and beta 2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood. 1993;81(12):3382–3387. [PubMed] [Google Scholar]
- 10.Fonseca R, Bailey RJ, Ahmann GJ, Rajkumar SV, Hoyer JD, Lust JA, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100(4):1417–1424. [PubMed] [Google Scholar]
- 11.Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. British journal of haematology. 2005 Jun;129(6):776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 12.Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006 Nov;91(11):1498–1505. [PubMed] [Google Scholar]
- 13.Rosinol L, Oriol A, Mateos MV, Sureda A, Garcia-Sanchez P, Gutierrez N, et al. Phase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: efficacy and clinical implications of tumor response kinetics. J Clin Oncol. 2007 Oct 1;25(28):4452–4458. doi: 10.1200/JCO.2007.12.3323. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Xie W, Mitsiades C, Chanan-Khan AA, Lonial S, Hassoun H, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009 Jul 20;27(21):3518–3525. doi: 10.1200/JCO.2008.18.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson K, Richardson P, Chanan-Khan A, Schlossman R, Munshi N, Oaklander A, et al. Single-agent bortezomib in previously untreated multiple myeloma (MM): Results of a phase II multicenter study. J Clin Oncol (Meeting Abstracts) 2006 Jun 20;24(18_suppl):7504. [Google Scholar]
- 16.Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007 Apr 15;109(8):3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 17.Gertz MA, Lacy MQ, Dispenzieri A, Greipp PR, Litzow MR, Henderson KJ, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood. 2005 Oct 15;106(8):2837–2840. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlowski RZ, Peterson BL, Sanford B, Chanan-Khan AA, Zehngebot LM, Watson PR, et al. Bortezomib and Pegylated Liposomal Doxorubicin as Induction Therapy for Adult Patients with Symptomatic Multiple Myeloma: Cancer and Leukemia Group B Study 10301. Blood. 2006 Nov 16;108(11):797. [Google Scholar]
- 19.Popat R, Oakervee HE, Hallam S, Curry N, Odeh L, Foot N, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma: updated results after long-term follow-up. British journal of haematology. 2008 May;141(4):512–516. doi: 10.1111/j.1365-2141.2008.06997.x. [DOI] [PubMed] [Google Scholar]
- 20.Oakervee H, Popat R, Cavenagh JD. Use of bortezomib as induction therapy prior to stem cell transplantation in frontline treatment of multiple myeloma: impact on stem cell harvesting and engraftment. Leukemia & lymphoma. 2007 Oct;48(10):1910–1921. doi: 10.1080/10428190701540991. [DOI] [PubMed] [Google Scholar]
- 21.Barlogie B, Haessler J, Pineda-Roman M, Anaissie E, van Rhee F, Kiwan E, et al. Completion of premaintenance phases in total therapies 2 and 3 improves clinical outcomes in multiple myeloma: an important variable to be considered in clinical trial designs. Cancer. 2008 Jun 15;112(12):2720–2725. doi: 10.1002/cncr.23487. [DOI] [PubMed] [Google Scholar]
- 22.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. The New England journal of medicine. 2008 Aug 28;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 23.Conner TM, Doan QD, LeBlanc AL, Walters IB, Beveridge RA. An Observational, Retrospective Analysis of Retreatment with Bortezomib of Mulitple Myeloma (MM) Patients. ASH Annual Meeting Abstracts. 2006 Nov 16;108(11):3531. [Google Scholar]
- 24.Hrusovsky I, Emmerich B, Engelhardt M, Kornacker M, Gann C-N, Pliskat H, et al. Bortezomib Retreatment in Relapsed Multiple Mycelia - A Retrospective Multicenter Survey. ASH Annual Meeting Abstracts. 2007 Nov 16;110(11):2720. [Google Scholar]
- 25.Petrucci MT, Blau IW, Corradini P, Dimopoulos MA, Drach J, Giraldo P, et al. Efficacy and Safety of Re-Treatment with Bortezomib (Velcade(C)) in Patients with Multiple Myeloma: Results from a Prospective International Phase II Trial. Blood (ASH Annual Meeting Abstracts) 2008 Nov 16;112(11):3690. [Google Scholar]
- 26.Mateos M-V, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib Plus Melphalan-Prednisone Continues to Demonstrate a Survival Benefit Vs Melphalan-Prednisone in the Phase III VISTA Trial in Previously Untreated Multiple Myeloma After 3 Years’ Follow-up and Extensive Subsequent Therapy Use. ASH Annual Meeting Abstracts. 2009 Nov 20;114(22):3859. [Google Scholar]


