Abstract
Purpose
We investigated whether replication-defective herpes simplex virus (HSV) vectors encoding genes of glutamic acid decarboxylase (GAD), the gamma-aminobutyric acid synthesis enzyme, can suppress detrusor-sphincter dyssynergia (DSD) in rats with spinal cord injury (SCI).
Materials and Methods
One week after spinalization, HSV vectors expressing GAD and green fluorescent protein (HSV-GAD) were injected to the bladder wall. SCI rats without HSV injection (sham) and those injected with LacZ-encoding HSV vectors (HSV-LacZ) were used as controls. Three weeks after viral injection, simultaneous recordings of urethral pressure and intravesical pressure were performed under an awake condition in three groups.
Results
In the HSV-GAD group, the urethral pressure rise during bladder contractions was significantly reduced by 77–79% compared with sham or HSV-LacZ groups, but bladder activity and urethral baseline pressure were not different among three groups. Intrathecal application of bicuculline, a GABAA antagonist, almost completely reversed the decrease in urethral pressure rise during bladder contractions whereas intrathecal saclofen, a GABAB antagonist, partially reversed it. In the HSV-GAD group, GAD67 mRNA was significantly increased in L6-S1 dorsal root ganglia, where bladder afferents originate, compared with the HSV-LacZ group.
Conclusions
HSV-based GAD gene transfer to bladder afferent pathway may represent a novel approach for the treatment of DSD in SCI.
Keywords: gene therapy, detrusor overactivity, detrusor-sphincter dyssynergia, GABA, spinalized, C-fiber bladder afferents
INTRODUCTION
Micturition depends on the coordination between the bladder and external urethral sphincter.1 However, in the chronic phase of spinal cord injury the bladder exhibits detrusor overactivity and bladder-sphincter coordination is impaired, leading to DSD, that is simultaneous contractions of the external urethral sphincter and bladder during the micturition reflex.2 These lower urinary tract dysfunctions then produce various problems, such as outflow obstruction, recurrent urinary tract infection and vesicoureteral reflux with or without upper urinary tract deterioration.
It has been reported that glutamate, glycine, opioids, and GABA can modulate the activity of the external urethral sphincter in the DSD condition.3–5 In our previous study, chronic SCI rats had reduced levels of GAD, the GABA synthesis enzyme, in L6-S1 DRG, where bladder afferent fibers originate, and the lumbosacral spinal cord.6 These animals showed DSD on simultaneous recordings of urethral pressure and intravesical pressure, which was suppressed by intrathecally applied GABA receptor agonists.6,7 In our recent study, bicuculline, a GABAA antagonist, increased the frequency of isovolumetric conditions and reduced urethral relaxation during bladder contraction in spinal intact rats.7 Therefore, hypofunction of inhibitory GABAergic neuronal activity in the spinal cord is likely to be involved in the genesis of DSD after SCI.
It has been reported that replication-defective HSV mediated gene transfer of GAD delivered by subcutaneous inoculation can reduce neuropathic pain in spinal cord hemisection or spinal nerve ligation rats.8–9 In our recent study, HSV-mediated GAD gene delivery into the bladder wall decreased C-fiber mediated non-voiding bladder contractions without affecting maximal voiding pressure in SCI rats.10 Also, HSV-GAD-treated SCI rats had decreased residual volume and increased voiding efficiency, compared with sham or HSV-LacZ-treated SCI rats. Therefore, we hypothesized that HSV-mediated GAD gene delivery can reduce not only detrusor overactivity, but also increased urethral resistance during bladder contractions (i.e., DSD).
Thus, in the present study, we investigated the feasibility of HSV vector mediated GAD gene therapy for the treatment of DSD following SCI. We examined bladder and urethral activity and also confirmed the GAD gene delivery by measuring expression of GAD67 mRNA in L6-S1 DRG following HSV vector administration.
MATERIALS AND METHODS
Animal model
A total of 38 adult female Sprague-Dawley rats weighing 236–270 g were used according to the experimental protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. To produce SCI, rats were anesthetized with pentobarbital (30 mg/kg i.p., Ovation, Deerfield, IL) and Th9 laminectomy was performed. The dura was opened and the complete transection of the Th9-Th10 spinal cord was performed with scissors and a sterile Surgifoam sponge (Ferrosan, Soeborg, Denmark) was placed between the cut ends of the spinal cord. The overlying muscle and skin were then sutured to close the wound. Rats were then put on an electric warmer to maintain body temperature and were allowed to recover from anesthesia. SCI animals were postoperatively treated with ampicillin (100 mg/kg, intramuscularly) for 5 days. The bladder of spinalized rats was emptied by abdominal compression twice a day until reflex voiding recovered, usually 10 to 14 days after spinalization. SCI rats were divided into following three groups: 1) SCI rats without HSV injection (sham, n = 10), 2) SCI rats with replication-defective HSV vector Q0ZHG (control vector) administration (HSV-LacZ, n = 8), and 3) SCI rats with replication-defective, GAD67-expressig HSV vector QHGAD67 administration (HSV-GAD, n = 20).
Viral vectors and in vivo viral administration
The QHGAD67 vector (HSV-GAD) was constructed by recombining the UL41-targeting plasmid that contains the human cytomegalovirus immediate early promoter driving expression of the GAD67 cDNA into the vector Q0ZHG as previously reported.8,10 Both QHGAD67 and Q0ZHG express the green fluorescent protein, while the Q0ZHG (HSV-LacZ) control vector also express LacZ.
In HSV-LacZ and HSV-GAD groups, animals were anesthetized with pentobarbital (30 mg/kg) 1 week after SCI. A lower abdominal incision was made to expose the bladder, and the vectors were injected into the bladder wall using a 30-gauge Hamilton syringe (10 μl, Hamilton, Reno, NV). A total of 40 μl viral suspension (2 × 107 plaque-forming units [PFU] in total) of either Q0ZHG or QHGAD67 was injected at four sites of the bladder wall around the bladder base. After the abdomen was closed, rats were allowed to recover from anesthesia and housed in an approved Biosafety level-2 animal facility.
Surgical procedure for experiments
In three groups, 4 weeks after SCI, the animals were anesthetized with 2% isoflurane, and the abdomen was opened via midline laparotomy in the Experiment #1 and #2. The ureters were transected at the level of the aortic bifurcation, and the distal ends were ligated.11–12 An especially fabricated double lumen catheter was inserted from the bladder dome for urethral pressure measurements.11–12 This catheter consisted of an outer cannula of PE-160 (Clay Adams) and an inner cannula of PE-50 (Clay Adams) tubing, which protruded for 1 mm. The tip was embedded and secured with glue in a cone-shaped plug fashioned from an Eppendorf pipette tip. The double lumen catheter tip was then seated in the bladder neck to measure urethral pressure independent of bladder pressure. A PE-90 (Clay Adams) catheter was also inserted through the lateral side of the bladder to record the intravesical pressure. After the abdomen was closed, the rats were placed in a restrainer (Braintree Scientific Inc., Braintree, MA) and were allowed to recover from anesthesia for 1 to 2 hours.
In 8 SCI rats of the HSV-GAD group (Experiment #2), a laminectomy was performed at the level of the 3rd lumbar vertebra under isoflurane anesthesia 1–2 days before cystometry. A PE-10 was implanted intrathecally at the level of the L6-S1 spinal cord through a small hole of the dura. The end of the catheter was heat sealed and placed subcutaneously. The implanted intrathecal catheter was exteriorized through the skin incision for drug administration when cystometry was performed.
Administration of drugs
For intrathecal application in the Experiment #2, 1 μl of drug solution was given via the implanted intrathecal catheter and flushed by 10 μl saline. After the experiments, the position of the intrathecal catheter was checked in all animals, and the extent of dye distribution in the subarachnoid space was confirmed by injection of 2 μl Evans blue flushed with 10 μl saline. (−)–Bicuculline methobromide and saclofen (Tocris Cookson Inc, Ellisville, MO), a GABAA and a GABAB antagonist, respectively, were dissolved in distilled water. Intrathecal vehicle (saline or distilled water) application did not alter bladder and urethral activity during cystometry in SCI rats (data not shown).
Experiment #1: Simultaneous recordings of urethral pressure and intravesical pressure in an awake condition (n = 8 in HSV-LacZ group, n = 10 in sham and HSV-GAD groups)
The urethra was perfused with physiological saline via the outer cannula at 0.075 ml/min using an infusion pump. Urethral pressure was recorded via the fluid filled inner cannula connected to a pressure transducer mounted at the level of the pubic symphysis. The intravesical catheter was connected to a pressure transducer and to an infusion pump through a three-way stopcock. The bladder was infused with physiological saline at a rate of 0.08 ml/min until isovolumetric rhythmic bladder contractions were induced and maintained (bladder volume range: 1.2–2.5 ml), and then saline infusion was stopped. When rhythmic bladder contractions became stable for at least 30 minutes, the interval and amplitude of isovolumetric bladder contractions, and baseline intravesical pressure were measured. Baseline urethral pressure and changes in urethral pressure from the baseline at the peak of bladder contractions were also measured to evaluate urethral activity. These urodynamic parameters were compared among three groups.
Experiment #2: Awake simultaneous recordings of urethral pressure and intravesical pressure before and after GABA receptor antagonists in the HSV-GAD group
In order to examine the effect of GAD delivery, (−)-bicuculline methobromide (GABAA antagonist; 0.1 μg; n = 5) or saclofen (GABAB antagonists; 1 μg; n = 5) were administered through the intrathecal catheter using a Hamilton microsyringe in the HSV-GAD group. Then cystometric/urethral parameters were compared before and after administration of GABA receptor antagonists.
Experiment #3: Quantification of GAD67 mRNA in HSV-LacZ and HSV-GAD groups (n = 4 each)
After bladder/urethral pressure measurements, L6-S1 DRG were quickly removed, and total RNA was extracted from the pooled DRG using TRIzol reagent (Invitrogen, Carlsbad, CA). One μg of RNA was reverse-transcribed into cDNA using Superscript II (Invitrogen). Primers for GAD67 (GAD67 forward primer, 5′-GCGGGAGCGGATCCTAATA-3′ and reverse primer, 5′-TGGTGCATCCATGGGCTAC-3′)10 and β-actin (Ambion, Austin, TX) were used. The GAD67 and β-actin mRNA levels were quantified with an MX3000P real-time PCR system (Stratagene, La Jolla, CA) in a 25 μl volume using SYBR Green PCR Master Mix (QIAGEN, Valencia, CA).10 Quantification of the samples was achieved from the threshold cycle by interpolation from the standard curve to calculate a copy number for GAD67, and ratio of GAD67 to β-actin mRNA was compared.
Statistical analysis
Data are expressed as the mean ± SE. Statistical comparisons were performed using paired or unpaired t-test where applicable with p < 0.05 considered statistically significant.
RESULTS
Experiment #1: Comparisons of bladder and urethral activity in sham, HSV-LacZ, and HSV-GAD groups
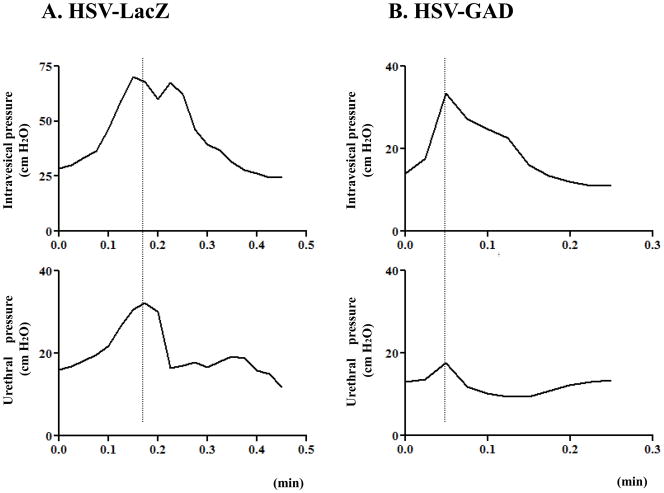
Representative traces of simultaneous recordings of urethral pressure and intravesical pressure in HSV-LacZ and HSV-GAD groups are shown in Fig. 1. In sham and HSV-LacZ groups, all SCI rats showed DSD (i.e., an increase in urethral pressure during bladder contraction) (Fig. 1A). There were no significant differences in cystometric/urethral parameters between sham and HSV-LacZ groups (Table 1). However, in the HSV-GAD group, the urethral pressure rise during bladder contractions was significantly reduced by 77–79% (p<0.01) compared with sham or HSV-LacZ groups (Table 1, Fig. 1B). There were no significant differences in bladder activity and baseline urethral pressure among three groups (Table 1).
Fig. 1.
Simultaneous recordings of intravesical pressure and urethral pressure in SCI rats with HSV-LacZ (A) or HSV-GAD treatment (B). A: DSD evidenced by urethral pressure rises during bladder contractions was shown. B: However, after the HSV-GAD treatment, increases in urethral pressure during bladder contractions were reduced.
Table 1.
Comparisons between sham, HSV-LacZ, and HSV-GAD groups in urodynamic parameters
| Bladder contraction |
Urethral contraction |
||||
|---|---|---|---|---|---|
| Interval (min) | Amplitude (cm H2O) | Baseline pressure (cm H2O) | Urethral pressure change (cm H2O) | Baseline pressure (cm H2O) | |
| Sham (n = 10) | 0.47 ± 0.07 | 26.0 ± 2.8 | 17.0 ± 1.3 | 17.2 ± 2.5 | 21.4 ± 3.1 |
| HSV-LacZ (n = 8) | 0.34 ± 0.04 | 28.0± 3.9 | 14.7 ± 0.9 | 18.7± 1.9 | 13.2± 2.3 |
| HSV-GAD (n =10) | 0.37 ± 0.03 | 21.6 ± 3.4 | 18.0 ± 1.4 | 4.0 ± 1.1**†† | 16.9 ± 4.1 |
Values are the mean ± SE. Significant differences when compared with sham are indicated by:
p < 0.01.
Significant differences when compared with HSV-LacZ are indicated by:
p < 0.01.
HSV, herpes simplex virus; GAD, glutamic acid decarboxylase
Experiment #2: Changes in bladder and urethral activity after intrathecal application of GABA receptor antagonists in the HSV-GAD group
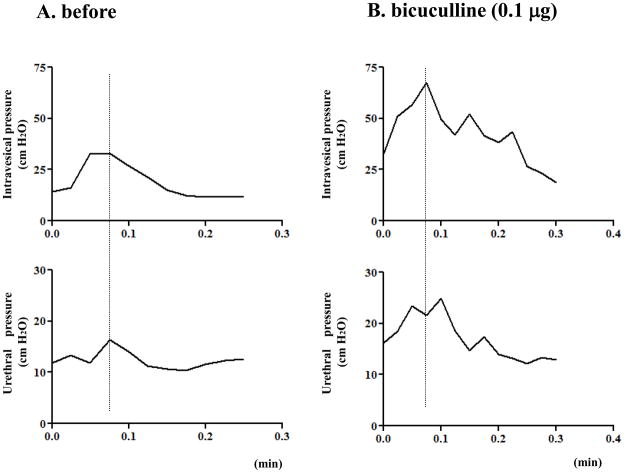
In the HSV-GAD group, simultaneous recordings of urethral pressure and intravesical pressure showed a reduction in the urethral pressure rise during bladder contractions as shown in the Experiment #1 (Fig. 2A). After intrathecal application of bicuculline (0.1 μg), the bladder contraction amplitude and urethral pressure changes during bladder contractions were significantly (p<0.05) increased (Table 2, Fig. 2B). There were no significant differences in these parameters between untreated HSV-LacZ and bicuculline-treated HSV-GAD rats. After intrathecal application of bicuculline, bladder contraction intervals or bladder and urethral baseline pressures were not changed in HSV-GAD-treated rats (Table 2).
Fig. 2.
Simultaneous recordings of intravesical pressure and urethral pressure in a HSV-GAD treated SCI rat before (A) and after 0.1 μg of intrathecal application of bicuculline (B). A: Small-amplitude increases of urethral pressure during bladder contractions were shown. B: After intrathecal bicuculline (0.1 μg), amplitudes of intravesical pressure and urethral pressure were increased.
Table 2.
Changes in bladder and urethral pressure after intrathecal administration of bicuculline or saclofen in the HSV-GAD group
| bicuculline (n = 5) |
saclofen (n = 5) |
|||
|---|---|---|---|---|
| before | after (0.1 μg) | before | after (1 μg) | |
| Bladder contraction interval (min) | 0.38 ± 0.03 | 0.31 ± 0.04 | 0.37 ± 0.04 | 0.39 ± 0.04 |
| Bladder amplitude (cm H2O) | 23.0 ± 3.8 | 37.5 ± 4.6* | 21.2 ± 3.6 | 25.4 ± 4.3 |
| Bladder baseline pressure (cm H2O) | 18.2 ± 1.8 | 13.8 ± 2.3 | 17.6 ± 1.8 | 14.0 ± 2.1 |
| Urethral pressure change (cm H2O) | 4.4 ± 1.2 | 19.2 ± 2.0** | 4.6 ± 1.2 | 10.1 ± 2.5* |
| Urethral baseline pressure (cm H2O) | 14.3 ± 4.0 | 15.5 ± 2.6 | 13.8 ± 3.6 | 16.5 ±3.6 |
Values are the mean ± SE. Significant differences when compared with before application are indicated by:
p < 0.05;
p < 0.01
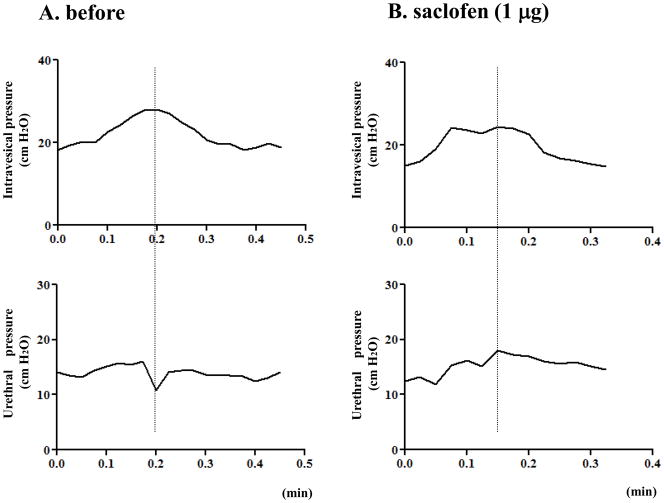
In a separate group of HSV-GAD-treated rats, intrathecal application of saclofen (1 μg) also significantly (p<0.05) increased urethral pressure changes during bladder contractions, but it was still lower compared with untreated HSV-LacZ rats (10.1 ± 2.5 vs. 18.7 ± 1.9, p < 0.05) (Fig. 3). After intrathecal application of saclofen, bladder activity or urethral baseline pressure were not changed in HSV-GAD-treated rats (Table 2).
Fig. 3.
Simultaneous recordings of intravesical pressure and urethral pressure in a HSV-GAD treated SCI rat before (A) and after 1 μg of intrathecal application of saclofen (B). A: A synergic pattern as evidenced by a negative shift of urethral pressure during bladder contractions was shown. B: After intrathecal 1 μg of saclofen, intravesical pressure did not change, but urethral pressure amplitude was increased.
Experiment #3: Comparisons of the GAD67 mRNA level of L6-S1 DRG between HSV-LacZ and HSV-GAD groups
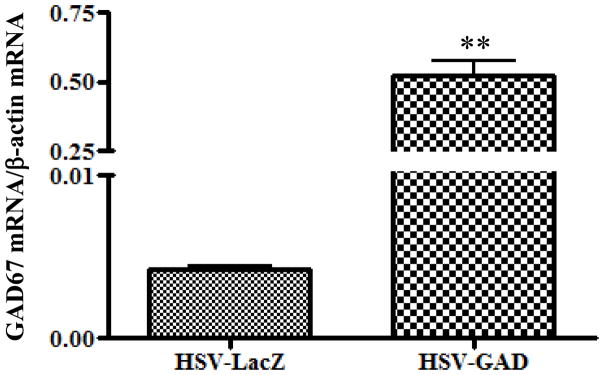
In the HSV-GAD group, the GAD67 mRNA/β-actin mRNA ratio in L6-S1 DRG was significantly higher (0.523 ± 0.050, p < 0.01) compared with the HSV-LacZ group (0.004 ± 0.001) (Fig. 4).
Fig. 4.
(A) GAD67 mRNA/β-actin mRNA ratio in L6-S1 DRG in HSV-LacZ and HSV-GAD-treated SCI rats. GAD67 mRNA/β-actin mRNA ratio in L6-S1 DRG was significantly increased in HSV-GAD-treated SCI rats compared with HSV-LacZ-treated SCI rats. Values are the mean ± SE. ** p<0.01 when compared between HSV-LacZ and HSV-GAD-treated SCI rats.
DISCUSSION
The organization of the micturition reflex undergoes marked changes after SCI. Following SCI, due to the loss of supraspinal micturition control, bladder-sphincter coordination is impaired, leading to DSD.2 The results of the present study indicate that; (1) the HSV-GAD treatment inhibits DSD as evidenced by a reduction in the urethral pressure rise during bladder contractions in SCI rats, (2) The GABAA receptor antagonist almost completely restores DSD, while GABAB receptor antagonist partially restores it, as evidenced by increase of the urethral pressure rise during bladder contractions after intrathecal bicuculline or saclofen in HSV-GAD-treated SCI rats, and (3) expression of GAD, the GABA synthesizing enzyme, is increased in L6-S1 DRG after the HSV-GAD treatment as evidenced by increase of GAD67 mRNA. Thus, it is assumed that HSV vectors can be efficiently transported to bladder afferent pathways and reduce DSD predominantly through GABAA receptor activation.
In our recent study, HSV-mediated GAD gene delivery into the bladder wall decreased residual volume and increased voiding efficiency, compared with sham or HSV-LacZ-treated SCI rats while there were no differences in maximal voiding pressure among sham, HSV-LacZ, and HSV-GAD groups.10 In the present study, the HSV-GAD treatment reduced the urethral pressure rise during bladder contractions in SCI rats. Therefore, it is plausible that HSV-mediated GAD gene delivery reduces urethral resistance during voiding, which was increased after SCI (i.e., DSD), without affecting voiding function, resulting in improving residual volume and voiding efficiency. Our previous study also indicated that hyperexcitability of C-fiber bladder afferents is involved in DSD after SCI because C-fiber desensitization by capsaicin pretreatment reduces urethral contraction pressure during bladder contraction in SCI rats.12 Thus, it is assumed that a local increase in GABA synthesis in bladder afferent pathways after HSV-GAD vector injection into the bladder wall could inhibit Onuf’s nucleus innervating the external urethral sphincter via suppression of C-fiber bladder afferent activity to reduce DSD in SCI rats.
The current study also showed that intrathecal bicuculline almost completely restored the urethral pressure rise during bladder contractions while intrathecal saclofen partially reversed it in HSV-GAD-treated SCI rats. In contrast, in our recent study, intrathecal bicuculline partially restored C-fiber mediated non-voiding bladder contractions, which was decreased by GAD gene delivery, while intrathecal saclofen did not affect cystometric parameters, in HSV-GAD-treated SCI rats.10 Our previous study also showed that the effective dose of GABA receptor agonists to induce inhibition of urethral activity was lower compared with the dose that inhibited bladder contractions in simultaneous recordings of urethral pressure and intravesical pressure in SCI rats.7 Therefore, HSV-GAD treatment in SCI rats might have inhibited more preferentially urethral activity rather than bladder activity predominantly via GABAA receptors. GABA reportedly inhibits bladder activity by acting through at least four distinct sites in spinalized rats; (1) at the spinal level by reducing afferent inputs from the detrusor through afferent nerves, (2) by inhibiting the neurons of the sacral parasympathetic nucleus, (3) at the pelvic ganglionic level by inhibiting excitatory neurotransmitters and (4) at the postganglionic level by reducing neurotransmitter release from neurons innervating the detrusor.13 In our previous study, intrathecal muscimol and baclofen, GABA receptor agonists, at the level of L6-S1 spinal cord reduced non-voiding bladder contractions in SCI rats, and the effects were antagonized by bicuculline and saclofen, respectively, suggesting that both GABAA and GABAB receptors are equally involved in suppression of detrusor overactivity in SCI, presumably at spinal cord sites including the sacral parasympathetic nucleus or Onuf’s nucleus.6 In contrast, as shown in the present study, GABAA receptor activation preferentially contributes to suppression of sensory inputs through C-fiber bladder afferent pathways in the spinal cord after the HSV-GAD treatment in SCI rats.
Our previous study showed that synergic urethral relaxation with high frequency oscillations (HFOs), which reflect the external urethral sphincter bursting activity, was observed during isovolumetric bladder contractions before drug application in spinal intact rat and that intrathecal bicuculline, but not saclofen, reduced urethral relaxation during bladder contractions and increased the amplitude of HFOs.7 Following spinal cord injury, the external urethral sphincter bursting activity during voiding is converted to tonic activity.11 In the present study, HSV-GAD treatment reduced the urethral pressure rise during bladder contractions by 77–79% compared with sham or HSV-LacZ groups. These findings suggest that under the normal condition GABAA receptor activation maintains synergic urethral relaxation and that decreased activation of GABAA receptors due to hypofunction of spinal GABAergic inhibitory mechanisms contributes at least in part to the emergence of DSD after SCI.
Prior works by our group6–7 and others14 have suggested that the GABA synthesis enzyme, GAD, was reduced following SCI, suggesting that hypofunction of the GABAergic inhibitory system is at least in part responsible for the development of DSD after SCI. Therefore, it is likely that GABA supplement therapy represents a reasonable approach for treating derusor overactivity and DSD after SCI. Previous clinical studies have also demonstrated that the GABAB receptor agonist baclofen administered intrathecally or intravenously reduces urethral resistance in humans with traumatic paraplegia and severe spasticity.15–16 However, the ubiquitous distribution of GABA receptors in the central nervous system results in side effects that impose severe restriction on the dose of baclofen. These complications may be avoided by administration of neurotransmitters directly to the cells that require it. In our pervious study, after HSV-GAD injection in the bladder wall, GAD expression was increased in L6-S1 DRG that contains bladder afferent neurons, and C-fiber-dependent detrusor overactivity was suppressed.10 Moreover, our prior works using HSV vectors have shown that the vectors administered to the rat bladder wall very efficiently transduce the bladder muscle layer and L6-S1 DRG neurons, but not adjacent DRG, as detected by expression of the reporter gene (E. coli lacZ) product, and is capable of expressing a variety of therapeutic gene products.17–19 Therefore, it is suggested that GAD gene therapy using HSV vectors that target the affected organ could be efficiently transported to its afferent pathways with limited side effects to treat DSD, as well as detrusor overactivity.
CONCLUSIONS
The present study provides the first evidence of the efficacy of GAD gene therapy using HSV vectors for DSD following SCI. GAD gene therapy mainly inhibits C-fiber bladder afferent pathways to exert its effects predominantly through GABAA receptors. Therefore, the novel GAD gene therapy using replication-defective HSV vectors could be effective for the treatment of DSD by restoring impaired GABA mechanisms in patients with SCI.
Acknowledgments
This work was supported by NIH DK057267, DK068557 and DK044935.
Key of Definitions for Abbreviations
- DSD
detrusor-sphincter dyssynergia
- GABA
Gamma-aminobutyric acid
- SCI
spinal cord injury
- GAD
glutamic acid decarboxylase
- DRG
dorsal root ganglia
References
- 1.Holstege G, Griffiths D, de Wall H, et al. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- 2.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 3.Miyazato M, Sugaya K, Nishijima S, et al. Intrathecal or dietary glycine inhibits bladder and urethral activity in rats with spinal cord injury. J Urol. 2005;174:2397. doi: 10.1097/01.ju.0000180415.69705.7a. [DOI] [PubMed] [Google Scholar]
- 4.Nishizawa O, Igawa Y, Satoh T, et al. Effects of glutamate receptor antagonists on lower urinary tract function in conscious unanesthetized rats. Adv Exp Med Biol. 1999;462:275. doi: 10.1007/978-1-4615-4737-2_21. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama O, Mita E, Akino H, et al. Roles of opiate in lower urinary tract dysfunction associated with spinal cord injury in rats. J Urol. 2004;171:963. doi: 10.1097/01.ju.0000105160.72711.83. [DOI] [PubMed] [Google Scholar]
- 6.Miyazato M, Sasatomi K, Hiragata S, et al. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol. 2008;179:1178. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazato M, Sasatomi K, Hiragata S, et al. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R336. doi: 10.1152/ajpregu.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Wolfe D, Hao S, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;10:57. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Hao S, Mata M, Wolfe D, et al. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol. 2005;57:914. doi: 10.1002/ana.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazato M, Sugaya K, Goins WF, et al. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009;16:660. doi: 10.1038/gt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakizaki H, de Groat WC. Reorganization of somato-urethral reflexes following spinal cord injury in the rat. J Urol. 1997;158:1562. [PubMed] [Google Scholar]
- 12.Seki S, Sasaki K, Igawa Y, et al. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004;171:478. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- 13.Maggi CA, Furio M, Santicioli P, et al. Spinal and supraspinal components of GABAergic inhibition of the micturition reflex in rats. J Pharmacol Exp Ther. 1987;240:998. [PubMed] [Google Scholar]
- 14.Moore KA, Kohno T, Karchewski LA, et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steers WD, Meythaler JM, Haworth C, et al. Effects of acute bolus and chronic continuous intrathecal baclofen on genitourinary dysfunction due to spinal cord pathology. J Urol. 1992;148:1849. doi: 10.1016/s0022-5347(17)37048-9. [DOI] [PubMed] [Google Scholar]
- 16.Hachen HJ, Krucker V. Clinical and laboratory assessment of the efficacy of baclofen (Lioresal) on urethral sphincter spasticity in patients with traumatic paraplegia. Eur Urol. 1977;3:237. doi: 10.1159/000472104. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura N, Franks ME, Sasaki K, et al. Gene therapy of bladder pain with herpes simplex virus (HSV) vectors expressing preproenkephalin (PPE) Urology. 2001;57 (6 Suppl 1):116. doi: 10.1016/s0090-4295(01)01060-3. [DOI] [PubMed] [Google Scholar]
- 18.Goins WF, Yoshimura N, Phelan MW, et al. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol. 2001;165:1748. [PubMed] [Google Scholar]
- 19.Yokoyama H, Sasaki K, Franks ME, et al. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum Gene Ther. 2009;20:63. doi: 10.1089/hum.2008.094. [DOI] [PMC free article] [PubMed] [Google Scholar]