Abstract
Objectives
The adult response to myocardial infarction results in inflammation, scar formation, left ventricular dilatation, and loss of regional and global function. Regenerative scarless healing has been demonstrated in fetal dermis and tendon and is associated with diminished inflammation. We hypothesized that following fetal myocardial infarction there would be minimal inflammation, regenerative healing, and preservation of function.
Methods
Anteroapical myocardial infarction encompassing 20% of the left ventricle were created in adult or early gestation fetal sheep. Myocardial function was serially assessed using quantitative echocardiography. Infarct architecture was examined histologically for evidence of scar formation. Cellular inflammation, cellular proliferation, and apoptosis were assessed using immunohistochemistry.
Results
In the adult sheep 4 weeks following myocardial infarction, there was a significant decline in ejection fraction (41±7.4% to 26±7.4%, p<0.05), and the akinetic myocardial segment increased in size (6.9±0.8 cm to 7.9±1.1 cm, p<0.05). In contrast, there was no decline in the fetal ejection fraction (53±8.1% to 55±8.8%) and no akinetic fetal myocardial segment 4 weeks post-infarction. The fetal infarcts lacked an inflammatory cell infiltrate and healed with minimal fibrosis, compared to the adults. Fetal infarcts also demonstrated BrdU+ proliferating cells, including cardiomyocytes, within the infarct.
Conclusions
These data demonstrate that the fetal response to myocardial infarction is dramatically different than the adult and is characterized by minimal inflammation, lack of fibrosis, myocardial proliferation, and restoration of cardiac function. Diminished inflammation is associated with fetal regenerative cardiac healing following injury. Understanding the mechanisms involved in fetal myocardial regeneration may lead to applications to alter the adult response following myocardial infarction.
Keywords: myocardial infarction, heart failure, inflammation, apoptosis
Introduction
The adult response to myocardial infarction (MI) has been well described and follows an orderly sequence of events. It is characterized by an early phase in which inflammatory cells, including neutrophils and T-cells, arrive at the infarct site within the first 72 hours [1,2]. Following the inflammatory phase, remodeling occurs within the infarct and is associated with degradation of the extracellular matrix by collagenases and matrix metalloproteinases [3]. The remodeling process of the ventricle following MI in the adult results in ventricular scar formation and is accompanied by ventricular wall thinning, increased wall stress, and a decline in cardiac function [2].
The role of the inflammatory response in post-infarction ventricular remodeling is not fully understood. However, it appears increased inflammation is associated with worse outcomes with neutrophilia being linked to impaired microvascular reperfusion and worsening wall motion abnormalities following MI [4]. In addition, it has been proposed that the benefit seen with antiplatelet therapy may be partially due to an anti-inflammatory effect targeted against neutrophils [5]. Laboratory studies targeting different components of the inflammatory response following MI have shown improvements in post-infarction left ventricular (LV) remodeling [1,6].
Further evidence to support the role of inflammation in the pathogenesis of the adult response to injury comes from studies in dermal and tendon wound healing. Wounds in adult dermis or tendon are associated with a brisk inflammatory cell infiltrate and heal with scar formation, whereas similar fetal dermal or tendon wounds are associated with minimal inflammation and heal by regeneration with a lack of scar formation [7–11]. These fetal wounds have also been shown to have a decrease in the proinflammatory cytokines interleukin-6 (IL-6) and interleukin-8 (IL-8) [10,11]. In addition, the deficiency of the anti-inflammatory cytokine interleukin-10 (IL-10) in fetal dermal wounds results scar formation, and we have recently shown that overexpression of IL-10 in adult dermal wounds decreases inflammatory mediators and inflammation resulting in regenerative or scarless healing [12,13].
To date, the fetal response to cardiac injury is unknown. We hypothesized that the fetal response to myocardial infarction would be associated with minimal inflammation and a lack of scar formation resulting in regenerative healing and restoration of myocardial function, thus preventing the negative sequelae of post-infarction LV remodeling.
Materials and methods
Experimental Design
A myocardial infarct model in fetal (n=15) and adult sheep (n=23) was used to investigate ventricular remodeling, the cellular inflammatory response to injury, and cellular proliferation within the infarct over time. Data generated from experimental animals were used for multiple experiments whenever possible in order to reduce the number of animals needed for the study.
Remodeling Experiments
Myocardial infarcts were created in early gestation fetal (n=11) and adult sheep (n=19). The animals were sacrificed at either 3 days (fetal n=4, adult n=7) or 4 weeks (fetal n=5, adult n=12). One set of fetal twins spontaneously aborted prior to sacrifice and was excluded from the study. Echocardiography was performed pre-infarction, post-infarction, and just prior to sacrifice to assess the LV function and infarct size. Hematoxylin & eosin (H&E) and Mason’s Trichrome staining were used to qualitatively assess the post-MI scar formation and ventricular remodeling. Immunohistochemistry for activated caspase-3 was performed to assess for evidence of apoptosis and cardiomyocyte cell death.
Cellular Inflammatory Response Experiments
Myocardial infarcts were created in early gestation fetal and adult sheep. The animals were sacrificed at 3 days (fetal n=4, adult n=7), 7 days (fetal n=4, adult n=4), or 4 weeks (fetal n=3, adult n=12), and immunohistochemistry for CD45, the common leukocyte antigen, was performed to assess the level of the cellular inflammatory response in the infarct. The 3 day and 4 week fetal and adult animals were also used in the remodeling experiments.
Fetal Myocardial Proliferation Experiments
Myocardial infarcts were created in fetal sheep (n=7), and 5-bromo-2’-deoxyuridine (BrdU) was given to the pregnant ewe for 48 hours prior to sacrifice at either 3 days or 4 weeks. Immunohistochemistry for BrdU was performed to assess for evidence of cell proliferation within the fetal infarcts. The animals used for fetal myocardial proliferation experiments were also used in the remodeling experiments.
Experimental Methods
Animal Procedures
All experiments were approved by the Institutional Animal Care and Use Committees of the Children’s Hospital of Philadelphia and the University of Pennsylvania and performed in compliance with National Institutes of Health Publication No. 85-23, revised 1996, and the European Convention on Animal Care. Pregnant ewes at 65–76 days gestation or adult sheep were used for all studies. Quantitative echocardiography was performed prior to infarction, immediately after infarction, and at the time of sacrifice. Animals were sedated with ketamine 11mg/kg IM, intubated, and anesthetized with inhaled isoflurane. Cefazolin 1g IV was given prior to incision and oxytetracycline 0.06mg/kg IM prior to extubation for antibiotic prophylaxis. For the fetal model a laparotomy and hysterotomy was performed to expose the fetus. In all animals a left thoracotomy was performed and the pericardium opened to expose the heart. The left anterior descending coronary artery (LAD) and appropriate diagonal branches were suture ligated (Figure 1a, 1b) to produce an infarct involving 20% of the LV mass [14]. The chest and skin incisions were closed. For the fetal cases the amniotic fluid was replaced with sterile normal saline plus 2 million units of penicillin-G added for antimicrobial prophylaxis; the uterus and abdominal incisions in the ewe were closed prior to emergence from anesthesia. Analgesia was provided with buprenorphine 0.005mg/kg IM prior to extubation and flunixin meglumine 2.5mg/kg IM 4 hours post-operatively. For the proliferation studies, 48 and 24 hours prior to sacrifice the pregnant ewes received 250mg of BrdU IV. For the remodeling experiments, animals were anesthetized at 3 days or 4 weeks and had echocardiography prior to sacrifice. The hearts were excised for histologic analysis from sheep sacrificed at 3 days, 7 days, and 4 weeks.
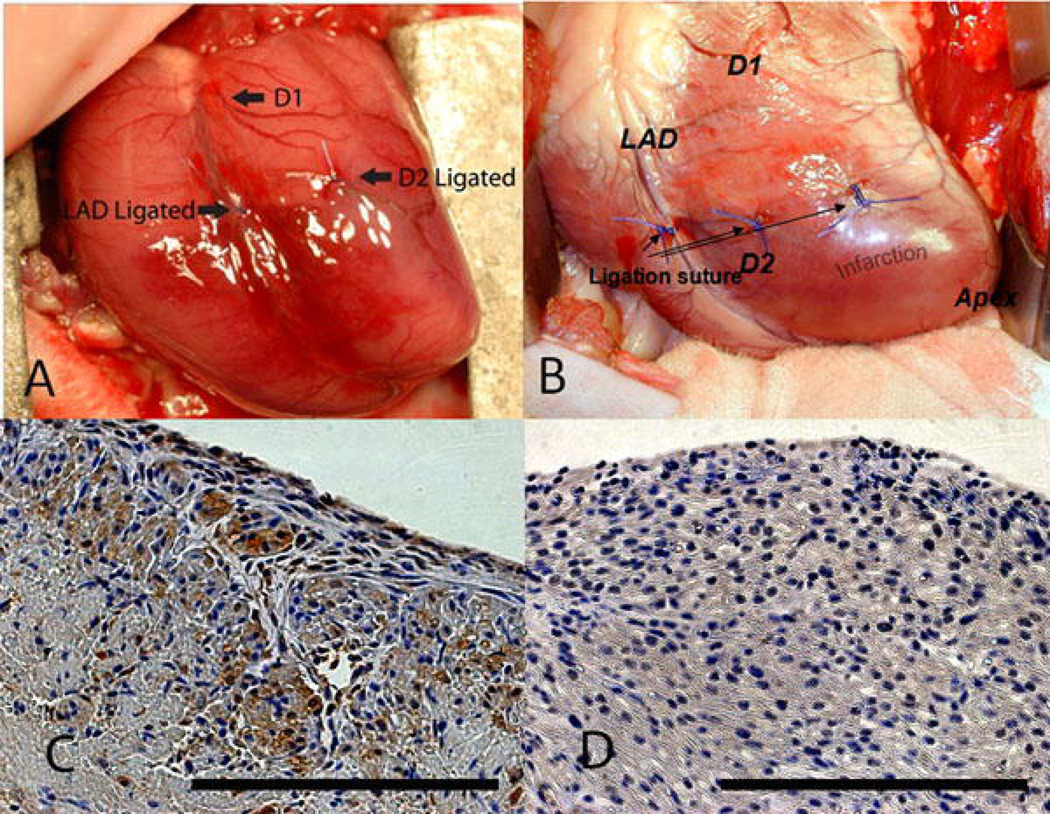
Figure 1.
Ligation of the distal LAD and D2 leads to a reproducible infarct in fetal and adult hearts. An anteroapical MI is created by ligating the LAD and D2 at a point 40% of the distance between the apex and the base in (a) fetal and (b) adult sheep. Activated caspase-3 staining of fetal hearts 3 days following infarction demonstrates (c) apoptosis in the area of infarction (400×) and (d) lack of apoptosis away from the area of infarction (400×). Scale bars equal 200µm.
Echocardiography
Quantitative two-dimensional subdiaphragmatic echocardiograms in the adult and quantitative two-dimensional transuterine echocardiograms in the fetus were obtained before coronary ligation (pre-MI), immediately after coronary ligation (post-MI), and at the time of sacrifice. Echocardiography was performed on a Phillips 7500 (3.5-MHZ probe in the adult and 7-MHZ in the fetus) and recorded on ½ inch video tape. Using a modified Simpson’s rule technique [15], apical 4-chamber views in both fetuses and adults were analyzed using an offline analysis system (TomTec Imaging System, GmbH, Unterschleissheim, Germany) to obtain LV volumes at end-systole and end-diastole. The ejection fraction (EF) was calculated from these volumes. Infarct length and LV long axis length were also measured. Infarct sized was estimated by the ratio of infarct length to LV length in diastole. This ratio has been found to correlate with quantitative postmortem assessments of infarct size in this sheep infarct model (unpublished data).
Histology
All tissues were fixed in 10% neutral buffer formalin (NBF) (Sigma-Aldreich) for greater than 72 hours and processed using a Leica 1050 histoprocessor (Leica Microsystems). 4µm paraffin sections were mounted on Fisher Plus slides (Fisher Scientific, Pittsburgh, PA) and incubated overnight at 52°F. Slides were deparaffinized in xylene for 10 minutes × 3 followed by rehydration by graded ethanol (2×100%, 95%, 75%) to distilled water. H&E and Masson’s Trichrome staining were performed.
Immunohistochemistry
Slides from distilled water were immersed in 0.3% H202 in distilled water for 30 minutes at room temperature to quench endogenous peroxidase. They were then rinsed well in distilled water. Antigen unmasking was done using target retrieval (pH 6.0, Dako) in the microwave (Ted Pella) for 4 minutes and then cooled to room temperature. Slides were rinsed well in distilled water and serum specific blocking was done with 10% serum for 30 minutes at room temperature. The serum was tipped off and sections were incubated with primary antibodies overnight at 4°C. Primary antibodies included CD45 (Serotec) mouse anti-ovine at 1:100 dilution, caspase-3 (Abcam) rabbit monoclonal at 1:50 dilution, and BrdU (Invitrogen) mouse monoclonal at 1:100 dilution. The following day, the primary antibody was tipped off and the slides washed in 0.1M PBS + Triton. Biotinylated specific secondary antibody, horse anti-mouse or goat anti-rabbit (Vector Lab), was applied at 1:200 dilution for 30 minutes at room temperature. The secondary antibody was tipped off, and the slides were rinsed in PBS + Triton followed by ABC Complex (Avidin-Biotin, Vector Lab) applied for 30 minutes at room temperature. After slides were washed in PBS + Triton, the developer with chromogenic substrate (ImmPACT DAB, Vector) was applied for 1–2 minutes. Slides were then rinsed in distilled water and counterstained with Harris Hematoxylin (Fisher Scientific, Pittsburgh, PA), eosin (Surgipath Medical Industries, Inc., Richmond, IL), or Lichgrün (Croma, Germany). Sections were then dehydrated through graded alcohol series for 2 minutes each (70%, 95%, 100% × 2), clarified in xylene, and a cover slip applied in Permount (Fisher Scientific, Pittsburgh, PA).
Statistical Analysis
Statistical analysis was performed using SPSS 15.0 (SPSS, Inc., Chicago, IL). Non-parametric statistical methods were used for data analysis due to uneven sample size between groups. To examine time-related changes in each of the four different animal groups (Fetal 3 day and 4 week, Adult 3 day and 4 week), a Friedman’s repeated measures test was used with three time points: pre-MI, post-MI and Final-MI. When a significant difference (p<0.05) was found within a group, post-hoc analysis was performed using the Wilcoxon matched-paired test to identify differences between mean values at each of the three time points. Absolute infarct length of the four groups (as listed above) was compared post-MI verses Final-MI using a Wilcoxon matched-paired test. Post-MI values of IL/DL were compared between fetal and adult sheep using a Mann-Whitney unrelated samples test. Significance was accepted as p<0.05. Values are presented as the mean ± standard deviation.
Results
Ventricular Remodeling in the Fetus and the Adult
Immediately after coronary ligation in both the fetus and the adult, there were easily identifiable wall motion abnormalities consistent with MI. The ratio of infarct length to LV long axis length in diastole (IL/DL) was 0.92±0.08 (n=19) in the adult sheep and 0.71±0.18 (n=9) in the fetal sheep (p<0.05). In this model the IL/DL correlates to the infarct size (unpublished data), and this demonstrates that a substantial infarct has been created in both the fetus and the adult when compared to the LV size. Activated caspase-3 staining in fetal infarcts at 3 days confirmed that there was myocyte death within the infarct (Figure 1c).
Qualitatively, all adult sheep had discrete and easily identifiable LV apical aneurysms at 4 weeks (Figure 2a). Apical aneurysms were not present in any of the fetal sheep 4 weeks after MI (Figure 2b).
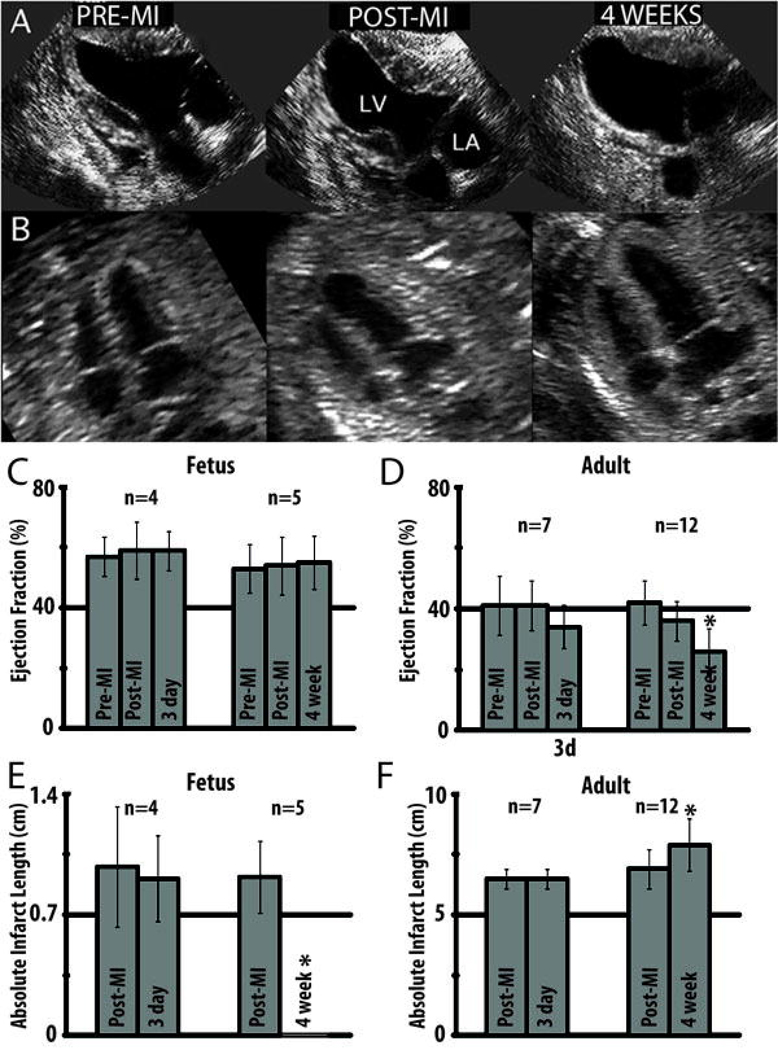
Figure 2.
Echocardioghraphic assessment of fetal and adult hearts following myocardial infarction demonstrates functional decline in the adult and restoration of function in the fetus. (a) Serial end-systolic echocardiographic views demonstrate dilation of the LV, 4 weeks following infarction with a large anteroapical infarct in the adult. (b) In the fetus, there is no evidence of LV dilation or infarcted myocardium at 4 weeks. (c) EF measured by quantitative echocardiography is unchanged in the fetus at 3 days (p=0.37) and 4 weeks (p=0.31) following infarction. (d) In the adult, the EF has significantly declined by 4 weeks following myocardial infarction (*p<0.05 vs. adult pre-MI and post-MI). (e) Absolute infarct length defined as the length of akinetic myocardium measured by echocardiography is unchanged at 3 days following infarction (p=0.72) but decreases to zero in the fetus at 4 weeks following infarction (*p<0.05 vs fetal post-MI). (f) In the adult, the absolute infarct length is also unchanged at 3 days following infarction (p=1.00) but increases over a period of 4 weeks following infarction (*p<0.05 vs adult post-MI).
EF in adult sheep sacrificed at 3 days following MI was 41±9.7% pre-MI, 41±8.2% post-MI, and 34±7.1% 3 days after MI (Figure 2d). There was no significant difference in the EF with respect to time of adult sheep sacrificed 3 days following MI (p=0.18). EF in adult sheep sacrificed at 4 weeks following MI was 42±7.4% pre-MI, 36±6.6% post-MI, and 26±7.4% 4 weeks after MI (Figure 2d). EF was shown to be significantly different with respect to time in the adult sheep sacrificed 4 weeks after MI (p<0.05). Post hoc analysis demonstrated that there was no significant difference between EF pre-MI and post-MI (p=0.10); however, there was a statistically significant decline in EF at 4 weeks compared to both pre-MI and post-MI (p<0.05). EF in fetal sheep sacrificed at 3 days following MI was 57±6.6% pre-MI, 59±9.4% post-MI, and 59±6.5% 3 days after MI (Figure 2c). EF in fetal sheep sacrificed at 4 weeks following MI was 53±8.1% pre-MI, 54±9.6% post-MI, and 55±8.8% 4 weeks after MI. There was no significant difference in EF with respect to time for fetal sheep sacrificed at 3 days (p=0.37) or 4 weeks (p=0.31) after MI.
Infarct length in the adult sheep sacrificed at 3 days following MI was 6.5±0.4 cm post-MI and 6.5±0.4 cm 3 days after MI (p=1.00) (Figure 2f). Infarct length in the adult sheep sacrificed at 4 weeks following MI significantly increased in size from 6.9±0.8 cm post-MI to 7.9±1.1 cm 4 weeks after MI (p<0.05) demonstrating progressive infarct expansion (stretching) (Figure 2f). Infarct length in fetal sheep sacrificed at 3 days following MI was 0.98±0.35 cm post-MI and 0.91±0.25 cm 3 days following MI (p=0.72) (Figure 2e). Infarct length in fetal sheep sacrificed at 4 weeks following MI was 0.92±0.21 cm post-MI, and no wall motion abnormality could be detected at 4 weeks following MI (p<0.05) (Figure 2e).
Gross inspection of the adult left ventricle 4 weeks following infarction demonstrates obvious ventricular wall thinning and scar formation in the area of the infarct (Figure 3b) whereas there was no identifiable gross abnormalities seen in the fetal heart (Figure 3a). H&E staining of fetal and adult hearts 4 weeks following MI confirms that there is ventricular wall thinning and myocyte loss in the adult infarcts (Figure 3d), which is not present in the fetal infarcts (Figure 3c). Histological assessment of scar formation using Mason’s Trichrome staining demonstrated myocyte loss with extensive fibrosis or scar formation in the adult infarcts (Figure 3f), which corresponds to the akinetic segment of the LV wall seen on echocardiogram. In contrast, the fetal infarcts demonstrated little histologic evidence of scar formation 4 weeks following infarction and had relatively normal appearing myocardium (Figure 3e) which corresponds with the restoration of myocardial function seen by echocardiogram.
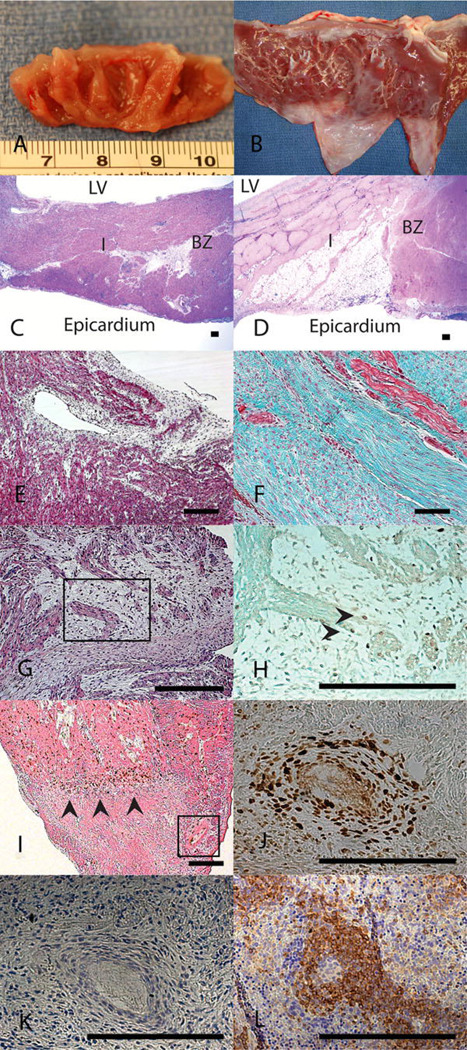
Figure 3.
Fetal cardiac ventricular remodeling following MI proceeds with regeneration of myocardium and without scar formation. 4 weeks after myocardial infarction (a) fetal hearts show no gross evidence of fibrosis while (b) adult hearts show apical fibrosis and ventricular wall thinning. H&E staining at 4 weeks demonstrates (c) no evidence of myocyte loss or ventricular wall thinning in the fetal heart (I) infarct or (BZ) borderzone and (d) significant myocyte loss and ventricular wall thinning in the adult infarct (I) (20×). Masson’s trichrome staining at 4 weeks following MI confirms that there is (e) minimal fibrosis in the fetal infarct (100×) and (f) an exuberant fibrotic response in the adult infarct (100×). (g) 4 weeks following myocardial infarction, H&E staining of the fetal infarct shows disordered clusters of cells with minimal surrounding fibrosis suggesting myocardial regeneration (200×). (h) BrdU immunostaining with Lichgrün counterstain on a serial section demonstrates that within these clusters, there are cycling, BrdU positive, cardiomyocytes marked by the arrowheads confirming myocardial proliferation within the infarct (400×). (i) BrdU immunostaining with eosin counterstain on a fetal heart 3 days following MI demonstrates a polarity for BrdU positive cells which accumulate around the area of infarction (marked with arrowheads, 50× optical, 2× digital) and (j) surrounding blood vessels within the infarct (serial section with Lichgrün counterstain, 400×). (k) CD45 immunostaining on a serial section of this blood vessel within the fetal infarct demonstrates that these BrdU positive cells are not positive for CD45, indicating they are not of hematopoetic lineage. (l) Positive control of CD45 immunohistochemistry on a fetal sheep spleen. Scale bars equal 200µm.
Fetal Myocardial Regeneration
At 4 weeks, BrdU staining demonstrated isolated areas with disorganized clusters of myocytes in the area of fetal infarction and numerous BrdU positive cardiomyocytes, consistent with cardiomyocyte proliferation (Figure 3g, 3h). At 3 days following infarction, BrdU staining demonstrated proliferating cells surrounding the area of infarction (Figure 3i) and surrounding blood vessels near the infarct (Figure 3j). These cells were CD45- and therefore likely not of hematopoietic origin (Figure 3k). This is distinctly different from the adult response following MI where there is minimal myocardial proliferation [16]. Myocardial proliferation within the fetal infarct (Figure 3g, 3h) and echocardiographic evidence of fully functional myocardium following infarction (normal EF and no wall motion abnormality) (Figures 2c, 2e) is consistent with fetal myocardial regeneration.
Inflammatory Response and Persistent Apoptosis
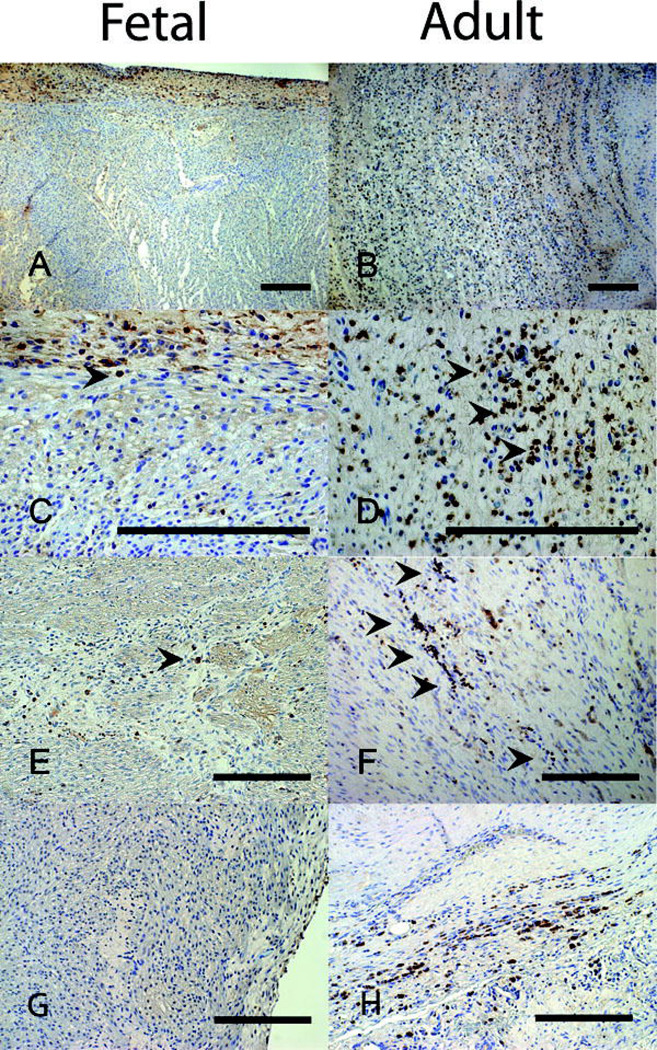
There was a minimal cellular inflammatory response in the fetal hearts at 3 days, 7 days, and 4 weeks following infarction. In the adult sheep, the cellular inflammatory response was greatest 7 days following infarction, and the adult had numerous CD45+ inflammatory cells in the area of infarction (Figure 4b, 4d) compared to relatively few seen in the fetus (Figure 4a, 4c). At 4 weeks, the adult infarcts continued to have persistent clusters of CD45+ inflammatory cells (Figure 4f) whereas there were minimal inflammatory cells seen in the fetal infarcts (Figure 4e). In order to assess the level of apoptosis over time following MI, we performed immunohistochemistry for activated caspase-3 staining. 3 days following infarction both fetal and adult infarcts demonstrated apoptosis. However, 4 weeks following MI adult infarcts demonstrated continued apoptosis (Figure 4h). In contrast, there was no observable activated caspase-3 staining in the fetal infarct 4 weeks after MI (Figure 4g).
Figure 4.
CD45 immunmohistochemistry following myocardial infarction demonstrates markedly less cellular inflammatory response in fetal verses adult hearts. 7 days following infarction the fetal heart (a-100×, c-400×) shows minimal numbers of inflammatory cells while the adult heart (b-100×, d-400×) shows a large inflammatory infiltrate. At 4 weeks following infarction, the number of inflammatory cells in both the (e) fetal and (f) adult hearts has decreased, but the adult heart has persistent scattered areas of inflammation not seen in the fetus (200×). (g) Caspase-3 staining in the fetal heart 4 weeks after infarction fails to show any apoptosis (200×). (h) Caspase-3 staining in the adult infarct 4 weeks after infarction demonstrates continuing apoptosis (200×). Scale bars equal 200µm.
Discussion
Our findings demonstrate a marked difference between the fetal and adult responses to myocardial infarction. The adult paradigm of healing by reparative scar formation was demonstrated by the replacement of myocardium with collagen, infarct expansion, and decline in cardiac function and was associated with a robust and prolonged cellular inflammatory response. The fetus, on the other hand, demonstrated the capability of healing by myocardial regeneration and was associated with minimal cellular inflammatory response and cellular proliferation both in the fetal infarct and borderzone. This provides the first model of regenerative healing in the heart following MI and demonstrates an association between the cellular inflammatory response, infarct expansion and ventricular remodeling following injury.
Acute MI afflicts over one million Americans each year, and despite modern reperfusion and pharmacologic therapy, the five year post-MI mortality remains near 20% with 70% of all heart failure cases due to post-MI LV remodeling [17–19]. Therefore, loss of myocardial function following MI is the cause of significant morbidity and mortality, and the fetal response to MI demonstrates that complete restoration of cardiac function with no pathologic LV remodeling is possible. We believe that this novel model of regenerative cardiac healing can be used to identify factors that are important in promoting the regenerative phenotype as has been done in other tissues. Based on our research in fetal scarless dermal healing, we have developed strategies to alter the wound environment and promote regenerative healing in the adult [12,13]. The same approach should be applied to investigate novel therapies to promote cardiac regeneration and minimize the pathologic LV remodeling that is seen in the adult.
Our model of fetal regenerative cardiac healing utilizes a permanent coronary artery ligation that was developed from an adult ovine MI model that has been shown to result in significant pathologic LV remodeling and decline in function [14]. The fetal and adult models both used ligation of the LAD and D2 at points 40% of the distance from the apex to the base. In the adult, we have previously correlated the size of the infarct with the IL/DL ratio. In this study we found that although we used an identical ligation strategy in the fetus and the adult, the IL/DL ratio is slightly smaller in the fetus. This difference may be the result of small differences in echocardiographic technique between adult and fetal animals. While it is possible that these data indicate that the fetal infarcts encompass a smaller portion of the LV mass, the consistence of our surgical technique as well as echocardiographic and direct visual inspection of a large wall motion abnormality strongly suggest that fetal hearts were subjected to a large infarction and a strong remodeling stimulus.
Our data demonstrates an association between decreased inflammation and fetal regenerative cardiac healing. Studies in other tissues have also demonstrated that fetal regenerative healing proceeds in the face of a diminished inflammatory response [7–11]. Myocardial infarction in the adult has been shown to induce a dramatic inflammatory response resulting in ventricular scar formation and leading to progressive infarct expansion and LV aneurysm formation (2,4,14]. The adult infarcts in this study also demonstrated a prolonged inflammatory cell infiltration and ongoing apoptosis. While our study does not prove that increased inflammation is causally linked to pathologic LV remodeling and continued cardiomyocyte cell death, it is reasonable to hypothesize that this is the case. Inflammation has been linked to fibrosis in multiple different organs including the heart [20–23]. Given the strong association we have shown between fetal regenerative cardiac healing and diminished inflammation, it is possible that cellular inflammation following MI in the adult affects downstream targets driving fibroplasia and potentiating ongoing cardiomyocyte apoptosis. However, further studies are needed to demonstrate a causal relationship. This study provides a basis for further research into therapies suppressing the inflammatory response following MI with the goal of minimizing ongoing apoptosis, promoting myocardial regeneration, preventing fibrosis, and preserving function.
While the inflammatory response may be one key difference between fetal regenerative cardiac healing and adult reparative cardiac healing, there are many other potential differences which may contribute to regenerative healing in the fetus and pathologic LV remodeling in the adult. We hypothesize that there are important differences in the extracellular matrix, cell migration, gene expression, and progenitor cell function between the fetal and adult hearts. Future studies are needed to identify additional factors that control fetal regenerative cardiac healing and adult pathologic LV remodeling following MI.
Our data clearly demonstrate regeneration of myocardium following MI in the fetus, but it is not clear what cells are most important in this process. Our findings suggest that proliferation of differentiated cardiomyocytes is partially responsible for the regenerated myocardium. Prior studies on fetal cardiac regeneration support this finding and have demonstrated that the majority of proliferating cells in the fetus are differentiated cardiomyocytes instead of either local or circulating progenitor cells [24]. The increased cellular proliferation that we observed surrounding the infarct 3 days after fetal MI is unlikely exclusively due cardiomyocyte proliferation in the borderzone. A portion of the cellular proliferation observed is likely due to progenitor cell populations and endothelial cells. Further studies are needed to better define the contributions of these cell populations to fetal myocardial regeneration following MI.
One caution when interpreting our study is that comparing fetal cellular processes with those of the adult may have a confounding variable of the stage of development. At this time, there is no model of fetal pathologic LV remodeling following MI. Development of such a model would eliminate this confounding variable; however, using the models described in our study to compare fetal regenerative cardiac healing with adult reparative cardiac healing still has the potential to identify important factors controlling cardiac regeneration following MI. Studies comparing fetal scarless wound healing with adult scar formation have identified important factors, such as IL-10, that can be used therapeutically to alter the adult healing response [13]. We propose that using this model of fetal regenerative cardiac healing in future research has the potential to further our knowledge of cardiac regeneration and lead to novel strategies to alter the adult response to MI. However, future investigations should also take into consideration the effect that stage of development has on cellular processes.
In conclusion, there has been little change in the natural history of post-MI ventricular remodeling and the associated morbidity and mortality over the last 50 years despite robust research efforts on the topic [25]. It is clear that following a large LV myocardial infarction, replacement of myocardium with collagen scar does not preserve function over time. Our research demonstrates that diminished inflammation is associated with fetal cardiac regenerative healing, and we propose that intensive research into other factors promoting fetal regenerative healing following MI will allow for the development of therapies that can promote regenerative healing in the adult.
Acknowledgments
The authors would like to recognize Mio Noma and Robert C. Lind for assisting with surgery and animal care, Theodore Plappert for his work performing and analyzing the echocardiograms, and Antoneta Radu for her contribution to the histology and immunohistochemistry presented in the manuscript.
This research was supported by National Institutes of Health Grants DK083085-01, HL63954, HL71137, HL76560 (Bethesda, MD), institutional development funds from the Children’s Hospital of Philadelphia, and individual Established Investigator Awards from the American Heart Association (Dallas, TX) to J. Gorman and R. Gorman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 23rd EACTS Annual Meeting, Vienna, Austria
References
- 1.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A Receptor Activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 2.St. John Sutton M, Sharpe N. Left ventricular remodeling after infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Cleutjens JPM, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Hiasa Y, Ohara Y, Miyazaki S, Ogura R, Miyajima H, Yuba K, Suzuki N, Hosokawa S, Kishi K, Ohtani R. Relation between neutrophil counts on admission, microvascular injury, and left ventricular functional recovery in patients with an anterior wall first acute myocardial infarction treated with primary coronary angioplasty. Am J Cardiol. 2007;100:35–40. doi: 10.1016/j.amjcard.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Åströ-Olsson K, Hedström E, Hultén LM, Wiklund O, Arheden H, Öhlin AK, Gottsäter A, Öhlin H. Dissociation of the inflammatory reaction following PCI for acute myocardial infarction. J Invasive Cardiol. 2007;19:452–456. [PubMed] [Google Scholar]
- 6.Ogino A, Takemura G, Kanamori H, Okada H, Maruyama R, Miyata S, Esaki M, Nakagawa M, Aoyama T, Ushikoshi H, Kawasaki M, Minatoguchi S, Fujiwara T, Fujiwara H. Amlodipine inhibits granulation tissue cell aptptosis through reducing calcineurin activity to attenuate postinfarction cardiac remodeling. Am J Physiol Heart Circ Physiol. 2007;293:2271–2280. doi: 10.1152/ajpheart.00303.2007. [DOI] [PubMed] [Google Scholar]
- 7.Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, Bradley SM, Stern R, Ferguson MWJ, Harrison MR. Studies in fetal wound healing: VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–69. doi: 10.1016/s0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- 8.Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, Scheuenstuhl H, Goodson WH., III Comparison of fetal, newborn and adult wound healing by histologic enzyme-histochemical and hydroxyproline determination. J Pediatr Surg. 1985;20:315–319. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- 9.Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: A study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 10.Liechty KL, Crombleholme TM, Adzick NS. Decreased IL-6 in Scarless Human Fetal Repair. Cytokine. 2000;12:671–676. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- 11.Liechty KW, Crombleholme TM, Cass DL, Martin B, MS, Adzick NS. Diminished Interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77:80–84. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- 12.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal Wound Repair Results in Scar Formation in Interleukin-10 (IL-10) Deficient Mice in a Syngeneic Murine Model of Scarless Fetal Wound Repair. J Pediatr Surg. 2000;35:866–873. doi: 10.1053/jpsu.2000.6868. [DOI] [PubMed] [Google Scholar]
- 13.Peranteau WH, Zhang L, Murvarak N, Badillo AT, Radu A, Zoltick PW, Liechty KW. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 14.Markovitz LJ, Savage EB, Ratcliffe MB, Bavaria JE, Kreiner G, Iozzo RV, Hargrove WC, 3rd, Bogen DK, Edmunds LH., Jr Large animal model of left ventricular aneurysm. Ann Thorac Surg. 1989;48:838–845. doi: 10.1016/0003-4975(89)90682-6. [DOI] [PubMed] [Google Scholar]
- 15.Folland ED, Parisi AF, Moynihan PF, Jones DR, Feldman CL, Tow DE. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionucleotide techniques. Circulation. 1979;64:760–766. doi: 10.1161/01.cir.60.4.760. [DOI] [PubMed] [Google Scholar]
- 16.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomuocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics 2008 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Bonow RO. Chronic heart failure in the United States. A manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 19.Kaul P, Armstrong PW, Chang WC, Naylor CD, Granger CB, Lee KL, Peterson ED, Califf RM, Topol EJ, Mark DB. Long-term mortality of patients with acute myocardial infarction in the United States and Canada. Comparison of the patients enrolled in global utilization of streptokinase and tPA for occluded arteries - Gusto-1. Circulation. 2004;110:1754–1760. doi: 10.1161/01.CIR.0000142671.06167.91. [DOI] [PubMed] [Google Scholar]
- 20.Elias JA, Freundlich B, Kern JA, Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990;97:1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Antonini JM, Rojanasakul Y, Castranova V, Scabilloni JF, Mercer RR. Potential role of apoptotic macrophages in pulmonary inflammation and fibrosis. J Cell Physiol. 2002;194:215–224. doi: 10.1002/jcp.10220. [DOI] [PubMed] [Google Scholar]
- 22.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, Carretero OA. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension. 2004;43:229–236. doi: 10.1161/01.HYP.0000107777.91185.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drenckhahn JD, Schwartz QP, Gray S, Laskowski A, Kiriazis H, Ming Z, Harvey RP, Du XJ, Thorburn DR, Cox TC. Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev Cell. 2008;15:521–533. doi: 10.1016/j.devcel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Longterm trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]