Abstract
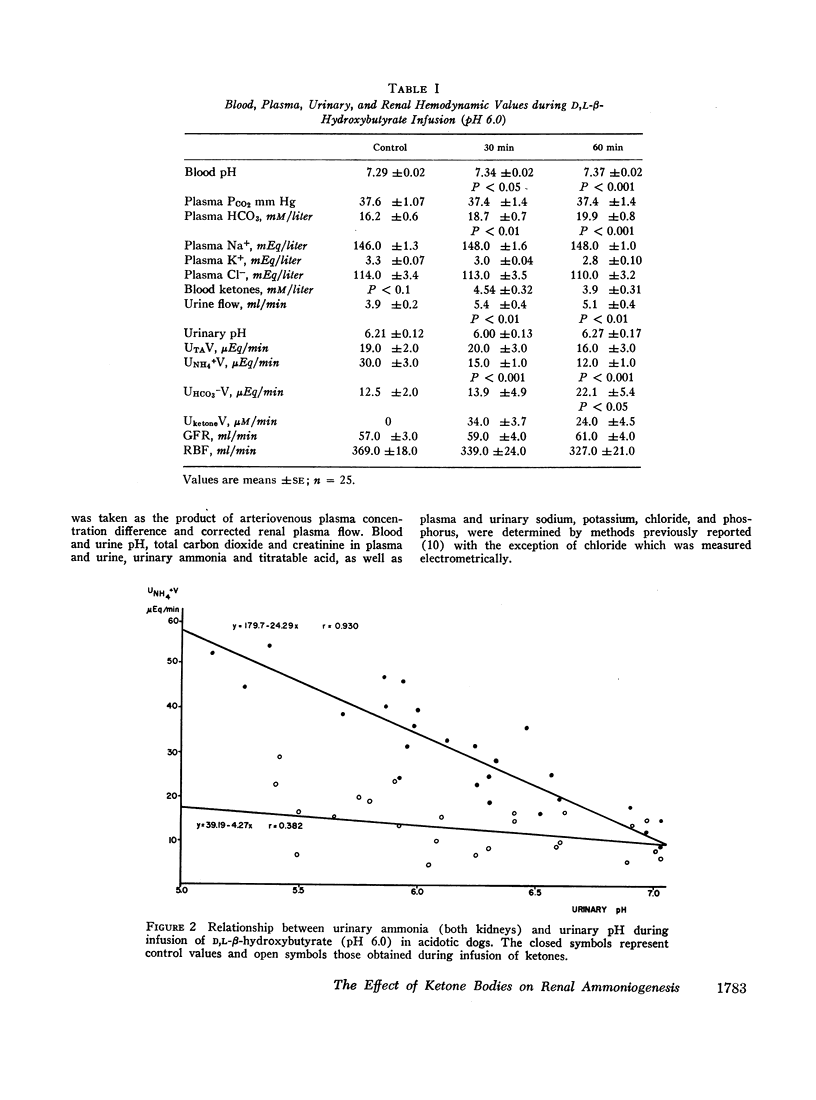
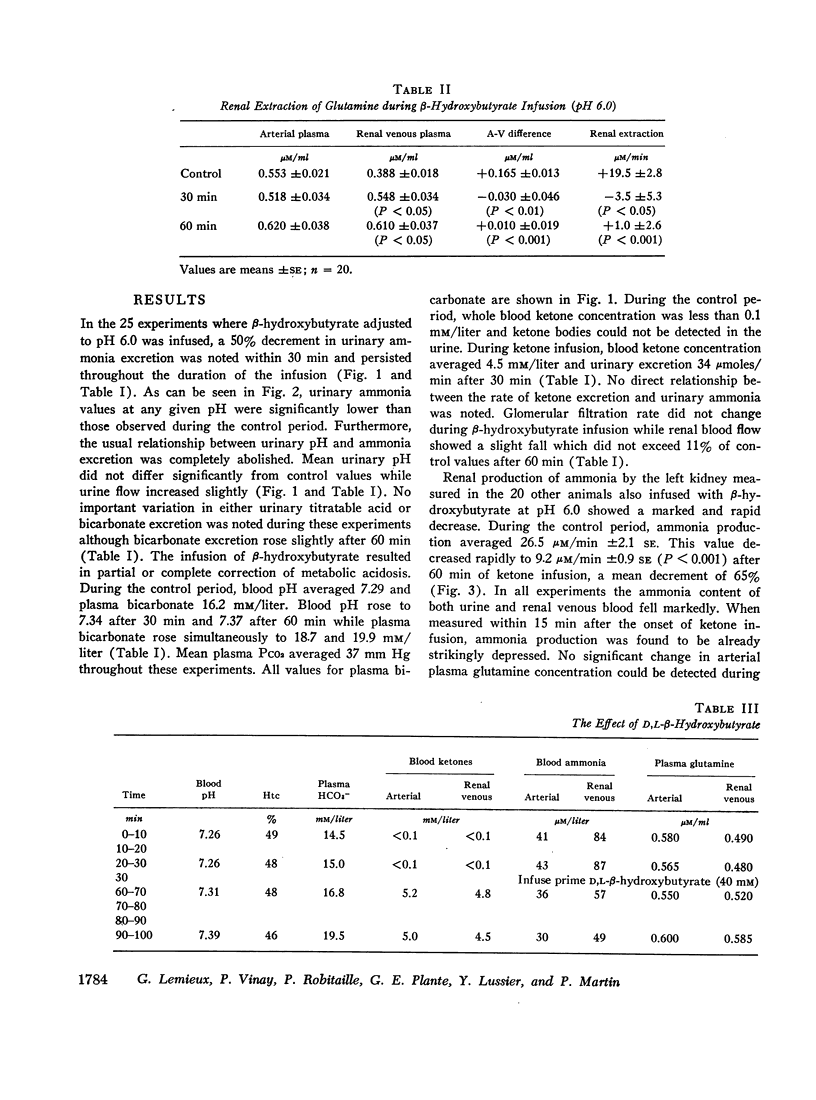
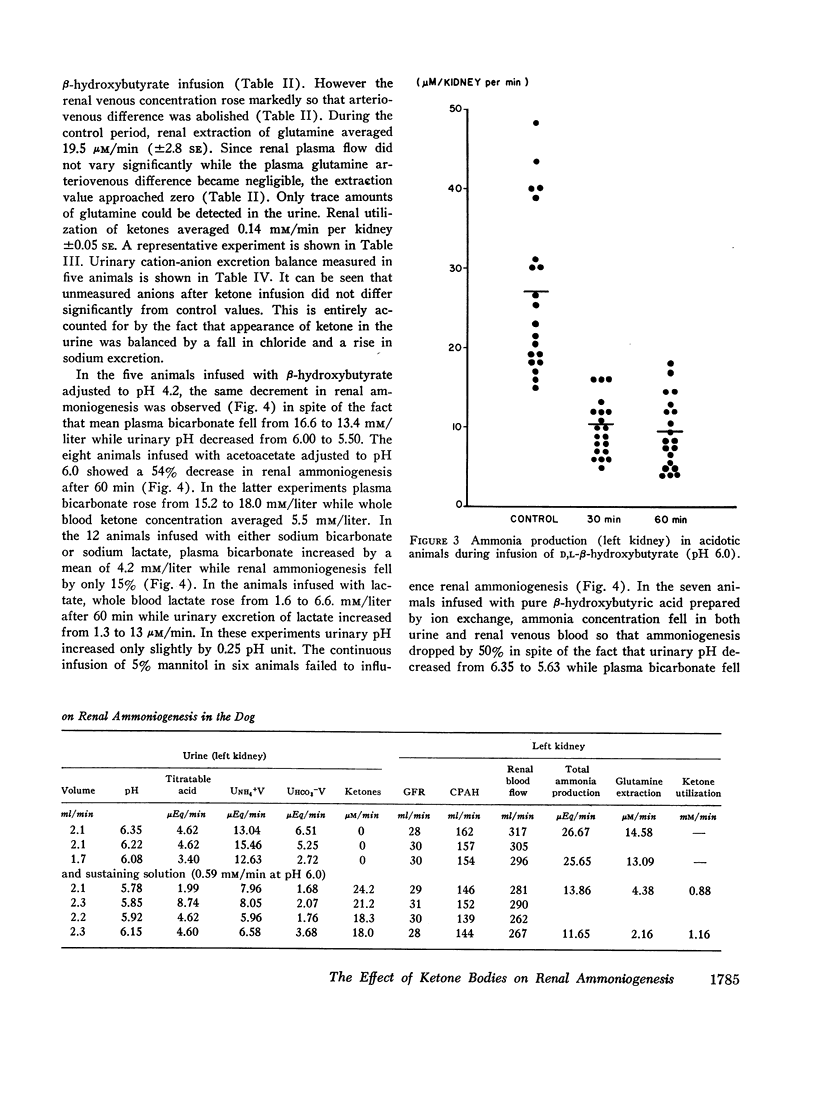
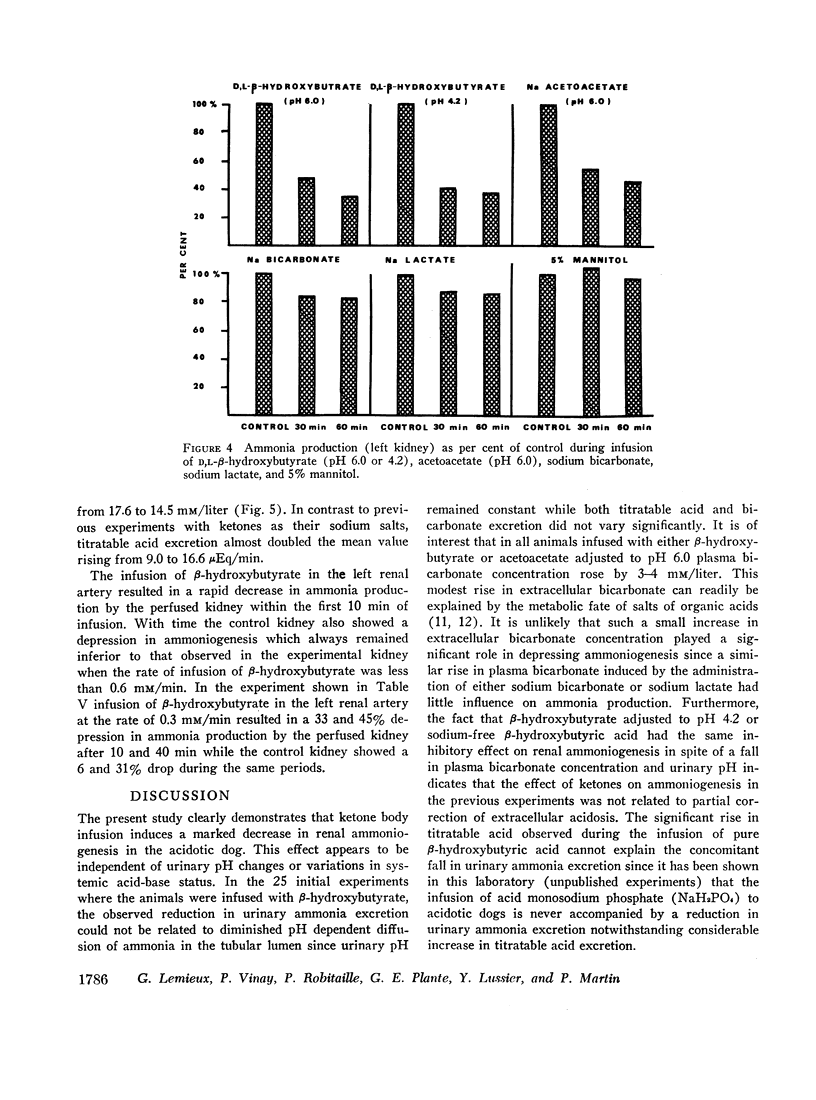
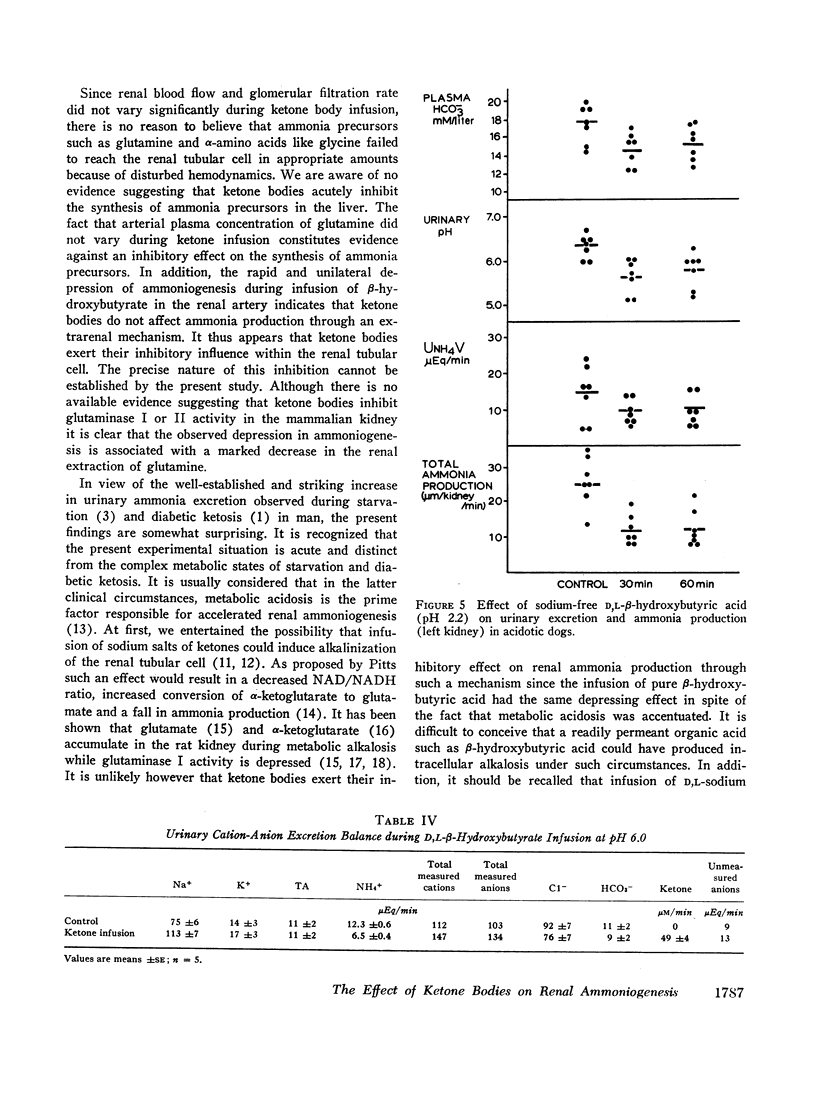
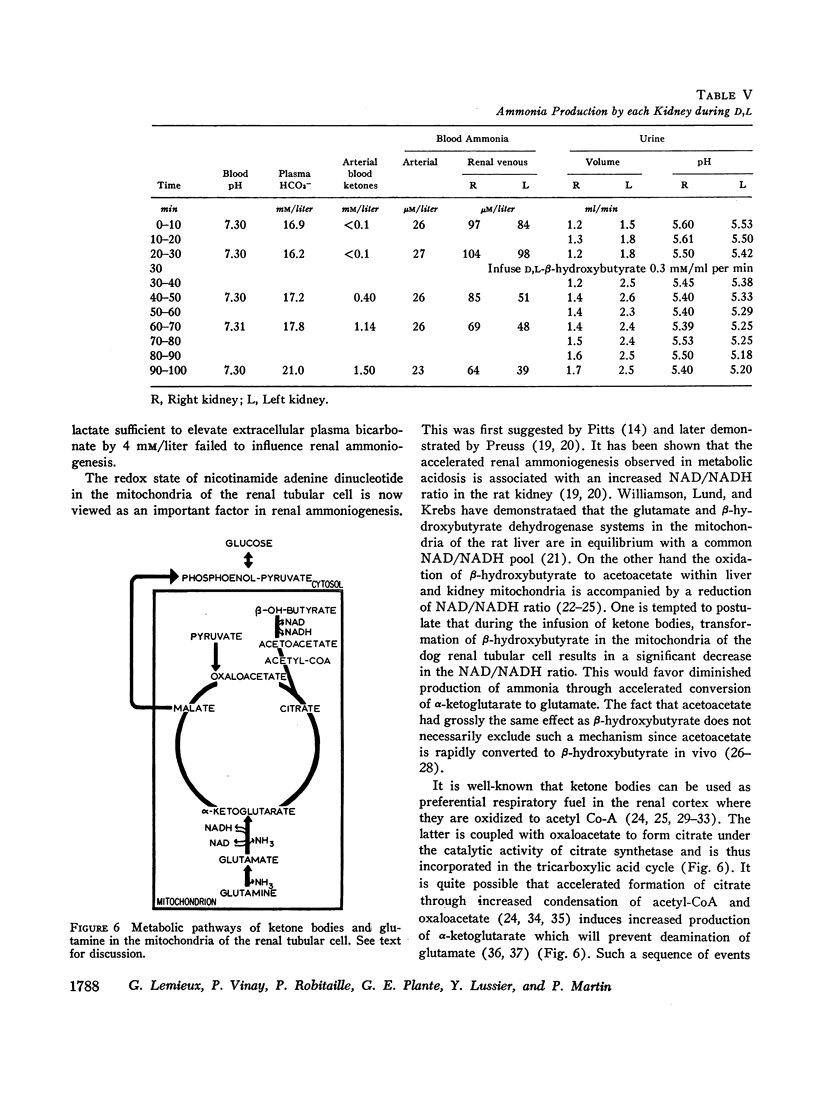
Infusion of ketone bodies to ammonium chloride-loaded acidotic dogs was found to induce significant reduction in urinary excretion of ammonia. This effect could not be attributed to urinary pH variations. Total ammonia production by the left kidney was measured in 25 animals infused during 90 min with the sodium salt of D,L-β-hydroxybutyric acid adjusted to pH 6.0 or 4.2. Ketonemia averaged 4.5 mM/liter. In all experiments the ammonia content of both urine and renal venous blood fell markedly so that ammoniogenesis was depressed by 60% or more within 60 min after the onset of infusion. Administration of equimolar quantities of sodium acetoacetate adjusted to pH 6.0 resulted in a 50% decrease in renal ammonia production. Infusion of ketone bodies adjusted to pH 6.0 is usually accompanied by a small increase in extracellular bicarbonate (3.7 mM/liter). However infusion of D,L-sodium lactate or sodium bicarbonate in amounts sufficient to induce a similar rise in plasma bicarbonate resulted in only a slight decrement in ammonia production (15%). The continuous infusion of 5% mannitol alone during 90-150 min failed to influence renal ammoniogenesis. Infusion of pure sodium-free β-hydroxybutyric acid prepared by ion exchange (pH 2.2) resulted in a 50% decrease in renal ammoniogenesis in spite of the fact that both urinary pH and plasma bicarbonate fell significantly. During all experiments where ketones were infused, the renal extraction of glutamine became negligible as the renal glutamine arteriovenous difference was abolished. Renal hemodynamics did not vary significantly. Infusion of β-hydroxybutyrate into the left renal artery resulted in a rapid decrease in ammoniogenesis by the perfused kidney. The present study indicates that ketone bodies exert their inhibitory influence within the renal tubular cell. Since their effect is independent of urinary or systemic acid-base changes, it is suggested that they depress renal ammoniogenesis by preventing the transformation of glutamine and glutamate into α-ketoglutarate in the mitochondria of the renal tubular cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNS F. H., HORN H. D. Quantitative Bestimmung von L(+)-Milchsäure mit Milchsäuredehydrogenase. Biochim Biophys Acta. 1956 Aug;21(2):378–380. doi: 10.1016/0006-3002(56)90023-3. [DOI] [PubMed] [Google Scholar]
- Balagura-Baruch S., Shurland L. M., Welbourne T. C. Effects of alpha-ketoglutarate on renal ammonia release in the intact dog. Am J Physiol. 1970 Apr;218(4):1070–1075. doi: 10.1152/ajplegacy.1970.218.4.1070. [DOI] [PubMed] [Google Scholar]
- Churchill P. C., Malvin R. L. Relation of renal gluconeogenesis to ammonia production in the dog. Am J Physiol. 1970 Jan;218(1):241–245. doi: 10.1152/ajplegacy.1970.218.1.241. [DOI] [PubMed] [Google Scholar]
- DEVLIN T. M., BEDELL B. H. Effect of acetoacetate on the oxidation of reduced diphosphopyridine nucleotide by intact rat liver mitochondria. J Biol Chem. 1960 Jul;235:2134–2139. [PubMed] [Google Scholar]
- GOLDFINGER S., KLINENBERG E., Jr, SEEGMILLER J. E. RENAL RETENTION OF URIC ACID INDUCED BY INFUSION OF BETA-HYDROXYBUTYRATE AND ACETOACETATE. N Engl J Med. 1965 Feb 18;272:351–355. doi: 10.1056/NEJM196502182720705. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L., COPENHAVER J. H., Jr Relation of glutaminase I activity to glutamic acid concentration in the rat kidney. Am J Physiol. 1960 Feb;198:227–229. doi: 10.1152/ajplegacy.1960.198.2.227. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Pathways of glutamine deamination and their control in the rat kidney. Am J Physiol. 1967 Oct;213(4):983–989. doi: 10.1152/ajplegacy.1967.213.4.983. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Goldstein L., Schooler J. M. Regulation of ammonia production in the rat kidney. Adv Enzyme Regul. 1967;5:71–86. doi: 10.1016/0065-2571(67)90009-x. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorno W. E., Rector F. C., Jr, Seldin D. W. Relation of renal gluconeogenesis to ammonia production in the dog and rat. Am J Physiol. 1967 Oct;213(4):969–974. doi: 10.1152/ajplegacy.1967.213.4.969. [DOI] [PubMed] [Google Scholar]
- HUMOLLER F. L., BARAK A. J., HOLTHAUS J. M. DISTRIBUTION OF AMMONIA IN CELLS AND PLASMA. Clin Chem. 1964 Jul;10:589–596. [PubMed] [Google Scholar]
- Hird F. J., Marginson M. A. The formation of ammonia from glutamine and glutamate by mitochondria from rat liver and kidney. Arch Biochem Biophys. 1968 Sep 20;127(1):718–724. doi: 10.1016/0003-9861(68)90282-8. [DOI] [PubMed] [Google Scholar]
- KLINGMAN J. D., HANDLER P. Partial purification and properties of renal glutaminase. J Biol Chem. 1958 May;232(1):369–380. [PubMed] [Google Scholar]
- KREBS H. A., SPEAKE R. N., HEMS R. ACCELERATION OF RENAL GLUCONEOGENESIS BY KETONE BODIES AND FATTY ACIDS. Biochem J. 1965 Mar;94:712–720. doi: 10.1042/bj0940712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. The physiological role of the ketone bodies. Biochem J. 1961 Aug;80:225–233. doi: 10.1042/bj0800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm D. E., Cahill G. F., Jr Effect of acid-base status on renal and hepatic gluconeogenesis in diabetes and fasting. Am J Physiol. 1969 May;216(5):1207–1212. doi: 10.1152/ajplegacy.1969.216.5.1207. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The regulation of the release of ketone bodies by the liver. Adv Enzyme Regul. 1966;4:339–354. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., GREVILLE G. D. The enzymic oxidation of alpha- and 2-beta-hydroxybutyrate. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):188–202. doi: 10.1016/0006-3002(53)90138-3. [DOI] [PubMed] [Google Scholar]
- LEMIEUX G., GERVAIS M. ACUTE CHLORIDE DEPLETION ALKALOSIS: EFFECT OF ANIONS ON ITS MAINTENANCE AND CORRECTION. Am J Physiol. 1964 Dec;207:1279–1286. doi: 10.1152/ajplegacy.1964.207.6.1279. [DOI] [PubMed] [Google Scholar]
- LIPSKY S. R., APLER B. J., RUBINI M. E., VAN ECK W. F., GORDON M. E. The effects of alkalosis upon ketone body production and carbohydrate metabolism in man. J Clin Invest. 1954 Sep;33(9):1269–1276. doi: 10.1172/JCI103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYON J. B., Jr, BLOOM W. L. The use of furfural for the determination of acetone bodies in biological fluids. Can J Biochem Physiol. 1958 Oct;36(10):1047–1056. [PubMed] [Google Scholar]
- Lea M. A., Weber G. Role of enzymes in homeostasis. 8. Inhibition of the activity of glycolytic enzymes by free fatty acids. J Biol Chem. 1968 Mar 25;243(6):1096–1102. [PubMed] [Google Scholar]
- Lemieux G., Plante G. E. The effect of starvation in the normal dog including the Dalmatian coach hound. Metabolism. 1968 Jul;17(7):620–630. doi: 10.1016/0026-0495(68)90021-8. [DOI] [PubMed] [Google Scholar]
- MCCANN W. P. The oxidation of ketone bodies by mitochondria from liver and peripheral tissues. J Biol Chem. 1957 May;226(1):15–22. [PubMed] [Google Scholar]
- McGarry J. D., Guest M. J., Foster D. W. Ketone body metabolism in the ketosis of starvation and alloxan diabetes. J Biol Chem. 1970 Sep 10;245(17):4382–4390. [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARMEGGIANI A., BOWMAN R. H. REGULATION OF PHOSPHOFRUCTOKINASE ACTIVITY BY CITRATE IN NORMAL AND DIABETIC MUSCLE. Biochem Biophys Res Commun. 1963 Aug 1;12:268–273. doi: 10.1016/0006-291x(63)90294-8. [DOI] [PubMed] [Google Scholar]
- Pitts R. F. The renal metabolism of ammonia. Physiologist. 1966 May;9(2):97–109. [PubMed] [Google Scholar]
- Preuss H. G. Pyridine nucleotides in renal ammonia metabolism. J Lab Clin Med. 1968 Sep;72(3):370–382. [PubMed] [Google Scholar]
- Preuss H. G. Renal glutamate metabolism in acute metabolic acidosis. Nephron. 1969;6(3):235–246. doi: 10.1159/000179731. [DOI] [PubMed] [Google Scholar]
- RAPOPORT A., FROM G. L., HUSDAN H. METABOLIC STUDIES IN PROLONGED FASTING. I. INORGANIC METABOLISM AND KIDNEY FUNCTION. Metabolism. 1965 Jan;14:31–46. doi: 10.1016/0026-0495(65)90079-x. [DOI] [PubMed] [Google Scholar]
- SAYRE F. W., ROBERTS E. Preparation and some properties of a phosphateactivated glutaminase from kidneys. J Biol Chem. 1958 Nov;233(5):1128–1134. [PubMed] [Google Scholar]
- SCHWAB L., LOTSPEICH W. D. Renal tubular reabsorption of acetoacetate in the dog. Am J Physiol. 1954 Feb;176(2):195–200. doi: 10.1152/ajplegacy.1954.176.2.195. [DOI] [PubMed] [Google Scholar]
- Sherrard D. J., Simpson D. P. An improved method for the microdetermination of glutamine in plasma and urine. J Lab Clin Med. 1969 May;73(5):877–882. [PubMed] [Google Scholar]
- Smith H. W., Finkelstein N., Aliminosa L., Crawford B., Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945 May;24(3):388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUBBS P. K. INHIBITION OF CITRATE FORMATION BY LONG-CHAIN ACYL THIOESTERS OF COENZYME A AS A POSSIBLE CONTROL MECHANISM IN FATTY ACID BIOSYNTHESIS. Biochim Biophys Acta. 1963 Oct 22;70:608–609. doi: 10.1016/0006-3002(63)90804-7. [DOI] [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Control of glycolysis and gluconeogenesis in rat kidney cortex slices. Biochem J. 1967 Jul;104(1):300–305. doi: 10.1042/bj1040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIELAND O., WEISS L. INHIBITION OF CITRATE-SYNTHASE BY PALMITYL-COENZYME A. Biochem Biophys Res Commun. 1963 Sep 10;13:26–31. doi: 10.1016/0006-291x(63)90156-6. [DOI] [PubMed] [Google Scholar]
- WIELAND O., WEISS L. Increase in liver acetyl-coenzyme A during ketosis. Biochem Biophys Res Commun. 1963 Feb 18;10:333–339. doi: 10.1016/0006-291x(63)90534-5. [DOI] [PubMed] [Google Scholar]
- Weber G., Lea M. A., Convery H. J., Stamm N. B. Regulation of gluconeogenesis and glycolysis: studies of mechanisms controlling enzyme activity. Adv Enzyme Regul. 1967;5:257–300. doi: 10.1016/0065-2571(67)90020-9. [DOI] [PubMed] [Google Scholar]
- Weber G., Lea M. A., Stamm N. B. Sequential feedback inhibition and regulation of liver carbohydrate metabolism through control of enzyme activity. Adv Enzyme Regul. 1968;6:101–123. doi: 10.1016/0065-2571(68)90009-5. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]



