Abstract
Objective
To examine both the source of follistatin-like protein 1 (FSTL-1) and factors that induce its expression in arthritis and to determine whether juvenile rheumatoid arthritis (JRA) is characterized by over-expression of FSTL-1.
Methods
FSTL-1 expression patterns were analyzed by immunohistochemistry on joints derived from mice with collagen-induced arthritis. Induction of FSTL-1 secretion was assessed in osteoblasts, adipocytes, and human fibroblast-like synoviocytes in response to TGF-β, IL-1β, TNF-α, and IL-6. Finally, sera and synovial fluids from children with oligoarticular, polyarticular, and systemic-onset JRA were assayed for FSTL-1 using a custom ELISA. FSTL-1 concentrations were correlated with erythrocyte sedimentation rate (ESR) and platelet count.
Results
Immunohistochemical staining of joint sections demonstrated expression of FSTL-1 in all cell types of the mesenchymal lineage, including osteocytes, chondrocytes, adipocytes, and fibroblasts. FSTL-1 could be induced in osteoblasts, adipocytes, and human fibroblast-like synoviocytes by TGF-β, IL-1β, TNF-α, and IL-6. The IL-1β response was significantly greater than the TNF-α response (p < 0.05). Only the systemic-onset JRA subtype had elevated serum and synovial fluid FSTL-1 concentrations. Synovial fluid concentrations were 2–3-fold higher than serum concentrations. The elevation in serum FSTL-1 concentrations seen in systemic-onset JRA correlated closely with elevated ESR and platelet count.
Conclusion
These findings demonstrate that the arthritic joint matrix is a major source of FSTL-1 and that IL-1β is a central mediator of FSTL-1 secretion. Furthermore, FSTL-1 may represent a useful biomarker of disease activity in systemic-onset JRA.
Juvenile rheumatoid arthritis (JRA) encompasses a heterogeneous group of diseases that are important causes of morbidity in children. JRA affects an estimated 250,000 children in the United States. The American College of Rheumatology (ACR) has classified JRA into a number of subtypes, including systemic-onset, polyarthritis, and oligoarthritis (1). Each of these subtypes has a different clinical presentation, prognosis, and response to specific therapies, suggesting that they differ in their pathogenesis and pathophysiology. For instance, polyarticular JRA responds well to anti-TNF therapy (2, 3) while systemic-onset JRA does not (4, 5). Systemic-onset JRA also differs from the other forms of JRA in that the arthritis is often accompanied by fever, rash, organomegally, leukocytosis, and other systemic features in addition to arthritis. These systemic features can precede the development of arthritis by months or years, making the diagnosis at times difficult.
A number of biomarkers exist for aiding in the diagnoses and monitoring of rheumatoid arthritis (RA), including rheumatoid factor (6) and anti-citrullinated proteins (CCP) (7, 8). However, these markers are usually not present in JRA. The most commonly used biomarkers used in JRA include elevation in erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and platelet count, but these are non-specific.
In an effort to identify novel biomarkers for JRA (and other forms of arthritis) we recently analyzed gene expression in the mouse model of collagen-induced arthritis (CIA) and discovered that a poorly characterized gene, follistatin-like protein 1 (FSTL-1), originally cloned from an osteoblast cell line as a TGF-β inducible gene (9), was highly-overexpressed in mouse paws during early arthritis, especially at the interface of synovial pannus and eroding bone (10). FSTL-1 is highly conserved across mammalian species. Human and mouse FSTL-1 share 92% identity in their amino acid sequence. FSTL-1 has been found in RA synovial tissue (11, 12) and anti-FSTL-1 antibodies have been detected in the serum and synovial fluid of RA patients (12). It was initially reported that administration of human FSTL-1 to Balb/c mice with antibody-induced arthritis ameliorated disease (13), possibly by reducing synovial production of matrix metalloproteinases (14). The effect was modest and our own group subsequently demonstrated that FSTL-1 is a novel pro-inflammatory molecule with a previously unrecognized role in inflammation (11, 15). Transfection of FSTL-1 into macrophages and fibroblasts lead to up-regulation of proinflammatory cytokines felt to play central roles in chronic arthritis, including IL-1β and TNF-α. Induction of FSTL-1 requires NFκB (11). Over-expression of FSTL-1 in mouse paws by gene transfer resulted in severe paw swelling and arthritis, while neutralization of FSTL-1 suppressed arthritis (11). FSTL-1 was also found to be upregulated in the synovium of patients with RA, suggesting clinical relevance to our findings in the mouse model.
The current study was designed to determine the source of FSTL-1 and factors that induce its expression in arthritis. In addition we sought to determine whether JRA is characterized by over-expression of FSTL-1.
MATERIALS AND METHODS
Patient samples
Banked sera and synovial fluids were obtained from patients with JRA defined according to criteria established by the ACR (1). Patient demographics are summarized in Table 1. The study patients were recruited from the rheumatology clinic at Children’s Hospital of Pittsburgh. Banked synovial fluids were also obtained from the Cincinnati Children’s Hospital Medical Center JRA Tissue Repository. Control synovial fluids were collected from children with no history of JRA or inflammatory disease who underwent an orthopedic procedure, such as ACL repair. The synovial fluid samples were placed on ice immediately after collection, centrifuged at 400 × g for 10 minutes to remove cells and debris and stored at −80° C. The sera were allowed to clot, centrifuged at 3,000 × g for 10 minutes to remove red blood cells, and stored at −80° C. The study was approved by the Institutional Review Board at the University of Pittsburgh. Informed consent was obtained from all guardians of patients and assent was obtained from the subjects when appropriate.
Table 1.
Demographic and clinical characteristics of the study population*
| Characteristic | Oligo (n = 54) | Poly (n = 26) | Systemic (n = 15) | Control (n = 15) |
|---|---|---|---|---|
| Age, years | 9 ± 5.17 | 12 ± 6.17 | 13 ± 6.81 | 12 ± 8.31 |
| Sex, no. (%) | ||||
| Male | 18 (33) | 9 (35) | 8 (53) | 6 (40) |
| Female | 36 (67) | 17 (65) | 7 (47) | 9 (60) |
| Disease Duration, years | 3 ± 4.35 | 7 ± 5.99 | 7 ± 8.42 | N/A |
Except where indicated otherwise, values are the mean ± SD. Oligo = oligoarticular JIA; Poly = polyarticular JIA; Systemic = systemic JIA
Mice
Male DBA/1 mice, 6–10 weeks of age, were purchased from Harlan (Indianapolis, IN). Mice were housed in the animal resource facility at the Children’s Hospital of Pittsburgh Rangos Research Center (Pittsburgh, PA). The study was approved by the Children’s Hospital of Pittsburgh’s Animal Research and Care Committee. CIA was induced by intra-dermal immunization of DBA/1 mice with bovine collagen type II (Elastin Products, Owensville, MO), as previously described (16).
FSTL-1 immunoassay
For detection of human FSTL-1 in sera and synovial fluids, standard bind plates (Meso Scale Discovery (MSD), Gaithersburg, Maryland) were coated with 0.2 μg per well goat anti-human FSTL1 (AF1694; R&D Systems, Minneapolis, MN) in 0.03% Triton-X100 overnight at 4° C. Plates were washed with PBS/0.05% Tween-20 and blocked with MSD Human Serum Cytokine Assay Diluent for 1 hour. Human sera and synovial fluids, diluted 1:2 in MSD Human Serum Cytokine Assay Diluent, were added overnight at 4° C. Plates were washed and 0.5 μg/ml custom sulfo-tagged polyclonal rabbit anti-FSTL-1 was added for 4 hours. Plates were washed, 150 μl/well of 2× MSD Read Buffer was added, and plates were imaged in a MSD SECTOR Imager 2400.
Immunohistochemistry
Knee joints from mice with CIA were frozen in liquid nitrogen-cooled isopentane. Seven micron sections were prepared with the Cryojane Tape Transfer System (Instrumedics, St. Louis, MO). Slides were fixed with 2% paraformaldehyde for 20 minutes and washed with PBS followed by BSA buffer (0.5% BSA and 0.15% glycine in PBS). Slides were blocked with a 1/20 dilution of normal donkey serum (Sigma-Aldrich, St. Louis, MO) in BSA buffer and washed three times with BSA buffer. Slides were incubated for 1 hour with 10 μg/ml affinity-purified polyclonal goat anti-mouse FSTL-1 (AF1738; R&D Systems, Minneapolis, MN). The slides were washed three times with BSA buffer, and bound antibody was visualized using 4 μg/ml Alexa Fluor 488-conjugated donkey anti-goat IgG (Invitrogen, Carlsbad, CA). For identification of fibroblasts, slides were reacted with 1.5 μg/ml rat anti-mouse CD90 (BD Pharmingen, Franklin Lakes, NJ) and bound antibody was visualized with 2 μg/ml Alexa Fluor 594-conjugated donkey anti-rat IgG. Nuclei were stained with Hoechst 3343 blue (Invitrogen). A coverslip containing a drop of gelvatol was added, and slides were stored at 4° C until observation. Slides were imaged using an Olympus Fluroview 1000 (Olympus) confocal microscope or a Nikon TE2000 inverted phase-fluorescence microscope using a 12-bit 1600×1200 element CCD array to capture images (Spot, Diagnostic Instruments, Sterling Heights, MI); color, where shown, is assigned to the channel indicated and reflects approximate output fluorescence unless indicated. Filters for green fluorescence were: excitation 450–490 nm, 510 nm dichroic mirror, 500–570 nm barrier; for red fluorescence: excitation 536–556 nm, 580 nm dichroic mirror, 580–650 nm barrier. Photographs used a NA 0.70 long working distance 40 × phase contrast objective.
In vitro induction and detection of FSTL-1
MC3T3, 3T3-L1, and human fibroblast like synoviocytes derived from patients with rheumatoid arthritis undergoing joint replacement were cultured at a concentration of 3 × 104 cells/well in 96-well flat bottom plates for 3 days in triplicate with or without the addition of TGF-® (2 ng/ml), IL-1® (10 ng/ml) TNF-α (10 ng/ml), or IL-6 (50 ng/ml). Cell culture supernatants were assayed for mouse or human FSTL-1 by coating Nunc Immunomodule MaxiSorp ELISA plates (Nalgene, Rochester NY) with 5 μg/ml rat anti-mouse FSTL-1 (MAB1738; R&D Systems, Minneapolis, MN) or goat anti-human FSTL-1 (AF1694; R&D Systems) overnight at 4°C. Plates were washed with PBS/0.05% Tween-20 and blocked with 1% BSA/5% sucrose/0.05% Tween-20 for 1 hour. Cell culture supernatants were added at appropriate dilutions and held at room temperature for 1 hour. After washing, 5 μg/ml biotin-labeled goat anti-mouse FSTL-1 (AF1738; R&D Systems) or rat-anti-human FSTL-1 (MAB 1694; R&D Systems) was added for 1 hour. Plates were washed and incubated with streptavidin-HRP (Invitrogen, Carlsbad, CA), developed with Peroxidase Substrate System ABTS (Kirkegaard & Perry, Gaithersburg, MD), and absorbance was read at 405 nm on a microplate reader.
RESULTS
FSTL-1 is produced in the joint space by cells of the mesenchymal lineage
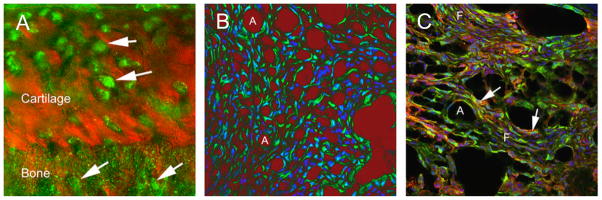
To determine whether FSTL-1 is produced in joint tissues, we used fluorescent antibody labeling in frozen sections of joints from mice with CIA. FSTL-1 protein was found in cells of the mesenchymal lineage, including osteocytes and chondrocytes (Figure 1A), adipocytes (Figure 1B) and fibroblasts (Figure 1C). No FSTL-1 expression was observed in cells of the hematopoietic lineage, such as macrophages, T cells, and B cells (data not shown).
Figure 1. FSTL-1 is over-expressed in mesenchyme-derived tissues in CIA.
FSTL-1 was visualized in knee joints from mice with CIA. (A) The bone-cartilage interface at high power, with the cartilage and bone visualized in phase contrast (red). Chondrocytes within amorphous matrix are seen in the upper part of the field; the cells label strongly for FSTL-1 (green; arrows). In the lower third of the field are osteocytes within the articular bone. These also label for FSTL-1 (arrows). The field shown is 100 μm square. (B) A high power field showing adipocytes (A) and fibroblast-like cells in synovial tissue. Anti-FSTL-1 antibody is labeled green; nuclei are visualized by Hoechst (blue fluorescence). The phase image is displayed in red with density inverted, so that the area of lipid, which is lost in processing, appear red. Field: 200 μm square. (C) FSTL-1 expression (green) in synovial fibroblasts (F) labeled with anti-CD90 (red); nuclei are blue. Note that many cells are labeled with both antibodies, and appear yellow (arrows). Adipocytes (A) appear as empty spaces; however, the phase image is inverted and merged as greyscale with the three colored fluorescent images. Field: 200 μm square.
FSTL-1 secretion is induced in mesenchymal lineage cells by arthritis-promoting cytokines
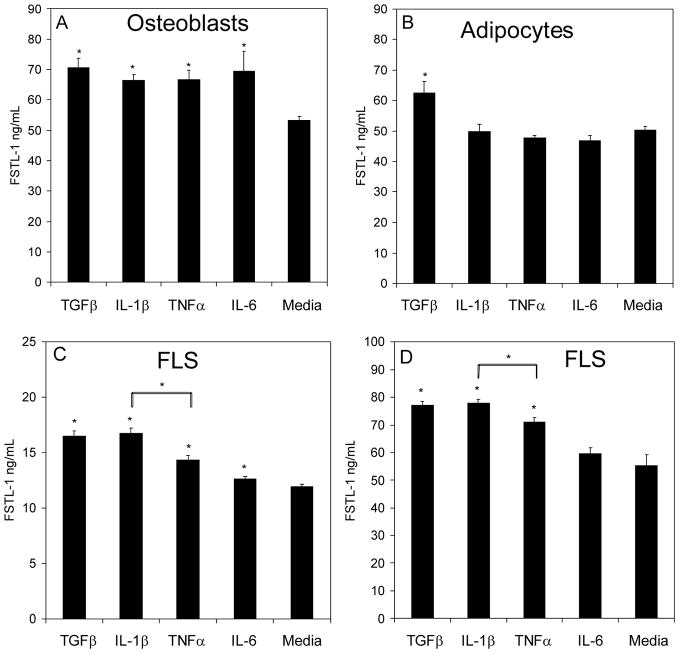
We next analyzed the ability of various inflammatory cytokines to induce FSTL-1 secretion from mesenchymal cells. For these experiments we utilized the mouse osteoblast cell line, MC3T3, the mouse adipocyte cell line, 3T3L1, and two human fibroblast-like synoviocyte cell lines derived from hip joints of two patients with RA. Cells were stimulated with IL-1β, TNF-α, or IL-6. TGF-β was used as a positive control, since FSTL-1 was originally described as a TGF-β inducible gene in MC3T3 cells (9). IL-1β, TNF-α, and IL-6 all stimulated FSTL-1 secretion from osteoblasts (Figure 2A), while the adipocytes responded only to TGF-β (Figure 2B). One of the 2 fibroblast-like synoviocyte lines responded to IL-1β, TNF-α, and IL-6 (Figure 2C) while the other line responded to IL-1β, and TNF-α, but not IL-6 (Figure 2D). In both fibroblast-like synoviocyte cell lines, IL-1β induced a significantly greater secretion of FSTL-1 than did TNF-α. Monocytic cells (U937), T cells (Jurkat) and B cells (A20) failed to make any detectable FSTL-1 (data not shown), demonstrating that FSTL-1 is not produced by cells of the hematopoietic lineage. FSTL-1 was also not produced by hepatocytes (data not shown).
Figure 2. FSTL-1 is induced by pro-inflammatory cytokines.
The osteoblast cell line, MC3T3 (A), the adipocyte cell line, 3T3-L1, (B) and 2 RA fibroblast-like synoviocyte (FLS) cell lines (C, D) were cultured for 3 days in the presence or absence of TGF-β, IL-1β, TNF-α, or IL-6 and supernatants were assayed for FSTL-1. Each bar represents the mean and S.E.M. of 6 replicates. *p < 0.05.
FSTL-1 is elevated in sera and synovial fluids of patients with systemic-onset JRA
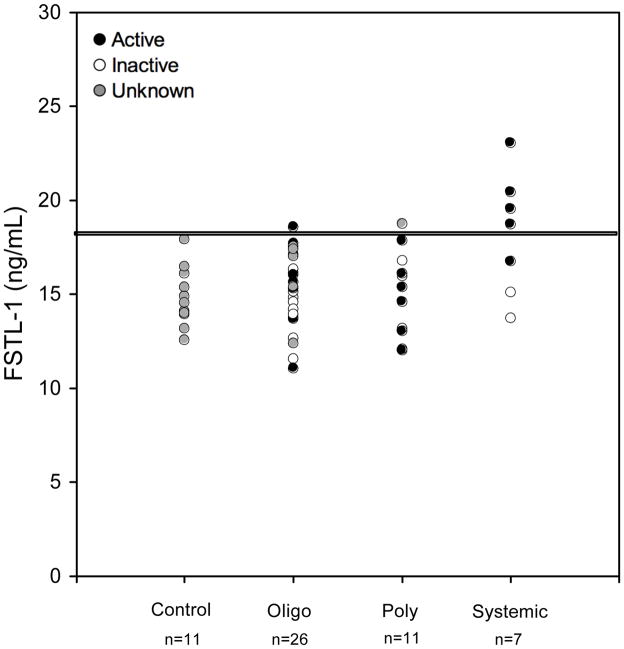
We have previously shown that FSTL-1 is overexpressed in synovial tissues of mice with arthritis (10). To explore the possibility that FSTL-1 might play a role in JRA, we measured FSTL-1 titers in banked sera (n=55) and synovial fluids (n=74) from children with JRA. Patient demographics are summarized in Table 1. The mean FSTL-1 concentration of control subjects was 15.2 ng/ml and all controls had concentrations below 18 ng/ml (Figure 3). Mean FSTL-1 concentration of the oligoarthritis and polyarthritis JRA subtypes (both 15 ng/ml) did not differ significantly from that of controls. In marked contrast, the mean concentration of the systemic-onset JRA subtype (18 ng/ml) was significantly elevated (p = 0.0007). Furthermore, in systemic-onset JRA, a striking correlation was observed between elevated FSTL-1 concentration and laboratory markers of inflammation, defined here as either an ESR ≥ 20 mm/hr or a platelet count ≥ 380×109/L (based on the upper limit of normal as reported by the laboratory). Four of the 7 sera from systemic-onset JRA subjects had concentrations above 18 ng/ml, with one as high as 23 ng/ml. All of these 4 subjects with elevated FSTL-1 concentrations had active disease, as defined above, while 2 of the 3 subjects with normal FSTL-1 concentrations had inactive disease. Thus, in all but 1 of the 7 samples from subjects with systemic-onset JRA, elevation of serum FSTL-1 correlated with laboratory evidence of active disease. In contrast, no correlation was observed between FSTL-1 concentrations and active disease in the oligoarthritis and polyarthritis subsets. These data suggest that elevation of serum FSTL-1 is a biomarker for systemic-onset JRA.
Figure 3. FSTL-1 is elevated in sera of patients with active systemic-onset JRA.
Sera from children with JRA, as well as pediatric control sera, were assayed for FSTL-1. Each circle represents an individual sample. Black circles indicate subjects with laboratory evidence of active disease (ESR ≥ 20 mm/hr or a platelet count ≥ 380×109/L). White circles indicate subjects with normal ESR and platelet count. Grey circles indicate subjects who did not have an ESR or platelet count. The horizontal line is drawn at a level of 18 ng/ml.
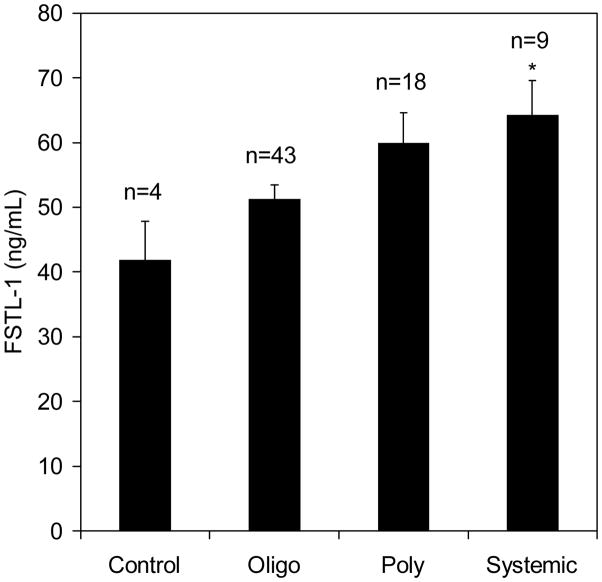
We next assayed FSTL-1 concentrations in JRA synovial fluids. Only synovial fluids from systemic-onset JRA patients were significantly higher than controls (Figure 4), again suggesting that elevated FSTL-1 is a marker of the systemic-onset subtype of JRA. Synovial fluid FSTL-1 concentrations were 2–3 fold higher than those observed in serum, indicating that the joint is a source of FSTL-1.
Figure 4. FSTL-1 is elevated in synovial fluids of patients with systemic-onset JRA.
Synovial fluids from children with JRA, as well as fluids from control subjects, were assayed for FSTL-1. Each bar represents the mean and S.E.M. of the indicated number of samples. *p < 0.05 compared to controls.
DISCUSSION
Together with our earlier studies, the data presented here support the conclusion that FSTL-1 is a major mediator of the inflammatory cascade that underlies arthritis. For instance, we have demonstrated that over-expression of FSTL-1 in mouse paws by gene transfer resulted in severe paw swelling and arthritis (15), while neutralization of FSTL-1 suppressed arthritis (11). Also, transfection of FSTL-1 into macrophages and fibroblasts lead to up-regulation of proinflammatory cytokines with central roles in chronic arthritis, including IL-1-β and TNF-α (11).
While expression of FSTL-1 in osteoblasts and fibroblasts has been previously reported, the finding that FSTL-1 can be produced by other mesenchymal cells, including adipoctyes and chondrocytes, is novel. These results, along with the observation that synovial fluid levels are 2–3 fold higher than serum levels, support the conclusion that the joint is a primary source of FSTL-1. Furthermore, it supports the concept that the joint matrix, including bone, cartilage, and adipose tissue, is not merely a passive target of destruction by blood-derived immune cells in arthritis; rather, this joint matrix plays an active role in perpetuating and amplifying the inflammatory response by releasing pro-inflammatory mediators, such as FSTL-1.
The present study suggests that systemic-onset JRA is characterized by elevated concentrations of serum and synovial fluid FSTL-1 that are not observed in other forms of JRA. Elevation of serum FSTL-1 correlated closely with markers of disease activity in systemic-onset JRA, including elevated ESR and platelet count, but we did not observe this correlation in oligoarthritis or polyarthritis. These data suggest that FSTL-1 might be useful as a biomarker of disease activity in this JRA subtype. An important caveat is that, although the results are statistically-significant, the number of systemic onset samples available to us was small. It will be important to validate these findings in a larger cohort of patients. Also, because we used banked samples, we had limited clinical data other than ESR and platelet counts. It will be of interest to compare FSTL-1 levels to additional measures of clinical activity.
The specificity for systemic JRA is interesting in light of our finding that FSTL-1 secretion from human fibroblast-like synoviocytes was significantly greater following incubation with IL-1β than with TNF-α. Systemic-onset JRA has recently been shown to have a strong IL-1β gene expression signature (17). Many patients with systemic-onset JRA respond well to the IL-1 receptor antagonist, Anakinra, (17, 18) but less well to anti-TNF therapy (4, 5). However, it is as yet unclear why patients with polyarticular and oligoarticular arthritis did not have elevated FSTL-1 titers, since TNF-α also induced FSTL-1 secretion from fibroblast-like synoviocytes, albeit to a lesser degree, and TNF-α is a central cytokine in polyarticular disease (2, 3). It is possible that the preferential induction of FSTL-1 by IL-1β is more pronounced in vivo than in vitro, although this is speculative and requires further study.
These findings suggest the possibility that FSTL-1 might be a useful biomarker in other disorders driven by IL-1β, such as the autoinflammatory syndromes, including Muckle-Wells and neonatal-onset multisystem inflammatory disease (NOMID) (19–22). We are currently investigating this possibility. None of the samples we evaluated were from subjects with macrophage activation syndrome (MAS), which is a serious complication of systemic-onset JRA that can lead to rapid deterioration and death if not treated aggressively. Whether FSTL-1 titers might be useful to screen for MAS is currently under investigation.
In conclusion, in this study FSTL-1 was found to be a biomarker of active disease in systemic JIA. Further studies are needed to confirm these observations in a larger cohort and to determine whether FSTL-1 might also be useful as a biomarker in other autoimmune and autoinflammatory conditions, particularly those with a strong IL-1β component. FSTL-1 may also represent a therapeutic target in certain forms of arthritis and other inflammatory diseases.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RO1 AI073556 (Hirsch), R01 AR056959 (Hirsch), P30 AR047363 (Thompson), and the Children’s Hospital of Pittsburgh of UPMC.
References
- 1.Cassidy JT, Levinson JE, Bass JC, Baum J, Brewer EJ, Jr, Fink CW, et al. A study of classification criteria for a diagnosis of juvenile rheumatoid arthritis. Arthritis Rheum. 1986;29(2):274–81. doi: 10.1002/art.1780290216. [DOI] [PubMed] [Google Scholar]
- 2.Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group [see comments] N Engl J Med. 2000;342(11):763–9. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 3.Lovell DJ, Giannini EH, Reiff A, Jones OY, Schneider R, Olson JC, et al. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthritis Rheum. 2003;48(1):218–26. doi: 10.1002/art.10710. [DOI] [PubMed] [Google Scholar]
- 4.Horneff G, Schmeling H, Biedermann T, Foeldvari I, Ganser G, Girschick HJ, et al. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63(12):1638–44. doi: 10.1136/ard.2003.014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, et al. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum. 2003;48(4):1093–101. doi: 10.1002/art.10885. [DOI] [PubMed] [Google Scholar]
- 6.Rose HM, Ragan C, et al. Differential agglutination of normal and sensitized sheep erythrocytes by sera of patients with rheumatoid arthritis. Proc Soc Exp Biol Med. 1948;68(1):1–6. doi: 10.3181/00379727-68-16375. [DOI] [PubMed] [Google Scholar]
- 7.Sebbag M, Simon M, Vincent C, Masson-Bessiere C, Girbal E, Durieux JJ, et al. The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1995;95(6):2672–9. doi: 10.1172/JCI117969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young BJ, Mallya RK, Leslie RD, Clark CJ, Hamblin TJ. Anti-keratin antibodies in rheumatoid arthritis. Br Med J. 1979;2(6182):97–9. doi: 10.1136/bmj.2.6182.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming- growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217(1):13–9. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 10.Thornton S, Sowders D, Aronow B, Witte DP, Brunner HI, Giannini EH, et al. DNA microarray analysis reveals novel gene expression profiles in collagen-induced arthritis. Clin Immunol. 2002;105(2):155–68. doi: 10.1006/clim.2002.5227. [DOI] [PubMed] [Google Scholar]
- 11.Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182(1):234–9. doi: 10.4049/jimmunol.182.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Ozaki S, Osakada F, Mori K, Okubo M, Nakao K. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol. 1998;10(9):1305–14. doi: 10.1093/intimm/10.9.1305. [DOI] [PubMed] [Google Scholar]
- 13.Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, et al. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50(2):660–8. doi: 10.1002/art.20023. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Ozaki S, Kawabata D, Kishimura M, Osakada F, Okubo M, et al. Potential preventive effects of follistatin-related protein/TSC-36 on joint destruction and antagonistic modulation of its autoantibodies in rheumatoid arthritis. Int Immunol. 2003;15(1):71–77. doi: 10.1093/intimm/dxg005. [DOI] [PubMed] [Google Scholar]
- 15.Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, et al. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177(7):4758–62. doi: 10.4049/jimmunol.177.7.4758. [DOI] [PubMed] [Google Scholar]
- 16.Hughes C, Wolos JA, Giannini EH, Hirsch R. Induction of T cell anergy in an experimental model of autoimmunity using non-mitogenic anti-CD3 monoclonal antibody. J Immunol. 1994;153:3319–3325. [PubMed] [Google Scholar]
- 17.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201(9):1479–86. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irigoyen PI, Olson J, Hom C, Ilowite NT. Treatment of systemic onset juvenile rheumatoid arthritis with anakinra. Arthritis Rheum. 2004;50:S437. [Google Scholar]
- 19.Hawkins PN, Bybee A, Aganna E, McDermott MF. Response to anakinra in a de novo case of neonatal-onset multisystem inflammatory disease. Arthritis Rheum. 2004;50(8):2708–9. doi: 10.1002/art.20357. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins PN, Lachmann HJ, McDermott MF. Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N Engl J Med. 2003;348(25):2583–4. doi: 10.1056/NEJM200306193482523. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364(9447):1779–85. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovell DJ, Bowyer SL, Solinger AM. Interleukin-1 blockade by anakinra improves clinical symptoms in patients with neonatal-onset multisystem inflammatory disease. Arthritis Rheum. 2005;52(4):1283–6. doi: 10.1002/art.20953. [DOI] [PubMed] [Google Scholar]