Abstract
Objective
To investigate whether cartilage degeneration is prevented or minimized in an anterior cruciate ligament (ACL) injury rat model following intra-articular injections of lubricin derived from human synoviocytes in culture (HSL), recombinant protein (rhPRG4), or from human synovial fluids (HSFL).
Methods
Unilateral ACL transection (ACLT) was performed in Lewis rats (n=45). Intra-articular injections (50μl/injection) of PBS (n=9), HSL (n=9; 200μg/ml), rhPRG4 (n=9; 200μg/ml) and HSFL (n=9; 200μg/ml) started on day 7 post-injury and continued twice weekly. Animals were harvested on day 32 post-injury. Histological analysis was performed using Safranin O/Fast green stain and blinded investigators graded articular cartilage degeneration using OARSI modified Mankin criteria. Histological specimens were immunoprobed for lubricin and sulphated glycosaminoglycans. 24 hour urine collection was performed on days 17 and 29 post-injury and urinary CTXII (uCTXII) levels were measured.
Results
Treatment with HSL resulted in significantly (p<0.05) lower OARSI scores for cartilage degeneration compared to no treatment or PBS treatment. Increased immunostaining for lubricin in the superficial zone chondrocytes and on the surface of cartilage was observed in lubricins-treated but not untreated or PBS-treated joints. On day 17, uCTXII levels of HSL and HSFL-treated animals were significantly lower than untreated (p=0.005; p=0.002) and PBS-treated (p=0.002; p<0.001) animals, respectively.
Conclusion
Across all types of lubricin evaluated in this study, a reduction in cartilage damage following ACLT was evident, combined with a reduction in collagen type II degradation. Intraarticular lubricin injection following an ACL injury may be beneficial in retarding cartilage degeneration and development of post-traumatic OA.
Keywords: ACL Injury, Lubricin, PRG-4, SZP, tribonectin, tribosupplementation
INTRODUCTION
Acute anterior cruciate ligament (ACL) injury is a significant risk factor for the development of post-traumatic osteoarthritis (OA) (1, 2). Contributing factors such as joint instability, altered joint loading (3, 4), enzymatic tissue degradation (5, 6), and possibly a lack of lubrication (7) are postulated to play a significant role in the pathogenesis of OA following injury. Synovial fluid (SF) lubricin concentrations in injured joints from patients with ACL injury were significantly lower than SF lubricin concentrations in uninjured contralateral joints (7). Inhibition of tumor necrosis factor-alpha (TNF-α) with etanercept in a rat ACL transection (ACLT) model up-regulated lubricin expression, reduced friction and reduced sulphated glycosaminoglycan (sGAG) loss from cartilage (8). Two delayed doses of etanercept led to both an increase in cartilage surface lubricin (superficial zone protein) and lubricin recoverable from synovial fluid lavage. However, the translational value of inhibiting TNF-α in preserving chondroprotection may be limited by confounding side-effects from immune system dysregulation. Thus the direct intra-articular re-introduction (i.e. supplementation) of lubricin, during the peri-injury period, may offer an opportunity to preserve cartilage by re-establishing a protective covering of lubricin. This approach appears effective in the rat meniscectomy model (9); disease modifying effects were noted using 3 injections per week (10).
The objective of this study was to determine if treatment with either full-length recombinant human lubricin (11), human synoviocyte lubricin (manufactured in culture) or lubricin from pooled human SF could preserve cartilage integrity following ACLT. ACL rupture is a common injury resulting in over 250,000 operative reconstructions per year in the US. These injuries place the knee at risk for early post-traumatic OA despite surgical treatment (12). We hypothesized that cartilage integrity, as measured using the modified Mankin Osteoarthritis Research Society International score, lubricin deposition on the surface of articular cartilage, as detected by immunostaining, and the detection of the degradation product of C-terminal telopeptides of type II collagen in the urine (uCTXII) would show improvement at 5 weeks in the rat ACLT model (13) following lubricin treatment as compared to no treatment or sham (PBS) treatment. The three forms of lubricin and PBS were administered twice weekly, where the human lubricin from SF served as a positive control.
METHODS
ACL Transection Animal Model
ACL transection was performed on the right knee joints of 8–10 weeks old male Lewis rats. A total of 45 animals underwent surgery, and they were randomly assigned to one of five treatment groups; 1) no-treatment (ACLT; n=9), 2) sham treatment (PBS; n=9), 3) human synoviocyte lubricin (HSL) (n=9), 4) recombinant human proteoglycan 4 (rhPRG4) (n=9), or 5) human synovial fluid lubricin (HSFL) (n=9). Animals were anesthetized with inhaled isoflurane, the skin was prepped with a topical antiseptic, and an incision was made in the skin laterally to the right knee joint. After the joint capsules were opened, the ACL was transected using a surgical scalpel. In all animals, the right knee joint was the operated joint and the left knee joint was the ACL-intact control joint. All experimental procedures were conducted at Biomodels, LLC which is AAALAC accredited. The study was approved by the Institutional Animal Care and Use Committee. Surgeries and injections were conducted by authors, KM and SA, who had extensive experience in performing and replicating this procedure. Control animals (n=4) did not undergo ACLT and were housed separately.
Lubricins
HSFL was obtained from patients undergoing knee and hip joint replacement surgery as described previously (14). Lyophilized HSL and rhPRG4 were obtained from SBH Sciences (Natick, MA) and reconstituted in PBS at pH 7.4. The manufacturer reports that both molecules are anti-adhesive in a cell-based assay (15) and lubricate a cartilage against cartilage bearing assay (16). Recombinant lubricin was full length PRG4 produced by Chinese hamster ovary (CHO) cells which has been observed to also lubricate a cartilage upon glass bearing previously (11).
Agarose Gel Electrophoresis (AGE)
Each of the lubricins and See Blue Plus 2 standards (Invitrogen, Carlsbad, CA) were loaded into horizontal 1.0% (w/v) agarose slab gels prepared in AGE buffer. Samples were reduced using β-mercaptoethanol. In a separate experiment, HSFL was exhaustively reduced and alkylated by adding 4 μl of 0.5M dithiothreitol (DTT) at 100°C for 4 mins, followed by 4 μl of 0.25M iodoacetamide (IA) and reheating at 100°C for progressively longer incubation periods of 10 mins to 12 hrs following the introduction of IA. Every 2 hrs, additional DTT and IA was added. Electrophoresis was conducted on ice, for 2.5 h at 50V in a horizontal gel apparatus (Bio-Rad; Hercules, CA) in the presence of AGE buffer (40 mM Tris, 1mM EDTA, 0.1% SDS adjusted to pH 8.0). Transfer from the gel to 0.2 μm nitrocellulose was performed by vacuum blotting in 0.6M NaCl/0.06M sodium citrate/pH 7.0 using VacuGene XL System (Pharmacia Biotech: Piscataway, NJ) at a suction pressure of 40 mbar, for 2 h. Membranes were blocked with 2% BSA in 20mM Tris Base, 137mM NaCl at pH 7.6 with 0.2% (v/v) Tween 20 for 60 min at room temperature. Probing with monoclonal antibody 9G3 was performed overnight at 4°C with rocking at 1:2,000 dilution. The secondary antibody was anti-mouse IgG-IR (800 CW) at 1:10,000 dilutions (Li-Cor Biosciences, Lincoln, NE) and was imaged by Odyssey Infrared Imager.
Lubricin Dosing
Seven days following ACLT, animals were re-anesthetized with inhaled isoflurane and were injected intra-articularly with 10 μg of one of the 3 lubricins in a 50 μl volume. This was repeated twice weekly for a total of 7 post-operative intra-articular treatments. Injections were performed through the patellar tendon of the operated knee joint each time while the knee was flexed. Confirmation of intra-articular administration was confirmed by a noticeable and palpable synovial fluid collection.
Histological Analysis and Immuno Staining
Paraffin-embedded coronal sections were taken from weight-bearing areas of the articular cartilage of ACL transected joints of each animal. Microtomed sections were collected every 250 μm to find a representative area showing both femoral condyles, tibial plateaus, and the menisci. Two adjacent sections were collected through this region and stained with Safranin O/Fast green stain for assessment of cartilage sGAG content. Another two adjacent sections were stained with hematoxylin and eosin, and subsequent sections immunoprobed as described below. Specific staining for a glycosylated epitope within the lubricin mucin domain was performed with monoclonal antibody 9G3 (provided by M. Warman) at 1:100 dilution. Biotinylated anti-mouse IgG at 1:200 dilution was utilized and detected using the Vectastain ABC kit (VECTOR Laboratories, Burlingame, CA).
Probing for proteoglycans was performed at 1:100 dilution with monoclonal antibodies 5D4, 3B3 and 2B6 (provided by B. Caterson) which are specific for keratan sulfate, chondroitin-6-sulphate and chondroitin-4-sulfate, respectively (17–19). Biotinylated anti-mouse IgG at 1:200 dilution was utilized for 5D4 and 2B6 antibodies and biotinylated anti-mouse IgM at 1:200 dilution for 3B3 antibody. Development was performed using the kit described above. Glycosaminoglycan immunoprobe staining was also counterstained by Fast green. Cartilage from control adult rats were processed the same way providing normal cartilage histology for comparison.
Flurorescence In Situ Hydridization (FISH) of Lubricin mRNA
Rat limbs from across treatment groups were decalcified prior to embedding and sectioning. The rat lubricin probe, a 373 bp fragment corresponded to nucleotides 1283 – 1655 of the rat PRG4 NCBI RGD:1308976 gene. The RT-PCR product was created using the forward primer 5' – GAATGGTGAGTTCAGGCTCCTTAG – 3' and reverse primer 5' – CCCAAGACTACAGCGGCAAAAC – 3' which are complimentary for rat lubricin mucin domain sequence. The PCR product was purifed via AGE using QIAquick Gel extraction kit (Qiagen), and quantitated against low DNA mass ladder standard (Invitrogen). In situ hybridization was performed using Random Primed DIG-labeled DNA probe as previously described (20–22). Hybridized probes were immunodetected with anti-DIG –fluorescein Fab fragments (Roche Applied Science) and viewed via fluorescence microscopy. Grayscale (16 bit) images were acquired with a Nikon E800 microscope (Nikon Inc., Melville NY) using a 40× Plan Fluor objective and a Spot II digital camera (Diagnostic Instruments, Sterling Heights, MI). Images were pseudocolored green in Photoshop CS4.
Histological Scoring
The Osteoarthritis Research Society International (OARSI) modified Mankin score (23) was used to measure tibial cartilage degeneration in each joint compartment and was averaged. A group of at least 3 scorers, blinded to treatment group, independently graded the best appearing Safranin O/Fast green stained section from each joint and arrived at a consensus score.
Determination of urinary CTXII concentrations in urines from untreated, PBS, HSL, rhPRG4 and HSFL-treated ACL transected animals
On days 17 and 29 following surgery, animals were housed in metabolic cages and 24 hour urine collections were performed. Urines were subsequently centrifuged at 5,000 rpm for 20 min. and stored at −20°C. Urinary CTXII concentrations were determined using the Pre-Clinical Urine Cartilaps ELISA (Immunodiagnostic Systems, Scottsdale, AZ). Urinary creatinine was determined using the QuantiChrom creatinine assay (BioAssay Systems, Hayward, CA). Urinary CTXII levels were normalized to urinary creatinine.
Statistical Analyses
Blinded OARSI modified Mankin scores were represented by box plots. The horizontal line within each plot represents the median, the box represents the interquartile range, and the error bars represent the 10th and 90th percentiles. Kruskall Wallis analysis of variance was used to determine differences in OARSI scores and urinary CTXII values across treatment groups. Statistical significance was set at α = 0.05 a priori.
RESULTS
Gel electrophoresis of HSFL, HSL and rhPRG4
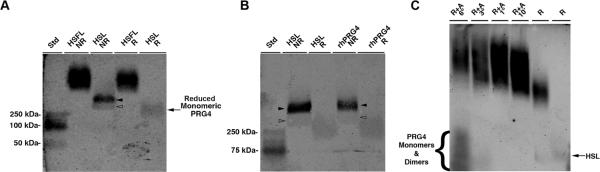
Purified HSFL demonstrated a higher molecular weight than HSL which showed co-existence of a dimer and monomer, since disulfide bond reduction resulted in appearance of monomer only (Fig 1; a). HSL and rhPRG4 both demonstrated dimers and monomers with similar molecular weight. After reduction, monomeric HSL and rhPRG4 had an apparent molecular weight of 250kDa (Fig 1; b). The much higher molecular weight of HSFL did not significantly change following standard reduction and alkylation (Fig 1; a). Exhaustive reduction and alkylation for up to 6 hrs resulted in monomeric and dimeric lubricin. A gradual increase in the molecular weight of the HSFL appeared proportional to reduction time with DTT and IA indicating the presence of an extensive network of disulfide bonded lubricin (Fig 1; c).
Figure 1.
Agarose gel electrophoresis of human synoviocyte lubricin (HSL), recombinant human proteoglycan 4 (rhPRG4), and human synovial fluid lubricin (HSFL) under reducing (R) and non-reducing (NR) conditions. A) Comparison of HSFL with HSL and B) HSL with rhPRG4. Dimerized HSL and rhPRG4 (solid arrowheads) and monomeric forms (open arrowheads) are reducible to a monomer with apparent MW 250 kDa. C) Progressive reduction and alkylation (R+A) measured in hours (°) or minutes (') of HSFL liberated monomeric and dimeric lubricin and resultant multimers assume a higher apparent molecular weight proportional to the duration of reduction and alkylation. The far right lane is HSL standard.
OARSI Histological Grading
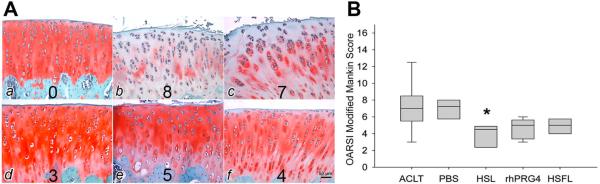
Representative Safranin O/Fast green stained tibial plateaus from control, untreated, PBS, HSL, rhPRG4, and HSFL-treated animals are shown in Figure 2A. Compared to untreated and PBS-treated, lubricin-treated tibial cartilage showed more intense staining that spanned the superficial, middle and deep cartilage zones indicating higher sGAG content, smoother, less fibrillated cartilage surfaces and preservation of superficial zone features including larger numbers of superficial zone chondrocytes. Lubricin-treated joints appeared similar to control cartilage in intensity of Safranin O staining. Cellularity was also similar between lubricin-treated joints and control cartilage. Less chondrocyte cloning was noted in cartilage from lubricin-treated joints than in untreated or PBS-treated joints. Staining with hematoxylin and eosin (Fig 3A) revealed that cartilage from lubricin-treated joints generally demonstrated flattened superficial zone chondrocytes which appeared absent or few in numbers in untreated or PBS-treated ACL transected joints. The median OARSI scores were lower in all of the lubricin-treated (HSL, rhPRG4, and HSFL) joints compared to the untreated and PBS-treated joints (Fig 2; b). Compared to PBS, HSL produced a 45% decrease in cartilage degeneration, as determined by OARSI score. rhPRG4 produced a 29% reduction in cartilage degeneration and HSFL produced a 33% reduction in cartilage degeneration, though these were not significant. Treatment with HSL resulted in significantly lower (p<0.05) cartilage degeneration compared to no treatment or PBS treatment. There were no significant differences in OARSI scores of rhPRG4 (p = 0.208) and HSFL-treated joints (p = 0.053) compared to untreated joints. There was no significant difference between untreated and PBS-treated joints' OARSI scores indicating lack of a placebo effect in this model.
Figure 2.
Safranin O stained tibial plateau cartilage and OARSI scores of control, untreated, PBS-treated, human synoviocyte lubricin (HSL), recombinant human proteoglycan 4 (rhPRG4), and human synovial fluid lubricin (HSFL). A. Representative tibial plateau cartilage from control (a), untreated (b), PBS-treated (c), HSL-treated (d), rhPRG4-treated (e), and HSFL-treated (f) animals harvested on day 32 post-ACLT. Associated consensus OASRI scores for these sections are illustrated at the bottom of each image. The tibial cartilage surface was rough along with inconsistent proteoglycan staining in untreated and PBS-treated joints, while the tibial cartilage surface was smooth, and proteoglycan staining was stronger in lubricin-treated joints. B. Box plot of OARSI modified Mankin scores of tibial plateaus from untreated (ACLT, n=9), PBS-treated (n=9), HSL-treated (n=9), rhPRG4-treated (n=9), and HSFL-treated (n=9) animals harvested on day 32 post-injury. Control cartilage was a criterion standard OARSI score = 0. *Indicates that HSL-treated joints had significantly lower (p<0.05) OARSI scores compared to ACLT and PBS-treated joints.
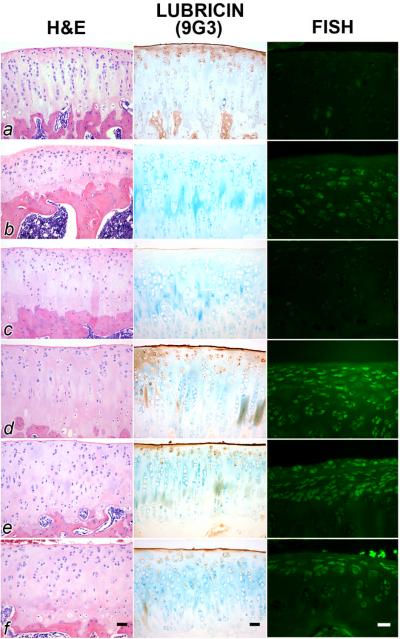
Figure 3.
Hematoxylin and eosin (H&E) staining, mAb 9G3 immunostaining for lubricin and in situ hybridization for lubricin mRNA from ACL transected joints from articular cartilage from representative control (a), untreated (b), PBS-treated (c), human synoviocyte lubricin (HSL)-treated (d), recombinant human proteoglycan 4 (rhPRG4)-treated (e) and human synovial fluid lubricin (HSFL)-treated (f) animals. For joints treated with HSL (d), rhPRG4 (e) and HSFL (f), there was a greater surface accumulation of lubricin, and more superficial zone staining and lubricin expression. ACL-transected joints (b) and those treated with PBS (c) showed either no or minimal surface staining for lubricin. Scale bars are 20 μm.
Lubricin Immuno Staining and FISH
ACL transected joints, treated with any of the 3 lubricins and probed with 9G3 shows greater surface staining of a smoother articular surface, and the presence of superficial zone chondrocytes which stain positive for lubricin intra-cellularly (Fig 3; d–f). Control cartilage is similar in appearance (Fig 3; a). These findings contrast with a representative untreated and placebo-treated ACL transected joint where lubricin immunostaining of the articular surface is absent or minimal (Fig 3; b, c). In situ hydridization indicated that lubricin expression is enhanced following treatment with any of the 3 lubricins (Fig 3; d–f). Control and PBS treated cartilage showed minimal lubricin expression (Fig 3; a, c). Cartilage from some specimens of untreated ACL transected joints showed more lubricin expression (Fig 3; b) but qualitatively less than cartilage from the ACL transected joints treated with any of the 3 lubricins (Fig 3; d–f).
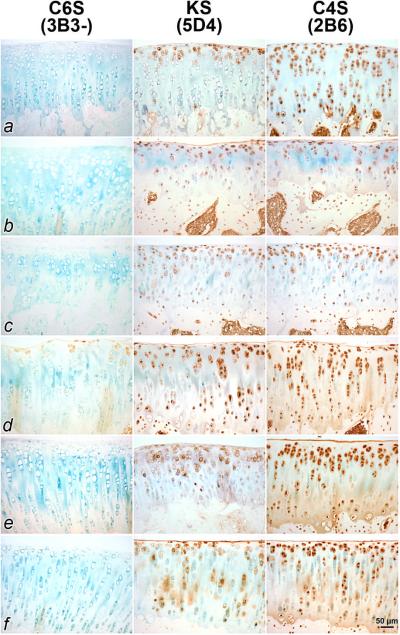
Mab 5D4, 3B3 and 2B6 Immunostaining
Representative ACL transected joints treated with HSL showed reactivity with 3B3 without chondroitinase digestion ((3B3 (−)) in the superficial and middle zones of articular cartilage, indicative of incompletely sulphated and thus immature chondroitin-6-sulfate (Fig 4; d). ACL transected joints which were untreated and treated with PBS, HSFL and rhPRG4, and control cartilage did not show 3B3 (−) reactivity (Fig 4; a–c, e, f). All ACL transected joints, regardless of treatment, showed reactivity to 5D4 and 2B6 which are reactive to keratan sulfate and chondroitin-4-sulfate respectively. The untreated and placebo treated ACL transected joints showed cellular and superficial zone staining but less in the extracellular matrix in the middle and deep zones (Fig 4; b, c). Counterstain was readily observable in cartilage from these animals in lieu of 5D4 and 2B6. By contrast joints treated by each of the 3 lubricins (Fig 4; d–f), and control cartilage (Fig 4; a), showed more immunostaining for glycosaminoglycan across the extracellular matrix of articular cartilage.
Figure 4.
Immunostaining of articular cartilage from ACL transected joints for chondroitin 6 sulfate (C6S), keratin sulfate (KS) and chondroitin 4 sulfate (C4S) from representative control (a), untreated (b), PBS-treated (c), human synoviocyte lubricin (HSL)-treated (d), recombinant human proteoglycan 4 (rhPRG4)-treated (e), and human synovial fluid lubricin (HSFL)-treated (f) animals. Controls and those treated with any lubricin generally showed more cellular staining for KS and C4S, than untreated or placebo-treated ACL transected joints. Greater matrix staining was noted for C4S across lubricin-treated joints. Joints treated with HSL (d) stained for C6S using 3B3 without chondroitinase digestion (3B3-) which may be indicative of repair activity.
Urinary CTXII concentrations
The mean uCTXII levels of HSL, rhPRG4, and HSFL-treated animals were lower than the corresponding mean uCTXII levels in untreated and PBS-treated animals on days 17, and 29 post-injury (Fig. 5). On day 17, the uCTXII levels of HSL-treated animal were significantly lower than the untreated (p=0.005) and PBS-treated (p=0.002) animals, respectively. Similarly, day 17 uCTXII levels of HSFL-treated animals were significantly lower than untreated (p=0.002) and PBS-treated (p<0.001) animals, respectively. There were no significant differences among day 17 uCTXII levels of untreated, PBS and rhPRG4-treated animals.
Figure 5.
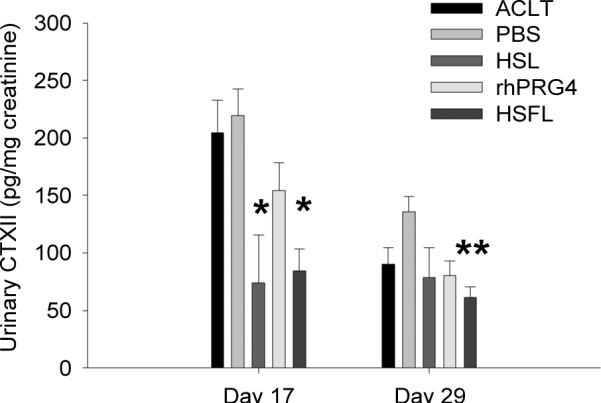
Urinary CTXII (uCTXII) levels, normalized to creatinine, of untreated ACL transected joints (ACLT, n=6), PBS-treated (PBS, n=6), HSL-treated (HSL, n=3), rhPRG4 treated (rhPRG4, n=6) and HSFL-treated (HSFL, n=6) animals on days 17 and 29 post-injury. *Indicates that day 17 uCTXII levels of HSL and HSFL-treated animals were significantly lower than corresponding uCTXII levels of untreated (p=0.005; p=0.002) and PBS-treated (p=0.002; p<0.001) animals, respectively. **Indicates that day 29 uCTXII levels of HSFL-treated animals were significantly lower (p<0.001) than PBS-treated animals.
The day 29 mean uCTXII levels of all animal groups were lower than the corresponding day 17 levels with the exception of HSL treated animals. The uCTXII levels of HSFL-treated animals were significantly lower than the uCTXII levels of PBS-treated animals (p<0.001). There were no significant differences among uCTXII levels of untreated, PBS, HSL and rhPRG4-treated animals.
DISCUSSION
Animal models of meniscectomy (9), medial collateral ligament and ACL disruption (24), and adjuvant induced arthritis (25) have all showed an association of cartilage degeneration with a decrease in either SF or cartilage bound lubricin. Among these, ACL injury is an acute traumatic injury leading to increased risk of long-term development of degenerative joint diseases (6). We have shown that many of the pre-OA degenerative changes following this injury can be mitigated or prevented by the intra-articular supplementation of lubricin. ACL transected joints treated with HSL demonstrated the lowest OARSI modified Mankin scores and was corroborated by a lowering in uCTXII levels. Immunohistochemical articular surface staining for lubricin was stronger among the lubricin treated joints and was present within superficial zone chondrocytes. Untreated and placebo treated ACL transected joints displayed minimal surface lubricin and fewer superficial zone cells. Lubricin treated joints also generally displayed greater sGAG preservation, as indicated by the intensity of Safranin O staining of the articular cartilage. The OARSI scoring system (23) is designed to describe early and late stages of degeneration. However, the present studies concern a pre-OA lesion which existing scoring systems may not adequately describe. Features of these lesions include loss of superficial zone chondrocytes, some fibrillation, and global sGAG loss in many instances which may be unique to OA trauma models. Zonal loss of sGAG is variably present in the ACL deficient joint at the time of analysis in this study. By contrast the intensity of sGAG staining may be a more reliable discriminator between lubricin treated and untreated joints. Whether sGAGs were simply more abundant in cartilage from lubricin treated joints is supported by the detection of partially sulfated chondrotin-6-sulfate via mAb 3B3 without a priori chondroitinase treatment in the HSL-treated joints. Proteoglycan containing this epitope at the non-reducing terminal of chondroitin-6-sulfate is thought of as a neoepitope of OA and indicative of aggrecan synthesis (17, 26). The 3B3(−) epitope appears during repair and remodeling in the superficial and intermediate zones (19, 27) and has also been observed in load induced injury (28). Keratan sulfate and chondroitin-4-sulfate appeared concentrated within or near chondrocytes in cartilage from untreated and placebo treated ACL transected joints. By contrast these two glycosaminoglycans were also detectable within the extra-cellular matrix separating chondrocytes in joints treated with any of the lubricins and appeared similar to normal control cartilage. Keratan and chondroitin-4-sulfate are proteoglycan components of mature aggrecan and appear to have been preserved in the extra-cellular matrix of lubricin-treated ACL transected joints. In summary, cartilage from traumatized joints, treated with any of 3 lubricins, show chondroprotection at the aggrecan level. In addition, all lubricin treated ACL transected joints showed evidence of superficial zone chondrocyte immunopositivity for lubricin which is likely related to native lubricin synthesis by superficial and intermediate zone chondrocytes. Overall, HSL may have demonstrated the most chondroprotective effects and apparent restorative effects as indicated by 3B3 (−) staining.
Fully rationalizing lubricin immunohistology with in situ hybridization results leads to speculation that lubricin expression could be enhanced following injury (Fig 3; b). However the accumulation of cartilage surface lubricin does not occur due to inflammation and proteolysis. Conversely, control cartilage shows a normal amount of lubricin immunostaining and low level of expression (Fig 3; a) which may be indicative of homeostasis. The lubricin residence half-life of 6 days (10) likely contributes to this balance. Introducing lubricin into the traumatized joint (Fig 3; d–f) protects the superficial zone and appears to augment its expression.
The observation that uCTX-II, a validated marker of collagen type II degradation (29), was less concentrated in the rat urine normalized for creatinine, among lubricin treated animals, is an important translational finding. 24 hr urine collections at two differing time points showed a diminution in uCTX-II in the non-lubricin treated joints. HSL and HSFL in particular however, led to statistically significant reductions in uCTX-II, when administered twice weekly. Although uCTX-II is enzymatically generated, the addition of an anti-adhesive like lubricin will lubricate the articular surface, and may also prevent the interaction of inflammatory cells and other proteins in SF with the articular surface. In the absence of lubricin, biofouling of the articular cartilage with globular proteins is suspected to occur in the lubricin null mouse (30). In inflammatory arthropathies, lubricin may reduce initial collagen type II cleavage by minimizing superficial zone degradation.
Lack of lubricating activity at the articular surface induces greater asperity contact friction (31) which superficial zone chondrocytes may sense as excessive tangential shear (32) due to stick-slip of loaded and non-lubricated surfaces attempting to slide past each other. This may have a catabolic effect resulting in the loss of superficial zone chondrocytes which also secrete lubricin (superficial zone protein). Superficial zone chondrocytes are also lacking in the lubricin null mouse (33) which recapitulates CACP syndrome (34) in humans. Cartilage from these mice show a progressive loss of Safranin O staining as they age (35) which occurs well after surface fibrillation has begun in the absence of boundary lubrication (30). These features occur in the absence of inflammation and suggest that the diarthrodial joints lacking its boundary lubricant undergoes a form of primary degeneration resulting in precocious joint failure. In traumatic injuries, it is possible that this process may co-exist with the inflammatory degradation from a traumatic origin, which is well known to enzymatically degrade lubricin (25). Loss of superficial zone chondrocytes could have lasting deleterious effects on articular cartilage since these cells serve as progenitor cells (36) for the limited self-reparative ability of this tissue.
Cartilage from PBS treated ACL transected joints showed fewer flattened superficial zone chondrocytes, as similarly reported in meniscectomized sheep (37) and appeared to be covered with a thin layer of lubricin (Fig 3), as compared to normal controls. Presumably this lubricin originates from synoviocytes within the joint capsule which continue to secrete lubricin as the acute inflammatory phase of injury abates. This finding contrasts with an earlier ACLT rat study (8) where etanercept was used to up-regulate lubricin. In the untreated ACL transected joint, in that study, there was no lubricin noted on the surface. This difference could be explained by differences in joint harvesting dates between the studies such that a longer post-operative period provides more time for joint homeostasis to become re-established and by differences in surgeons and surgical technique. The earlier study using etanercept (8) used a #11 scalpel directed into the joint to transect the ACL which may have been more traumatic than hooking the ACL and severing.
Recent studies of introducing human recombinant lubricin with a truncated mucin domain in the meniscectomized rat joint showed preservation of cartilage thickness but was limited since a control using full length wild type human lubricin was not studied (9). Thus it is difficult to know ultimately how disease modifying exogenous lubricin may be in that OA animal model. Despite this, the lubricin construct with a truncated mucin domain reduced pain in this model as assessed by the differential weight shifting of the lubricin treated rat between two balances which measured weight bearing of the affected and contralateral limb (10). Lubricin with a truncated mucin domain was chosen as a test construct since its expression from CHO cells was enhanced by virtue of eliminating many of the degenerate repeating KEPAPTT sequences. It is unknown if this truncated lubricin is suitable for use in larger joints. The length of this lubricin is similar to the native length of rat lubricin. The present study investigated 3 lubricins: HSFL, HSL and full-length rhPRG4. Among these HSL appeared to offer the most chondroprotection and would suggest that maintaining or approximating the normal length of a lubricin construct is an important factor in therapeutic efficacy.
HSFL, HSL and rhPRG4 all appear to have similar lubricating and anti-adhesive properties in vitro by preventing up to 40% of calcein labeled synoviocytes in suspension from adhering to cell culture plastic (data not shown, SBH Sciences specifications). This cell-based assay (15) can be useful as a surrogate marker for biological activity in lieu of measuring friction reduction (16). On agarose gels lubricin appears as a monomer and dimer (38); reduction and alkylation results in monomer only (39) in the case of HSL and rhPRG4. This is not the case for HSFL which is recovered from SF by filtering over a 0.22μm membrane. HSFL is a gel-like network of lubricin (30), which lubricates artificial bearings like latex upon glass (40), mica (41), idealized surfaces (42) and graphite (30) and requires exhaustive disulphide bond reduction in order to visualize lubricin monomer. In the present study, HSL and HSFL produced chondroprotective effects, suggesting that a gel-like network of lubricin is not necessary. Lubricin interacts with collagen (43), hydrophobic (42) and hydrophilic surfaces via its C-terminus (44), and likely forms a loop in its mucin domain allowing the N-terminus to also interact with the same surface (39, 42). Surface bound layers of lubricin appear as both single end-grafted and double-end grafted molecules. The O-linked glcosylations which decorate the mucin domain are important in helping to create steric repulsion and hydration shells which manifest as repulsive forces (45); working in an anti-adhesive capacity and keep cartilage asperities from direct contact. Lateral translation of one bearing against its apposed bearing is thus `lubricated' under conditions which characterize mammalian diarthrodial joints, including very slow sliding speed, high pressures and compliant bearing materials (cartilage) (46). This becomes the predominant mode of joint lubrication once loaded and pressurized cartilage has stayed in position for several minutes and the coefficient of friction has reached an asymptotic level of 0.14 (31, 47). At that point cartilage has reached zero pore pressure by exuding extracellular matrix fluid. Preventing asperity contact, adhesions and resultant damage is provided by this “carpet” (41) of end-grafted lubricin molecules. This is a critical system in chondroprotection and can be re-introduced to weight bearing diarthrodial joints since lubricin is surface active and rapidly adsorbs on surfaces.
In conclusion, this study indicates that lubricin is a disease modifying protein that may prevent the fundamental processes which can lead to OA following ACL injury. Lubricin is a promising biologic candidate since it is a replacement for a normally occurring mucinous glycoprotein and thus has a low toxicity profile. Patients on average seek medical attention 6.7 days following an acute joint injury (48) which was part of the justification for delayed treatment in this study. Injecting lubricin (tribosupplementation) into the intra-articular space could also be an adjunct or replacement for viscosupplementation. This study may have been limited by inadequate power, particularly since OARSI scores across lubricin treated limbs were similar in magnitude and near the lower end of the OARSI scale, creating the possibility of a `floor effect'. Thus, we cannot rule out that rhPRG4 and HSFL-treated joints were different from the untreated ACLT and PBS-treated groups. Additional longer term animal studies are needed to determine the full extent of disease modifying features in an OA model.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Matthew Warman from Boston Children's Hospital for providing lubricin specific mAb 9G3, Dr. Bruce Caterson from Cardiff University for providing mAb's 3B3, 5D4 and 2B6 and Dr. Deborah Ciombor from Brown University for review of data. This research was funded by NIH/NIAMS R21AR055937, RO1AR050180, R41AR057276 and NCRR COBRE P20 RR024484.
REFERENCES
- 1.Aichroth PM, Patel DV, Zorrilla P. The natural history and treatment of rupture of the anterior cruciate ligament in children and adolescents. A prospective review. J Bone Joint Surg Br. 2002;84(1):38–41. doi: 10.1302/0301-620x.84b1.11773. [DOI] [PubMed] [Google Scholar]
- 2.Fithian DC, Paxton LW, Goltz DH. Fate of the anterior cruciate ligament-injured knee. Orthop Clin North Am. 2002;33(4):621–36. v. doi: 10.1016/s0030-5898(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 3.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88(8):1826–34. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 4.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–7. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 5.Vasara AI, Jurvelin JS, Peterson L, Kiviranta I. Arthroscopic cartilage indentation and cartilage lesions of anterior cruciate ligament-deficient knees. Am J Sports Med. 2005;33(3):408–14. doi: 10.1177/0363546504268040. [DOI] [PubMed] [Google Scholar]
- 6.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament injury, reconstruction and osteoarthritis. Curr Opin Orthop. 2005;16(5):354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–15. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009;60(10):2997–3006. doi: 10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–7. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 10.Glasson SSR-BT, Mark LS, Whiteside G, Resmini C, et al. Intra-articular lubricin supplementation modifies disease progression and ameliorates pain in a rat model of osteoarthritis. 55th Orthopaedic Research Society; Las Vegas, NV. 2009. [Google Scholar]
- 11.Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res. 2009;27(6):771–7. doi: 10.1002/jor.20798. [DOI] [PubMed] [Google Scholar]
- 12.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–73. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoop R, Buma P, van der Kraan PM, Hollander AP, Billinghurst RC, Meijers TH, et al. Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthritis Cartilage. 2001;9(4):308–15. doi: 10.1053/joca.2000.0390. [DOI] [PubMed] [Google Scholar]
- 14.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. Journal of Rheumatology. 2000;27(3):594–600. [PubMed] [Google Scholar]
- 15.Braut-Boucher F, Pichon J, Rat P, Adolphe M, Aubery M, Font J. A non-isotopic, highly sensitive, fluorimetric, cell-cell adhesion microplate assay using calcein AM-labeled lymphocytes. J Immunol Methods. 1995;178(1):41–51. doi: 10.1016/0022-1759(94)00239-s. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56(3):882–91. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 17.Richardson JB, Caterson B, Evans EH, Ashton BA, Roberts S. Repair of human articular cartilage after implantation of autologous chondrocytes. J Bone Joint Surg Br. 1999;81(6):1064–8. doi: 10.1302/0301-620x.81b6.9343. [DOI] [PubMed] [Google Scholar]
- 18.Kavanagh E, Osborne AC, Ashhurst DE, Pitsillides AA. Keratan sulfate epitopes exhibit a conserved distribution during joint development that remains undisclosed on the basis of glycosaminoglycan charge density. J Histochem Cytochem. 2002;50(8):1039–47. doi: 10.1177/002215540205000806. [DOI] [PubMed] [Google Scholar]
- 19.Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res. 2002;20(1):40–50. doi: 10.1016/S0736-0266(01)00071-7. [DOI] [PubMed] [Google Scholar]
- 20.Roche PJ. Preparation of template DNA and labeling techniques. In: Darby Ian A., Hewitson Tim D., editors. In situ Hybridization Protocols. 3rd ed. Humana Press; Totowa, NJ: 2006. pp. 9–16. [DOI] [PubMed] [Google Scholar]
- 21.Tesch GH, Lan HY, Nikolic-Paterson DJ. Treatment of tissue sections for in situ hybridization. In: Darby Ian A., Hewitson Tim D., editors. In situ Hybridization Protocols. 3rd ed. Humana Press; Totowa, NJ: 2006. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 22.Ge NL, Kocan KM, Murphy GL, Blouin EF. Detection of Anaplasma marginale DNA in bovine erythrocytes by slot-blot and in situ hybridization with a PCR-mediated digoxigenin-labeled DNA probe. J Vet Diagn Invest. 1995;7(4):465–72. doi: 10.1177/104063879500700407. [DOI] [PubMed] [Google Scholar]
- 23.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis and Rheumatism. 2005;52(6):1746–1755. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 25.Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56(1):108–16. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 26.Caterson B, Hughes CE, Roughley P, Mort JS. Anabolic and catabolic markers of proteoglycan metabolism in osteoarthritis. Acta Orthop Scand Suppl. 1995;266:121–4. [PubMed] [Google Scholar]
- 27.Slater RR, Jr., Bayliss MT, Lachiewicz PF, Visco DM, Caterson B. Monoclonal antibodies that detect biochemical markers of arthritis in humans. Arthritis Rheum. 1995;38(5):655–9. doi: 10.1002/art.1780380513. [DOI] [PubMed] [Google Scholar]
- 28.Lin PM, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis Cartilage. 2004;12(6):485–96. doi: 10.1016/j.joca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Garnero P, Landewe R, Boers M, Verhoeven A, Van Der Linden S, Christgau S, et al. Association of baseline levels of markers of bone and cartilage degradation with long-term progression of joint damage in patients with early rheumatoid arthritis: the COBRA study. Arthritis Rheum. 2002;46(11):2847–56. doi: 10.1002/art.10616. [DOI] [PubMed] [Google Scholar]
- 30.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56(11):3662–9. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coles JM, Blum JJ, Jay GD, Darling EM, Guilak F, Zauscher S. In situ friction measurement on murine cartilage by atomic force microscopy. J Biomech. 2008;41(3):541–8. doi: 10.1016/j.jbiomech.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong BL, Bae WC, Chun J, Gratz KR, Lotz M, Sah RL. Biomechanics of cartilage articulation: effects of lubrication and degeneration on shear deformation. Arthritis Rheum. 2008;58(7):2065–74. doi: 10.1002/art.23548. [DOI] [PubMed] [Google Scholar]
- 33.Rhee DK, Marcelino J, Baker MA, Gong YQ, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. Journal of Clinical Investigation. 2005;115(3):622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nature Genetics. 1999;23(3):319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 35.Coles JM, Zhang L, Blum JJ, Warman ML, Jay GD, Guilak F, et al. Loss of cartilage, stiffness, and frictional properties in mice lacking Prg4. Arthritis Rheum. 2010 Feb 26; doi: 10.1002/art.27436. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes AJ, Tudor D, Nowell MA, Caterson B, Hughes CE. Chondroitin sulfate sulfation motifs as putative biomarkers for isolation of articular cartilage progenitor cells. J Histochem Cytochem. 2008;56(2):125–38. doi: 10.1369/jhc.7A7320.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, Read RA, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8(2):R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt TA, Plaas AH, Sandy JD. Disulfide-bonded multimers of proteoglycan 4 (PRG4) are present in normal synovial fluids. Biochim Biophys Acta. 2009;1790(5):375–84. doi: 10.1016/j.bbagen.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Zappone B, Greene GW, Oroudjev E, Jay GD, Israelachvili JN. Molecular aspects of boundary lubrication by human lubricin: Effect of disulfide bonds and enzymatic digestion. Langmuir. 2008;24(4):1495–1508. doi: 10.1021/la702383n. [DOI] [PubMed] [Google Scholar]
- 40.Jay GD. Characterization of a bovine synovial fluid lubricating factor .1. Chemical, surface-activity and lubricating properties. Connective Tissue Research. 1992;28(1–2):71–88. doi: 10.3109/03008209209014228. [DOI] [PubMed] [Google Scholar]
- 41.Zappone B, Ruths M, Greene GW, Jay GD, Israelachvili JN. Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys J. 2007;92(5):1693–708. doi: 10.1529/biophysj.106.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang DP, Abu-Lail NI, Guilak F, Jay GD, Zauscher S. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir. 2008;24(4):1183–1193. doi: 10.1021/la702366t. [DOI] [PubMed] [Google Scholar]
- 43.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, et al. Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J Orthop Res. 2009;27(2):257–63. doi: 10.1002/jor.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones ARC, Gleghorn JP, Hughes CE, Fitz LJ, Zollner R, Wainwright SD, et al. Binding and localization of recombinant lubricin to articular cartilage surfaces. Journal of Orthopaedic Research. 2007;25(3):283–292. doi: 10.1002/jor.20325. [DOI] [PubMed] [Google Scholar]
- 45.Jay GD, Harris DA, Cha C-J. Boundary lubrication by lubricin is mediated by O-linked β(1–3)Gal-GalNAc oligosaccharides. Glycoconjugate Journal. 2002;18(10):807–815. doi: 10.1023/a:1021159619373. [DOI] [PubMed] [Google Scholar]
- 46.McCutchen CW. The frictional properties of animal joints. Wear. 1962;5:1–17. [Google Scholar]
- 47.Park S, Costa KD, Ateshian GA. Microscale frictional response of bovine articular cartilage from atomic force microscopy. Journal of Biomechanics. 2004;37(11):1679–1687. doi: 10.1016/j.jbiomech.2004.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jay GD, Elsaid KA, Zack J, Robinson K, Trespalacios F, Cha CJ, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31(3):557–64. [PubMed] [Google Scholar]