Abstract
Despite existing guidelines, dietary sodium intake among people worldwide often exceeds recommended limits. Research evidence is growing in both animal and human studies showing indirect and direct adverse consequences of high dietary sodium on the kidney. In patients with kidney disease, dietary sodium may have important effects on proteinuria, efficacy of antiproteinuric pharmacologic therapy, hypertension control, maintaining an optimal volume status, and immunosuppressant therapy. Dietary sodium intake is an important consideration in patients with all stages of chronic kidney disease, including those receiving dialysis therapy or those who have received a kidney transplant. We review in detail the dietary sodium recommendations suggested by various organizations for patients with kidney disease. Potential barriers to successfully translating current sodium intake guidelines into practice include poor knowledge about the sodium content of food among both patients and providers, complex labeling information, patient preferences related to taste, and limited support for modifications in public policy. Finally, we offer existing and potential solutions that may assist providers in educating and empowering patients to effectively manage their dietary sodium intake.
“Knowing is not enough; we must apply. Willing is not enough; we must do”
--Goethe
Epidemiology & Background
The United States guidelines for daily sodium intake generally recommend a limit of 2.3 grams (g) per day (1, 2). Despite guidelines, dietary sodium intake among people in the United States remains high. In the National Health and Nutrition Examination Survey (NHANES, 2005–06), individuals 50 years and older had daily sodium consumption between 3.1–3.9 g for males, and 2.4–3.0 g for females (3). Thus, approximately 95% of U.S. male adults and 75% of female adults consume more than recommended. Similar observations have been made worldwide as reported in the British National Dietary and Nutritional Survey (NDNS, 2000–01) (4), and in the INTERSALT study(5), where the highest daily sodium consumption was in northern China (5.5 g per day).
Though little is described about the actual sodium intake of patients with kidney disease, one small study of 60 patients showed those receiving dialysis consumed an average of 2.1 (SD: 1.3) grams of sodium per day and non-dialysis patients an average of 2.3 (SD: 1.1) grams (6). As these are averages, it suggests a significant portion of patients with kidney disease may at times be above the recommended limits.
Research evidence is growing in both animal and human studies showing adverse effects of high dietary sodium intake on the kidney. The aim of this paper is to briefly review some of the proposed mechanisms of kidney injury caused by high sodium intake, the potential clinical implications of this injury to patients, and to summarize current recommendations for limiting daily sodium intake. Recognizing potential barriers of translating these guidelines into practice, we additionally offer possible solutions that may assist providers in educating and empowering patients to effectively manage dietary sodium intake.
Mechanisms of Injury Due to High Dietary Sodium
Potential models of kidney injury include indirect and direct mechanisms. Models of indirect mechanisms suggest a complex relationship between increased sodium load, increased blood pressure, and proteinuria (7). Increased blood pressure and proteinuria then lead to vascular and renal injury, potentially causing progression of disease, as shown in Figure A (8) (This model refers to sodium in the form of sodium chloride, i.e. “salt”).
Fig. A.

Interplay between dietary salt, blood pressure, proteinuria and kidney disease progression. Reprinted with permission (8).
One example of indirect mechanisms contributing to increasing blood pressure is seen in those individuals who are considered salt (where sodium is coupled specifically to a chloride anion) sensitive. Figure B shows pressure naturiesis curves for: 1.) normal, non hypertensive subjects, 2.) subjects with hypertension whose blood pressure is not significantly increased with salt loading (salt resistant) and, 3.) those with hypertension whose blood pressure changes significantly with salt loading (salt sensitive). Although actual changes in blood pressure are continuous, these different curves suggest the three subgroups adapt differently to sodium chloride intake (9). In one study, about 60% of patients with known essential hypertension were deemed salt sensitive (10). Although there is not empirical evidence from randomized trials, available data suggest as kidney disease progresses, salt sensitivity increases exponentially (11), supporting reductions in daily sodium intake in this population.
Fig. B.
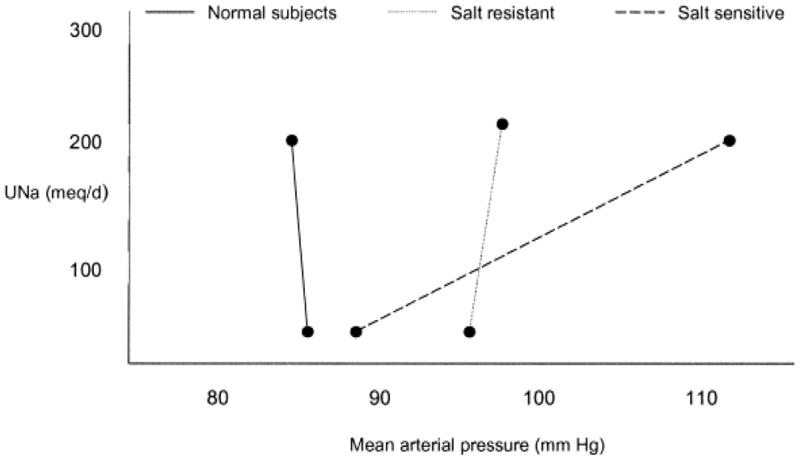
Pressure naturiesis curves for normal, salt resistant, and salt sensitive subjects. The salt sensitive show a significant change in mean arterial pressure associated with changes in salt loading. Reprinted with permission (9, 10).
There is also evidence of high sodium intake affecting kidney and vascular systems directly, and independently of blood pressure (7). High dietary sodium increases oxidative stress in the mammalian kidney by increasing generation and decreasing the breakdown of reactive oxygen species (12, 13). Evidence supports a direct effect of sodium intake on endothelium, mediated through changes in shear stress, modulating the production of TGFβ1 and nitric oxide. In turn, this results in vascular and glomerular fibrosis with potential decline in kidney function (14).
Endogenous inhibitors of Na/K ATPase may also be implicated in renal injury models. Data suggest, that ouabain, in small doses, causes partial Na/K ATPase inhibition within renal epithelial cells, and acts as an inducer of regular, low frequency calcium oscillations. The oscillations lower the threshold for activation of calcium dependent transcription factor NF-Kβ (15), which has a role modulating the expression of immunoregulatory genes relevant in inflammatory disease and apoptosis (16, 17). Oubain itself may also contribute to hypertension. In patients with significantly decreased glomerular filtration rates, impaired renal excretion of sodium is implicated as a major culprit of hypertension, and some data supports that oubain plays a role in this process, by raising intracellular sodium, subsequently increasing intracellular stores of calcium in vascular smooth muscle through changes in Na/Ca exchange. The end result is increased arterial tone and peripheral vascular resistance contributing to hypertension (18).
Clinical Relevance
General Population
There are important potential benefits of sodium reduction in improving overall health in the U.S. population while reducing medical costs. A recent study used a computer simulation of heart disease in U.S. adults age 35 to 84 years old, to explore the impact of modest reductions in dietary sodium on population health. The analysis suggested dietary salt reductions of 1.2 g per day would save up to 392,000 quality adjusted life years and $24 billion dollars in healthcare costs annually (19).
In addition to being linked to cardiovascular morbidity and mortality (20), high sodium intake is associated with increased risk of incident hypertension (21). Blood pressure control, however, can be improved with sodium reduction. Follow up to the Dietary Approaches to Stop Hypertension (DASH) Trial showed that both sodium reduction and a diet rich in fruits, vegetables and low in fat (DASH diet) lower blood pressure. Each diet modification independently contributed to blood pressure reductions with greater effects in combination (22).
Patients with Kidney Disease
Reducing sodium intake in patients with chronic kidney disease (CKD) is important to maximize benefits of other therapies. Proteinuria is a marker of kidney disease and contributes to progression, therefore, controlling and reducing protein in the urine is a mainstay of treatment. High dietary sodium is related to increased urinary albumin excretion (23), and has been shown to abate the anti-proteinuric benefits of angiotensin converting enzyme (ACE) inhibitor therapy (24). More recently, supplemental oral sodium chloride was shown to blunt the albumin excretion rate response to telmisartan in those patients with diabetes mellitus, albuminuria, and habitual low sodium intake (25).
As previously noted, hypertension is a potential indirect mechanism for risk of CKD progression due to increased dietary sodium intake. One example of this is shown in a study of over 23,000 individuals in Maryland where hypertension had a strong graded relationship with the future risk of CKD in both men and women (26).
Patients on Renal Replacement Therapy
Increased dietary sodium has implications in those with end stage renal disease (ESRD). Sodium accumulation is one of the consequences of renal failure, resulting in increased water intake, increases in the extracellular volume, and accompanying rise in blood pressure (27). At the beginning of maintenance hemodialysis, Scribner recommended dietary sodium restriction and ultrafiltration to control extracellular volume (28), citing research in nephrectomized dogs in which hypertension appeared to be influenced by the size of the extracellular space. Indeed, studies on humans show strict volume control is associated with improved control of hypertension, and prolonged survival in those on hemodialysis (29, 30). In one cross sectional study, dialysis centers placing strong emphasis on sodium restriction showed reduced prescriptions of antihypertensive drugs, less left ventricular hypertrophy, and decreased intradialytic hypotension, as compared to centers focusing more on antihypertensive based therapy (31).
Volume homeostasis is an important predictor of outcome in peritoneal dialysis patients as well (32). In patients utilizing continuous peritoneal dialysis, excessive dietary sodium intake is cited as a major cause of extracellular volume expansion (33). Those who are hypertensive are more fluid overloaded, despite higher fluid and sodium removal as compared to normotensives (34).
Lower sodium intake also benefits those with kidney transplants. In kidney transplant patients being treated for hypertension, low sodium intake in combination with antihypertensive therapy results in significantly decreased blood pressure versus those on therapy without sodium restriction (35). Some immunosuppressants are associated with total body sodium retention (36), and thus it is often recommended to reduce dietary sodium as an adjunct in managing patients with increased extracellular volume and/or hypertension.
Debate Regarding Clinical Implications
In reviewing this literature, it would take significant effort not to recognize that there exists some controversy regarding high dietary sodium and its clinical implications. On one hand is the evidence that high dietary sodium potentially leads to poor outcomes, as previously discussed. However, others suggest there is no proven benefit to restricting sodium intake and that it may not be a prudent use of resources to attempt to alter sodium intake, as it may be a predefined, physiologically set parameter in humans (37). Indeed, the largest world-wide observational study to examine blood pressure and sodium consumption did not find a significant relationship with median blood pressure or prevalence of high blood pressure across 48 centers. However, there was a weak association between sodium intake and rise in blood pressure with age (5); later re-analysis with a correction factor did confirm a strong, positive association of sodium intake with systolic pressure of individuals (38). One study looking at data from the NHANES III showed lower sodium intake had a modest association with higher mortality, and that cardiovascular disease was increased in the lowest quintile of sodium intake versus the highest (39). The authors note the observed associations were mostly not statistically significant, but stated that their findings suggest higher sodium intake is unlikely to be independently associated with increased mortality or cardiovascular disease in the general US adult population (39).
For one’s own conclusion, it is important to consider the attributes and limitations of study designs, especially when comparing randomized controlled trial findings to data from observational studies. As an example, the dietary sodium intake in the NHANES III population was determined by participant twenty four hour recall and not confirmed with 24 hour urine sodium excretion measurements. There could be significant limitations in the study design because of potential recall bias, which may be somewhat supported in looking at the reported daily caloric intake of participants: on average, those with overweight body mass indices (25.8 and 26.4) reported daily energy intake that was in fact low at 1200–1700 kcal per day (39).
A final point for consideration is that a negative impact of dietary sodium on health outcomes may occur at extremes of intake. One patho-mechanistic model suggests that both low and high sodium may produce reactive oxygen species and oxidative products, and it is likely there is a “right balance” to strike between too little and too much (12).
Recommendations for Clinical Practice
Based upon growing evidence showing associations of high dietary sodium with poor health outcomes, several organizations recommend a reduction in sodium intake (Table 1). For healthy people, the World Health Organization (WHO) recommends that sodium intake be reduced by 20% and ultimately to a goal of < 2.0 g sodium per day, except where lower levels have been set (40). The United States Department of Agriculture (USDA)(1), the Institute of Medicine (IOM)(2), and the American Heart Association (AHA) Nutrition Committee (41) recommend at most, a consumption of 2.3 g of sodium per day. In addition, the USDA has also recommended more restrictions to 1.5 g of sodium per day for people of middle or older age, black race, or those who have hypertension.
Table 1.
Comparison of Daily Recommended Limits for Sodium Intake. Food examples that account for the entire daily limit provide a potential reference point for patients (45, 46).
| Recommended Limit for Healthy Adults | Additional Considerations | Food examples that could account for the entire daily limit | |
|---|---|---|---|
| World Health Organization | 2.0 g/day | Lower if specified by national targets | 1 large taco + 1 cup of refried beans |
| United States Department of Agriculture | 2.3 g/day | 1.5 g/day in those who may benefit (hypertensive, Black race, middle older age adults | 1 teaspoon of table salt (NaCl) |
| Institute of Medicine | 1.5 g/day Adequate Intake (AI) 2.3 g/day Upper Limit (UL) |
AI to ensure nutrient adequacy, not to prevent chronic disease. AI not applicable to certain populations (e.g. highly active with large sweat losses) |
6–8 fast food batter fried shrimp One 6″ tuna salad sub sandwich + 1 cup chicken noodle soup |
| American Heart Association | 2.3 g/day | Recommended as an achievable target | 5 fast food chicken select strips + 1 large fry + 1 chocolate shake |
| Kidney Disease Not Specifically Noted Above | |||
| NKF K/DOQI Guidelines | 2.4 g/day | 2.0 g/day Hemodialysis Reductions in Peritoneal Dialysis | 1 cup beans & franks + 1 cup potato salad |
Kidney disease is not specifically noted in these recommendations. However, guidelines from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) state there is strong evidence to support the recommendation that non dialysis patients with CKD adhere to a goal of less than 2.4 g of sodium per day (42). Sodium restriction is not recommended for patients with salt-wasting nephropathies. For patients receiving hemodialysis, NKF KDOQI has specific recommendations of no more than 2 g of sodium per day, and also recommends reducing sodium intake for those on peritoneal dialysis (43, 44).
Identifying & Addressing Barriers to Dietary Sodium Reduction
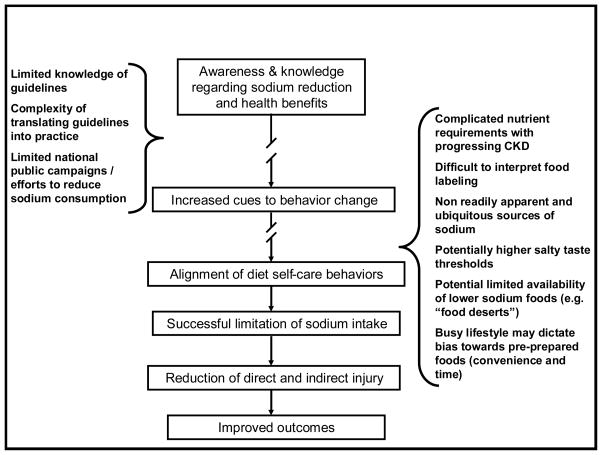
There are many potential barriers to reducing dietary sodium for patients with kidney disease. Figure C presents a suggested model of how decreasing daily sodium intake may lead to improved outcomes, and where some of the potential barriers may impact steps in this process. We expand discussion regarding some of these barriers further.
Figure C.
Potential Barriers (in sidebars) to successfully translating sodium guidelines into practice
Limited Knowledge in Patients and Providers
There is some evidence showing limited patient knowledge about sodium content in food. For example, patients with heart failure who are less knowledgeable about sodium are more likely to have a higher weekly consumption of high-sodium foods and also increased readmission after hospitalization (47). Although there is little known about the sodium knowledge of patients with kidney disease, less knowledge may be a contributing factor in this population, as well.
Providers may also be unclear regarding the amount of sodium in foods. One study of providers showed their knowledge of the sodium content of common food items was no different than the knowledge of the general population (48). Patients commonly indicate that their health care provider (i.e. physician) counsels them to adhere to a low sodium diet, but usually does not offer the detailed advice needed to execute this important recommendation (49). At a minimum, patients should be instructed to reduce intake of processed, canned, and “fast” foods. The natural sodium content of food is estimated at 10%, while more than 75% is added during the manufacturing of processed foods (50).
Limited Public Campaigns/Efforts to Regulate Food Sodium Content
There is little regulation of the sodium content in foods, and since the US Food and Drug Administration (FDA) considers salt to be a substance “generally regarded as safe (GRAS)”, manufacturers are not limited in the amount of sodium added to processed foods. Interestingly, the American Medical Association has recommended twice to the FDA to revoke the GRAS status of salt without success. Since the majority of kidney patients are affected by hypertension and likely to be salt-sensitive, a low sodium diet is usually agreed upon as a worthy therapeutic effort. However, despite several “Calls to Action” in the U.S. there remains a hesitancy and vocal debate for systematic efforts to reduce population dietary sodium intake (7, 51).
Difficult to Interpret Labeling Complicates Dietary Sodium Management
Interpretation of the sodium content in foods remains a challenge, especially in the United States. As mentioned earlier, most sodium in food is added during the manufacturing of processed foods. However, this remains hidden and elusive to most people. In addition, time constraints, convenience, and limited access to fresh produce may lead some individuals to select pre-prepared, or “fast foods”, which often do not include readily available labeling at the point of service.
Even when foods are labeled with sodium content, limiting daily intake is a complex task. The current U.S. food label includes sodium content, however patients simply cannot navigate the dense information contained on it, nor can they calculate the total nutritional content of the food item (52). In the United Kingdom, the majority of the food industry has adopted a “traffic-light” type labeling system that indicates if the food is high (red-light), medium (yellow-light), or low (green-light) sodium content (4). Similar clear labeling efforts have been instituted in New Zealand and Australia. In Finland, sodium intake has decreased by nearly 40% attributed these efforts (53). Clear labeling may simplify the complicated task of limiting sodium intake somewhat, but it requires national efforts towards this end.
Changes in Taste
Limiting dietary sodium content to less than 1.3 g per day has been described as unpalatable and intolerable. The ability of patients with kidney disease to detect, or taste salt in food is often impaired (54). This may contribute to an increase in the dietary consumption of sodium to reach levels that are palatable. Habitual high dietary sodium consumption, diabetic nephropathy, and the use of diuretics also further increases the sodium taste threshold (55, 56). However, this taste threshold may be significantly lowered by the institution of sodium restriction, even within just one week (56, 57).
For patients receiving dialysis, the dietitian has been noted to be the most trusted and informative source to guide adherence to both sodium and fluid restrictions (58). In the care of all stages of kidney disease, increasing education and awareness through a multidisciplinary team may better empower patients and guide their dietary self-care. As of 2010, the Center for Medicare and Medicaid Services reimbursement will further support face-to-face educational services provided by qualified professionals, to patients with chronic kidney disease. Sessions may be provided individually, or in a group and may include up to six visits (59). Medical nutrition therapy is an important component of this education.
If multidisciplinary teams are not readily available, there may be alternative educational resources, for example those offered online by the National Kidney foundation (60), the Centers for Disease Control and Prevention (61), and the American Association of Kidney Patients (62) websites. The Patient resource “Sodium and Your CKD Diet: How to Spice Up Your Cooking” offers helpful, straightforward advice on integrating daily sodium intake goals into menu options (60). Other valuable sources of dietary advice for patients are listed elsewhere (11).
Summary
Much data support limiting dietary sodium intake for patients with kidney disease. By effectively communicating tangible sodium intake limits with our patients, we begin to lay the foundation for building optimal dietary self care behaviors. Reinforcement of these behaviors can be provided with public campaigns and efforts directed at clear labeling and regulation of sodium content in foods. Patient follow up with their providers may be augmented with multidisciplinary teams used to confirm appropriate application of concepts and if needed, provide further individualized education. Although there are many potential barriers to successfully keeping sodium intake within current guidelines, we can use existing resources to optimize educational efforts with our patients, in order to achieve improved outcomes.
Acknowledgments
Dr. Cavanaugh is supported by a NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K23DK080952 career development award. Dr. Wright is supported by an American Kidney Fund Clinical Scientist in Nephrology award. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of either funding organization.
References
- 1.U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans, 2005. 6. Washington D.C: U.S. Government Printing Office; 2005. [Google Scholar]
- 2.Institute of Medicine. Water, Potassium, Sodium, Chloride and Sulfate. Washington, D.C: National Academy Press; 2004. Dietary Reference Intakes. [Google Scholar]
- 3.U.S. Department of Agriculture, Agricultural Research Service. Nutrient Intakes from Food. [Accessed on: 1/10/2010];Mean Amounts Consumed per Individual, One Day, 2005–2006. Available: www.ars.usda.gov/ba/bhnrc/fsrg.
- 4.Vennegoor MA. Salt restriction and practical aspects to improve compliance. J Ren Nutr. 2009;19:63–68. doi: 10.1053/j.jrn.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12:17–31. doi: 10.1053/jren.2002.29598. [DOI] [PubMed] [Google Scholar]
- 7.Aviv A. Salt and hypertension: the debate that begs the bigger question. Arch Intern Med. 2001;161:507–510. doi: 10.1001/archinte.161.4.507. [DOI] [PubMed] [Google Scholar]
- 8.Weir MR. Is it the low-protein diet or simply the salt restriction? Kidney Int. 2007;71:188–190. doi: 10.1038/sj.ki.5002066. [DOI] [PubMed] [Google Scholar]
- 9.Weir MR, Fink JC. Salt intake and progression of chronic kidney disease: an overlooked modifiable exposure? A commentary. Am J Kidney Dis. 2005;45:176–188. doi: 10.1053/j.ajkd.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int. 1982;21:371–378. doi: 10.1038/ki.1982.32. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox C. Dietary Salt Intake for Patients with Hypertension or Kidney Disease. In: Mitch WE, Ikizler TA, editors. Handbook of Nutrition and the Kidney. 6. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 233–242. [Google Scholar]
- 12.Ritz E, Mehls O. Salt restriction in kidney disease--a missed therapeutic opportunity? Pediatr Nephrol. 2009;24:9–17. doi: 10.1007/s00467-008-0856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–2782. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- 14.Sanders PW. Dietary salt intake, salt sensitivity, and cardiovascular health. Hypertension. 2009;53:442–445. doi: 10.1161/HYPERTENSIONAHA.108.120303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci U S A. 2001;98:13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aperia A. New roles for an old enzyme: Na, K-ATPase emerges as an interesting drug target. J Intern Med. 2007;261:44–52. doi: 10.1111/j.1365-2796.2006.01745.x. [DOI] [PubMed] [Google Scholar]
- 17.Sun Z, Andersson R. NF-kappaB activation and inhibition: A review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Blaustein M. Endogenous ouabain: role in pathogenesis of hypertension. Kidney International. 1996;49:1748–1753. doi: 10.1038/ki.1996.260. [DOI] [PubMed] [Google Scholar]
- 19.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected Effect of Dietary Salt Reductions on Future Cardiovascular Disease. N Engl J Med. doi: 10.1056/NEJMoa0907355. Epub 1/20/2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe LP, Greenland P, Ruth KJ, Dyer AR, Stamler R, Stamler J. Impact of major cardiovascular disease risk factors, particularly in combination, on 22-year mortality in women and men. Arch Intern Med. 1998;158:2007–2014. doi: 10.1001/archinte.158.18.2007. [DOI] [PubMed] [Google Scholar]
- 21.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 23.Verhave JC, Hillege HL, Burgerhof JG, Janssen WM, Gansevoort RT, Navis GJ, de Zeeuw D, de Jong PE. Sodium intake affects urinary albumin excretion especially in overweight subjects. For the Prevend Study Group. J Intern Med. 2004;256:324–330. doi: 10.1111/j.1365-2796.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- 24.Heeg JE, de Jong PE, van der Hem GK, de Zeeuw D. Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int. 1989;36:272–279. doi: 10.1038/ki.1989.190. [DOI] [PubMed] [Google Scholar]
- 25.Ekinci EI, Thomas G, Thomas D, Johnson C, Macisaac RJ, Houlihan CA, Finch S, Panagiotopoulos S, O’Callaghan C, Jerums G. Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care. 2009;32:1398–1403. doi: 10.2337/dc08-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14:2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 27.Raimann J, Liu L, Tyagi S, Levin NW, Kotanko P. A fresh look at dry weight. Hemodial Int. 2008;12:395–405. doi: 10.1111/j.1542-4758.2008.00302.x. [DOI] [PubMed] [Google Scholar]
- 28.Scribner BH, Buri R, Caner JE, Hegstrom R, Burnell JM. The treatment of chronic uremia by means of intermittent hemodialysis: a preliminary report. Trans Am Soc Artif Intern Organs. 1960;6:114–122. [PubMed] [Google Scholar]
- 29.Charra B, Calemard E, Cuche M, Laurent G. Control of hypertension and prolonged survival on maintenance hemodialysis. Nephron. 1983;33:96–99. doi: 10.1159/000182920. [DOI] [PubMed] [Google Scholar]
- 30.Ozkahya M, Ok E, Toz H, Asci G, Duman S, Basci A, Kose T, Dorhout Mees EJ. Long-term survival rates in haemodialysis patients treated with strict volume control. Nephrol Dial Transplant. 2006;21:3506–3513. doi: 10.1093/ndt/gfl487. [DOI] [PubMed] [Google Scholar]
- 31.Kayikcioglu M, Tumuklu M, Ozkahya M, Ozdogan O, Asci G, Duman S, Toz H, Can LH, Basci A, Ok E. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant. 2009;24:956–962. doi: 10.1093/ndt/gfn599. [DOI] [PubMed] [Google Scholar]
- 32.Van Biesen W, Vanholder R, Veys N, Lameire N. Improving salt balance in peritoneal dialysis patients. Perit Dial Int. 2005;25 (Suppl 3):S73–75. [PubMed] [Google Scholar]
- 33.Tzamaloukas AH, Raj DS, Onime A, Servilla KS, Vanderjagt DJ, Murata GH. The prescription of peritoneal dialysis. Semin Dial. 2008;21:250–257. doi: 10.1111/j.1525-139X.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Cheng LT, Wang T. Salt and fluid intake in the development of hypertension in peritoneal dialysis patients. Ren Fail. 2007;29:427–432. doi: 10.1080/08860220701260461. [DOI] [PubMed] [Google Scholar]
- 35.Keven K, Yalcin S, Canbakan B, Kutlay S, Sengul S, Erturk S, Erbay B. The impact of daily sodium intake on posttransplant hypertension in kidney allograft recipients. Transplant Proc. 2006;38:1323–1326. doi: 10.1016/j.transproceed.2006.02.103. [DOI] [PubMed] [Google Scholar]
- 36.Helderman JH, Schaefer H, Langone AJ, Goral S. Homeostasis of Solute and Water by the Transplanted Kidney. In: Alpern RJ, Hebert SC, editors. Seldin and Giebisch’s The Kidney Physiology and Pathophysiology. 4. New York: Elsevier Academic Press; 2008. pp. 2737–2761. [Google Scholar]
- 37.McCarron DA, Geerling JC, Kazaks AG, Stern JS. Can dietary sodium intake be modified by public policy? Clin J Am Soc Nephrol. 2009;4:1878–1882. doi: 10.2215/CJN.04660709. [DOI] [PubMed] [Google Scholar]
- 38.Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ. 1996;312:1249–1253. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III) J Gen Intern Med. 2008;23:1297–1302. doi: 10.1007/s11606-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Report of a WHO Forum and Technical Meeting 5–7 October 2006, Paris, France. Geneva: WHO Press; 2007. Reducing salt intake in populations. [Google Scholar]
- 41.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 42.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–290. [PubMed] [Google Scholar]
- 43.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48 (Suppl 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 44.Clinical practice guidelines for peritoneal adequacy, update 2006. Am J Kidney Dis. 2006;48 (Suppl 1):S91–97. doi: 10.1053/j.ajkd.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Department of Agriculture, Agricultural Research Service. [Accessed on: 1/25/2010];USDA National Nutrient Database for Standard Reference, Release 22. Available: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 46.McDonald’s USA. [Accessed on: 11/6/2009];Nutrition Facts for Popular Menu Items. Available: http://nutrition.mcdonalds.com/nutritionexchange/nutritionfacts.pdf.
- 47.Kollipara UK, Jaffer O, Amin A, Toto KH, Nelson LL, Schneider R, Markham D, Drazner MH. Relation of lack of knowledge about dietary sodium to hospital readmission in patients with heart failure. Am J Cardiol. 2008;102:1212–1215. doi: 10.1016/j.amjcard.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 48.Heidrich FE, Bergman JJ. Physician knowledge of sodium content of common foods. J Fam Pract. 1982;14:693–697. [PubMed] [Google Scholar]
- 49.Sheahan SL, Fields B. Sodium dietary restriction, knowledge, beliefs, and decision-making behavior of older females. J Am Acad Nurse Pract. 2008;20:217–224. doi: 10.1111/j.1745-7599.2008.00307.x. [DOI] [PubMed] [Google Scholar]
- 50.James WP, Ralph A, Sanchez-Castillo CP. The dominance of salt in manufactured food in the sodium intake of affluent societies. Lancet. 1987;1:426–429. doi: 10.1016/s0140-6736(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 51.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 52.Rothman RL, Housam R, Weiss H, Davis D, Gregory R, Gebretsadik T, Shintani A, Elasy TA. Patient understanding of food labels: the role of literacy and numeracy. Am J Prev Med. 2006;31:391–398. doi: 10.1016/j.amepre.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Havas S, Dickinson BD, Wilson M. The urgent need to reduce sodium consumption. JAMA. 2007;298:1439–1441. doi: 10.1001/jama.298.12.1439. [DOI] [PubMed] [Google Scholar]
- 54.Fernstrom A, Hylander B, Rossner S. Taste acuity in patients with chronic renal failure. Clinical nephrology. 1996;45:169–174. [PubMed] [Google Scholar]
- 55.Huggins RL, Di Nicolantonio R, Morgan TO. Preferred salt levels and salt taste acuity in human subjects after ingestion of untasted salt. Appetite. 1992;18:111–119. doi: 10.1016/0195-6663(92)90188-c. [DOI] [PubMed] [Google Scholar]
- 56.Kusaba T, Mori Y, Masami O, Hiroko N, Adachi T, Sugishita C, Sonomura K, Kimura T, Kishimoto N, Nakagawa H, Okigaki M, Hatta T, Matsubara H. Sodium restriction improves the gustatory threshold for salty taste in patients with chronic kidney disease. Kidney international. 2009;76:638–643. doi: 10.1038/ki.2009.214. [DOI] [PubMed] [Google Scholar]
- 57.Mattes RD. The taste for salt in humans. The American journal of clinical nutrition. 1997;65:692S–697S. doi: 10.1093/ajcn/65.2.692S. [DOI] [PubMed] [Google Scholar]
- 58.Smith K, Coston M, Glock K, Elasy TA, Wallston KA, Ikizler TA, Cavanaugh KL. Patient Perspectives on Fluid Management in Chronic Hemodialysis. J Ren Nutr. 2009 doi: 10.1053/j.jrn.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CMS Manual System. [Accessed on: 1/25/2010];Pub 100–04 Medicare Claims Processing. :1–16. Available: http://www.cms.hhs.gov/transmittals/downloads/R1876CP.pdf.
- 60.Sodium and Your CKD Diet. [Accessed on: 11/3/2009];How to Spice Up Your Cooking. Available: http://www.kidney.org/atoz/content/sodiumckd.cfm.
- 61.Centers for Disease Control and Prevention. Nutrition for Everyone. Health Facts Sodium and Potassium; [Accessed on: 1/25/2010]. Your online source for credible health information. Available: http://www.cdc.gov/nutrition/everyone/index.html. [Google Scholar]
- 62.American Association of Kidney Patients. Brochures. [Accessed on: 1/25/2010];AAKP Nutrition Counter: A Reference for the Kidney Patient. Available: http://www.aakp.org/brochures/