Abstract
A method has been described for the direct measurement of proinsulin in human plasma. The method makes use of an insulin-degrading enzyme designated “insulin-specific protease (ISP)”, which is obtained from rat skeletal muscle. Under the conditions used, this enzyme rapidly degrades insulin and insulin-like polypeptides to nonimmunoassayable components, whereas proinsulin and proinsulin cleaved at position B54,55 are not appreciably affected. The incubation of plasma with ISP results in the disappearance of insulin, but not proinsulin, as demonstrated by column chromatography. Immunoassay of the plasma, therefore, before and after incubation, determines the values for the total immunoreactive substance (TIR) and for immunoreactive proinsulin (IRP), respectively. The values obtained for proinsulin levels are reproducible and compare closely with the more complicated column fractionation methods.
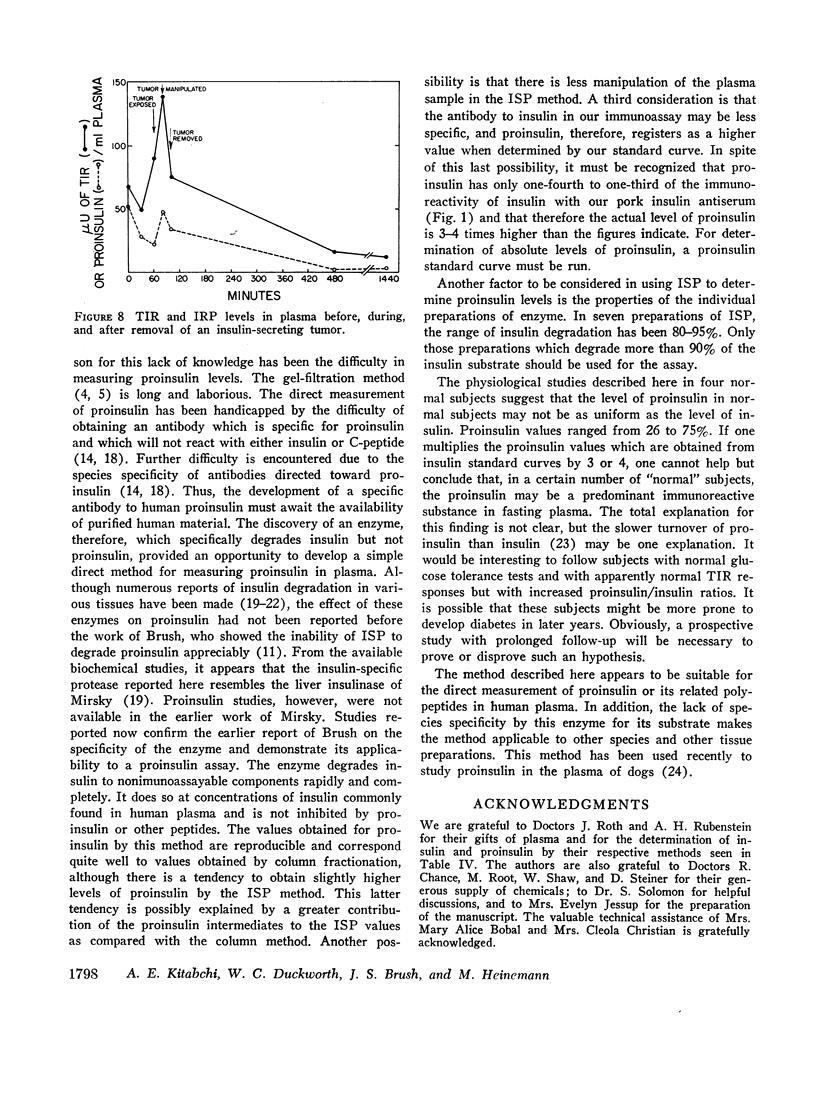
Proinsulin responses were studied in four normal subjects and one patient with an insulinoma after a glucose load. Fasting proinsulin levels varied widely in the normal subjects, and the levels rose more slowly than TIR levels after glucose. IRP levels in the patient with an insulinoma were very high and fell to normal after removal of the tumor.
The ISP method, therefore, appears to be suitable for the direct, accurate, and rapid determination of proinsulin and proinsulin-like materials in human plasma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brush J. S., Kitabchi A. E. Metabolic disposition of [131I] iodoinsulin within the rat diaphragm. Biochim Biophys Acta. 1970 Jul 21;215(1):134–144. doi: 10.1016/0304-4165(70)90396-x. [DOI] [PubMed] [Google Scholar]
- Brush J. S. Purification and characterization of a protease with specificity for insulin from rat muscle. Diabetes. 1971 Mar;20(3):140–145. [PubMed] [Google Scholar]
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- Goldsmith S. J., Yalow R. S., Berson S. A. Significance of human plasma insulin Sephadex fractions. Diabetes. 1969 Dec;18(12):834–839. doi: 10.2337/diab.18.12.834. [DOI] [PubMed] [Google Scholar]
- Gorden P., Roth J. Plasma insulin: fluctuations in the "big" insulin component in man after glucose and other stimuli. J Clin Invest. 1969 Dec;48(12):2225–2234. doi: 10.1172/JCI106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZEN H. M., TIETZE F., STETTEN D., Jr Further studies on the properties of hepatic glutathione-insulin transhydro-genase. J Biol Chem. 1963 Mar;238:1006–1011. [PubMed] [Google Scholar]
- Kitabchi A. E., Buchanan K. D., Vance J. E., Williams R. H. Effect of adrenocorticotropin and glucocorticoids on insulin secretion. J Clin Endocrinol Metab. 1968 Oct;28(10):1479–1486. doi: 10.1210/jcem-28-10-1479. [DOI] [PubMed] [Google Scholar]
- Kitabchi A. E. The biological and immunological properties of pork and beef insulin, proinsulin, and connecting peptides. J Clin Invest. 1970 May;49(5):979–987. doi: 10.1172/JCI106317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus N. R., Tanese T., Gutman R., Recant L. Synthesis and release of proinsulin and insulin by human insulinoma tissue. J Clin Endocrinol Metab. 1970 Mar;30(3):273–281. doi: 10.1210/jcem-30-3-273. [DOI] [PubMed] [Google Scholar]
- MIRSKY I. A. Insulinase, insulinase-inhibitors, and diabetes mellitus. Recent Prog Horm Res. 1957;13:429–471. [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Steiner D. F. Human serum proinsulin. J Clin Invest. 1970 Mar;49(3):497–507. doi: 10.1172/JCI106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Gorden P., Pastan I. "Big insulin": a new component of plasma insulin detected by immunoassay. Proc Natl Acad Sci U S A. 1968 Sep;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. H., Cho S., Steiner D. F. Evidence for proinsulin in human urine and serum. Lancet. 1968 Jun 22;1(7556):1353–1355. doi: 10.1016/s0140-6736(68)92040-0. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Melani F., Pilkis S., Steiner D. F. Proinsulin. Secretion, metabolism, immunological and biological properties. Postgrad Med J. 1969 Jul;45(Suppl):476–481. [PubMed] [Google Scholar]
- Stoll R. W., Touber J. L., Winterscheid L. C., Ensinck J. W., Williams R. H. Hypoglycemic activity and immunological half-life of porcine insulin and proinsulin in baboons and swine. Endocrinology. 1971 Mar;88(3):714–717. doi: 10.1210/endo-88-3-714. [DOI] [PubMed] [Google Scholar]
- TOMIZAWA H. H., NUTLEY M. L., NARAHARA H. T., WILLIAMS R. H. The mode of inactivation of insulin by rat liver extracts. J Biol Chem. 1955 May;214(1):285–294. [PubMed] [Google Scholar]



