Abstract
Rational
Exposing animal models of mental illness to addictive drugs provides an approach to understanding the neural etiology of dual diagnosis disorders. Previous studies have shown that neonatal ventral hippocampal lesions (NVHL) in rats produce features of both schizophrenia and addiction vulnerability.
Objective
This study investigated ventral and dorsal striatal dopamine (DA) efflux in NVHL rats combined with behavioral sensitization to cocaine.
Methods
Adult NVHL vs. SHAM-operated rats underwent a 5-day injection series of cocaine (15 mg/kg/day) vs. saline. One week later, rats were cannulated in nucleus accumbens SHELL, CORE, or caudate–putamen. Another week later, in vivo microdialysis sampled DA during locomotor testing in which a single cocaine injection (15 mg/kg) was delivered.
Results
NVHLs and cocaine history significantly increased behavioral activation approximately 2-fold over SHAM-saline history rats. DA efflux curves corresponded time dependently with the cocaine injection and locomotor curves and varied significantly by striatal region: Baseline DA levels increased 5-fold while cocaine-stimulated DA efflux decreased by half across a ventral to dorsal striatal gradient. However, NVHLs, prior cocaine history, and individual differences in behavior were not underpinned by differential DA efflux overall or within any striatal region.
Conclusion
Differences in ventral/dorsal striatal DA efflux are not present in and are not required for producing differential levels of acute cocaine-induced behavioral activation in NVHLs with and without a behaviorally sensitizing cocaine history. These findings suggest other neurotransmitter systems, and alterations in striatal network function post-synaptic to DA transmission are more important to understanding the interactive effects of addictive drugs and mental illness.
Keywords: Dual diagnosis, Dopamine, Nucleus accumbens shell, Core, Dorsal striatum, Microdialysis, Behavior, Hippocampus, Schizophrenia, Sensitization
Introduction
Co-morbidity of addictions with schizophrenia and other mental illnesses increases medical and psychiatric disability, homelessness, criminal incarceration, and premature death in these populations (Drake and Wallach 2000; O’Brien et al. 2004). Emerging findings suggest that the high prevalence of substance disorders in psychiatric illnesses reflects an involuntary neurobiological vulnerability to drug addiction imparted by the same neuropathological substrates that generate psychiatric symptoms (Chambers et al. 2001). This integrated circuit theory of dual diagnosis has appeal especially in the case of schizophrenia, given a wealth of data implicating sub-cortical dopamine (DA) transmission in this disorder and addictions.
Preclinical studies that combine neonatal ventral hippocampal lesions (NVHLs) in rats—an extensively characterized neurodevelopmental model of schizophrenia—and drug addiction models support a unified neural basis for psychiatric illness and addiction vulnerability. NVHLs produce a spectrum of developmental, clinical, and neurobiological features of human schizophrenia (Tseng et al. 2009). In this model, positive-like symptoms (e.g., behavioral hyper-reactivity to novelty, stress, and psychostimulants) emerge after adolescence and are reducible with neuroleptics; cognitive deficits and negative symptom traits are detectable earlier and are not treatment responsive. Sensory gating, cortical electrophysiological patterns, and various markers of prefrontal cortical/neuronal function and anatomy, involving glutamatergic and GABAergic transmission, are abnormal (Flores et al. 2005; Tseng et al. 2008, 2007; Vohs et al. 2010). Upon exposure to reinforcing drugs, NVHLs show augmented addiction-related phenotypes: Impulsivity in conditioned approach to natural rewards is worsened by cocaine history; long-term behavioral sensitization to cocaine, alcohol, and nicotine are all accentuated (Berg and Chambers 2008; Chambers et al. 2005; Chambers and Taylor 2004; Conroy et al. 2007); drug-seeking in cocaine and methamphetamine self-administration are both enhanced (Chambers and Self 2002; Brady et al. 2008). The ventral hippocampus, developmentally altered in NVHLs and functionally and structurally altered in schizophrenia, sends direct glutamatergic projections to the nucleus accumbens (NAC) and prefrontal cortical areas that project to the NAC (O’Donnell and Grace 1995). As a primary receptive field of mesolimbic DA projections, where addictive drugs potentiate DA concentrations, the NAC is involved in the selection and adaptability of motivated behavior (Kalivas and Volkow 2005). Thus, understanding separate and combined effects of NVHLs and addictive drug history on ventral striatal DA circuits may provide important clues about the genesis of dual diagnosis.
Previous work demonstrates that a behaviorally sensitizing cocaine history and NVHLs produce differential patterns of prefrontal cortical/striatal network activation. When combined in the same animals, these conditions show compounding effects on dorsal striatal neural activation that directly correlates with degree of long-term behavioral sensitization (Chambers et al. 2010). The present study replicates this experimental design, while assessing DA efflux into ventral striatal (NAC) and dorsal striatal (caudate–putamen, CAPU) regions. Advancing from prior studies that examined NAC DA efflux in living NVHL rats (Brake et al. 1999; Corda et al. 2006; Lillrank et al. 1999; Wan et al. 1996), the present study utilized a substantially larger number of rats in a microdialysis–HPLC quantification of DA efflux while examining (a) the separate and combined impacts of NVHLs and a behaviorally sensitizing cocaine history, (b) DA efflux across three ventral–dorsal striatal compartments, and (c) DA concentration changes measured simultaneously with behavior during acute cocaine challenge.
Materials and methods
Subjects and surgeries
Sprague–Dawley dams arriving at 14–17 days gestation and housed under standard conditions produced litters of eight to 14 pups on post-natal day (PD) 0. On PD 7, males weighing 16–19 g were randomized for surgeries with balancing of NVHL/SHAM assignments approximating 5:3 ratio within litters. Post-operative pups were returned to their litters where they grew undisturbed under standard conditions through weaning (PD 21), when they were pair-housed. Pups remained in pairs though the initial injection series and then were singly housed (PD 71) after cannulation. All procedures accorded with NIH guide for Care and Use of Laboratory Animals and the Indiana University Institutional Animal Care and Use Committee.
PD 7 pups underwent NVHL/SHAM surgeries under hypothermic anesthesia as originally described (Lipska et al. 1993), with delivery of ibotenic acid (3.0 μg in 0.3 μl in artificial CSF) vs. artificial CSF (0.3 μl) into the ventral hippocampus bilaterally (AP −3.0, ML ±3.5, DV −5.0; from bregma (millimeters)). Cannulation surgeries were conducted on PD 71 under isofluorane anesthesia. Rats were randomized to placement of a guide cannula (18 G/11 mm; Plastics One) in the NAC SHELL (AP +2.1, ML +2.0, DV −4.5), NAC CORE (AP +2.0, ML +2.6, DV −3.9), or CAPU (AP +1.9, ML +3.1, DV −2.5; from bregma (millimeters) all 10° off midline). Cannula was affixed to the skull with three screws and a microdialysis tubing tether (Instech) with cranioplastic cement. Unilateral (right) rather than dual cannulations were used to minimize non-specific injury of neural systems that might support the experimental phenotypes. Five days after cannulations (PD 76), rats underwent brief isofluorane anesthesia for placement of loop style probes (Kohl et al. 1998; 216 μm e.d.), with dialysis loops (Spectrum Laboratories, Rancho Dominguez, CA, USA) parallel with the AP plane, and inserted to extend (4 mm at the tip) below the guide cannula and cemented in place. The active membrane dialysis length of the probe loop was 1.5 mm (i.e., 3.0 mm total length) for all target regions.
Behavioral sensitization and microdialysis
Upon reaching adulthood (PD 60), rats were randomized to 5 days of once daily cocaine (15 mg/kg (NIDA) in 1 ml/kg saline i.p.) or saline injections (1 ml/kg i.p.) in home cages (Fig. 1). Two weeks later (PD 78), all rats underwent a single cocaine injection (15 mg/kg) within a 210-min locomotor arena session (under low red light) during microdialysis collection. Arenas ( ) with 16 × 16 infrared beam detectors (Med Associates) were equipped with microdialysis tubing swivel arms mounted above the field with dual channel swivels (Instech). Microdialysis was conducted as previously described (Engleman et al. 2003). Dialysis tubing coursed from infusion pump-mounted syringes (Harvard Apparatus) through swivels to dialysis probes protected by a spring sheath (Instech). Outlet tubing coursed back through the swivel, terminating at the elbow of the swivel arm where dialysate was collected in microfuge tubes. Sessions began with hookup of probe tubing and securing of tethers to the dialysis sheath. Probes were perfused at 1.5 μl/min with aCSF containing (in millimolars) 140 NaCl, 3 KCl, 2.5 CaCl2, 1.0 MgCl2, 2.0 Na2PO4, and 0.2 ascorbate adjusted to pH 7.4 with 0.1 N NaOH. For the first 90 min of the 210-min session, rats habituated to the setup, followed by a 120-min recording phase measuring distance ambulated in 10-min bins. Cocaine injections were delivered at the 30-min point of the 120-min recording phase. Sixteen microdialysis samples (15 μl) were collected in 10 min intervals. Based on empirically verified flow rates and length of outlet tubing, these collections corresponded to four washout samples (during habituation), three baseline samples (pre-injection locomotion), and nine post-injection samples (post-injection locomotion).
) with 16 × 16 infrared beam detectors (Med Associates) were equipped with microdialysis tubing swivel arms mounted above the field with dual channel swivels (Instech). Microdialysis was conducted as previously described (Engleman et al. 2003). Dialysis tubing coursed from infusion pump-mounted syringes (Harvard Apparatus) through swivels to dialysis probes protected by a spring sheath (Instech). Outlet tubing coursed back through the swivel, terminating at the elbow of the swivel arm where dialysate was collected in microfuge tubes. Sessions began with hookup of probe tubing and securing of tethers to the dialysis sheath. Probes were perfused at 1.5 μl/min with aCSF containing (in millimolars) 140 NaCl, 3 KCl, 2.5 CaCl2, 1.0 MgCl2, 2.0 Na2PO4, and 0.2 ascorbate adjusted to pH 7.4 with 0.1 N NaOH. For the first 90 min of the 210-min session, rats habituated to the setup, followed by a 120-min recording phase measuring distance ambulated in 10-min bins. Cocaine injections were delivered at the 30-min point of the 120-min recording phase. Sixteen microdialysis samples (15 μl) were collected in 10 min intervals. Based on empirically verified flow rates and length of outlet tubing, these collections corresponded to four washout samples (during habituation), three baseline samples (pre-injection locomotion), and nine post-injection samples (post-injection locomotion).
Fig. 1.
Experimental timeline according to animal age (post-natal day, PD)
HPLC detection of DA content
Samples were collected in microfuge tubes containing 5.0 μl of 0.1 N perchloric acid, immediately frozen on dry ice then stored at −80° until analysis. HPLC/EC analysis of DA was performed as described elsewhere (Franklin et al. 2009). The system comprised an amperometric microbore/Unijet cell with a 6-mm glassy carbon electrode (BAS, Lafayette, IN, USA) coupled with a Princeton Applied Research/EG&G electrochemical detector (Princeton Applied Research, Princeton, NJ, USA). Samples with volumes of ~20 μl (including preservative) were loaded into a 10-μl loop (sample loops were overfilled with twice their volume as recommended to optimize precision of sample analysis) and injected onto an analytical column (BDS Hypersil C18, 3 μm, 1 × 100 mm; Keystone Scientific, Bellefonte, PA, USA) with a mobile phase of sodium acetate 6.32 g/l, EDTA 0.15 g/l, sodium octyl-sulfate 0.75 g/l, and 6% acetonitrile at pH 4.0. A single 6-mm glassy carbon electrode was used at a potential of +500 mV, and DA was detected at a sensitivity of 2.0 nA/V to optimize detection of both baseline DA and several fold changes. This setup had a minimum level of detection of approximately 0.1 nM (1.0 fmol/10 μl). Output from HPLC detector was computer compiled (ChromPerfect, Justice Innovations, Inc., Palo Alto, CA, USA), and DA levels were determined by comparison with a standard curve of known quantities of DA in similar un-frozen preservative/aCSF solutions.
Lesion and cannulation verification
Rats were sacrificed immediately after microdialysis, after which probes were injected with methylene blue, followed by whole brain removal and rapid freezing in isopentane. Cryostat-cut coronal brain sections (40 μm) were mapped for probe placements (blue tracks) rostrally and for lesion verification caudally. Sections cut through the hippocampus were dehydrated, fixed, and thionin-stained. Lesion verification, mapping of probe placement, and examination of the fidelity of HPLC/DA detection were all conducted independently and blind from behavioral results. All of these conditions were assessed as sources of attrition. Exclusions were called on lesioned animals with only unilateral ventral hippocampal dysmorphology or significant extra-hippocampal damage, extra-striatal probe placement or related intracranial hemorrhage, dialysis probe blockage/bursting, operational failure of HPLC, or contaminants obscuring DA signal. A total of 211 rats were carried though at least cannulation in 27 cohorts of eight over a 3-year period toward a goal of reaching seven to nine non-excluded rats per group (indexed by lesion status, drug history, and cannulation site). Cohorts were balanced by lesion status and drug history, while two of three cannulation regions were rotated in across cohorts. Dialysate from each cohort was HPLC analyzed in the same batch, 2–4 weeks after microdialysis. At study completion, 92 of 211 (44%) rats survived the sources of attrition allowing subsequent analysis of sub-group Ns as indexed in Table 1. Success rates of lesions or the behavioral microdialysis/HPLC analysis were 74% and 59%, respectively. Lesion and cannulation mappings of included animals are shown in Fig. 2.
Table 1.
Study group sizes
| Lesion/drug history | Cannulation site | |||
|---|---|---|---|---|
| SHELL | CORE | CAPU | ||
| SHAM-SAL | 7 | 9 | 8 | 24 |
| NVHL-SAL | 7 | 7 | 7 | 21 |
| SHAM-COC | 8 | 7 | 9 | 24 |
| NVHL-COC | 9 | 7 | 7 | 23 |
| 11 | 31 | 30 | 31 | Total N = 92 |
Fig. 2.
Mapping of lesions and cannulation tracks of animals included in the study. Upper left sections show the largest (black) and smallest (white inset) lesion extents of NVHL animals used in the study. Upper right micrographs show typically sized NVHL damage vs. SHAM. Lower sections show cannulation placement for all rats included in the study (N = 92) by cannulation region. Coordinates are relative to bregma (Swanson 2004)
Data analysis
ANOVA examined independent factors of lesion status, drug history, and cannulation region in a repeated measures design with bins the within-subjects time factor. This 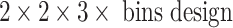 was applied in separate examinations of pre-injection and post-injection activities for behavioral and DA data. Secondary ANOVAs of lower order and/or post hoc Tukey tests characterized specific sub-group differences where appropriate. Dependent behavioral measures were assessed per rat as pre-injection distance/bin and post-injection distance/bin. Dependent DA measures were DA concentration/bin (three pre-injection bins) and %DA efflux (% increase over mean pre-injection DA concentration, at each of the nine post-injection bins). To more directly explore relationships between post-injection behavior and DA efflux, we focused on three additional summary dependent variables: (a) mean post-injection locomotion (calculated per rat as its average post-injection activity across nine bins), (b) individual mean post-injection %DA efflux/bin over the nine bins, and (c) peak %DA efflux (determined per rat as its maximum amplitude %DA efflux post-injection). Selecting the two treatment groups that showed the most mutually extreme behavioral differences, we performed simple two-way ANOVAs (group × region), on each of these dependent variables. Finally, while including all 92 rats in the study regardless of lesion status/drug history, we examined Pearson correlations between the behavioral measures and each of the %DA efflux measures. All data are presented as means ± SEM; significance was recognized at p < 0.05.
was applied in separate examinations of pre-injection and post-injection activities for behavioral and DA data. Secondary ANOVAs of lower order and/or post hoc Tukey tests characterized specific sub-group differences where appropriate. Dependent behavioral measures were assessed per rat as pre-injection distance/bin and post-injection distance/bin. Dependent DA measures were DA concentration/bin (three pre-injection bins) and %DA efflux (% increase over mean pre-injection DA concentration, at each of the nine post-injection bins). To more directly explore relationships between post-injection behavior and DA efflux, we focused on three additional summary dependent variables: (a) mean post-injection locomotion (calculated per rat as its average post-injection activity across nine bins), (b) individual mean post-injection %DA efflux/bin over the nine bins, and (c) peak %DA efflux (determined per rat as its maximum amplitude %DA efflux post-injection). Selecting the two treatment groups that showed the most mutually extreme behavioral differences, we performed simple two-way ANOVAs (group × region), on each of these dependent variables. Finally, while including all 92 rats in the study regardless of lesion status/drug history, we examined Pearson correlations between the behavioral measures and each of the %DA efflux measures. All data are presented as means ± SEM; significance was recognized at p < 0.05.
Results
Behavior
NVHLs showed increased locomotion in the 30-min pre-injection phase (lesion: F(1, 80) = 5.5, p < 0.05), whereas cocaine history, cannulation region, and bins had no effects (Fig. 3, left). There were no interactions between any of these main effects. In contrast, over the 90-min post-injection phase, locomotor activity was significantly increased by both NVHLs (F(1, 80) = 11.3, p < 0.01) and cocaine history (F(1, 80) = 4.7, p < 0.05). There was also a significant effect of bins (F(8, 640) = 81.9, p < 0.001) and a bins × drug history interaction (F(8, 640) = 2.2, p < 0.05) indicative of behavioral sensitization (Fig. 4). Cannulation site had no effect on post-injection locomotion, and there were no interactions between any other factors.
Fig. 3.
Pre- and post-injection locomotion. Left Distance traveled per 10-min bin for the lesion/drug history groups. Lesions increase pre-injection activity (−2, −1, 0 bins; *p < 0.05) and increased further in significance in the post-injection phase (bins 1–9; **p < 0.01) where drug history effects also emerged (*p < 0.05). Right Total post-injection locomotion. Data depicted as means ± SEM (error bars below only for clarity) include all rats in the study regardless of cannulation region (SHAM-SAL (N = 24); NVHL-SAL (N = 21); SHAM-COC (N = 24); NVHL-COC (N = 23))
Fig. 4.
Locomotor response to cocaine injections according to presence or absence of cocaine history. Significant effects of drug and bins × drug history interaction (*p < 0.05) were identified. Data depicted as means ± SEM, include all rats in the study regardless of cannulation region or lesion status (cocaine history rats (N = 47); saline history rats (N = 45))
Dopamine efflux
DA concentrations over the three pre-injection bins showed a significant main effect of cannulation region (F(2, 80) = 6.5, p < 0.01) but not lesion or drug history. There was also a significant effect of bins (F(2, 160) = 4.3, p < 0.05) associated with a small uptick (~10%) in DA concentration in the last pre-injection bin, likely due to a slight retrograde diffusion of increased DA from the adjacent post-injection volume in the dialysis line. There were no interactions between any main factors or bins. The non-significant mean baseline DA concentrations of the four treatment groups listed by cannulation region are shown in Table 2. Post hoc examination of baseline DA concentrations according to cannulation region, irrespective of lesion status or drug history, revealed an anatomical gradient of DA concentrations, where in comparison with the CAPU region (N = 31; 50.1 ± 13.6 fmol/10 μl), DA levels were marginally lower in the CORE (N = 30; 22.3 ± 3.8; p = 0.051) and significantly lower in the SHELL (N = 31; 8.9 ± 1.5; p < 0.01).
Table 2.
Baseline (pre-injection) DA concentrations (fmol/10 μl). Data reflect group means of the means of the 3 × 10 min pre-injection bin (± SEM)
| Lesion/drug history | Cannulation site | ||
|---|---|---|---|
| SHELL | CORE | CAPU | |
| SHAM-SAL | 8.1 ± 2.9 | 16.6 ± 5.7 | 87.9 ± 49.5 |
| NVHL-SAL | 13.2 ± 4.1 | 27.2 ± 9.4 | 52.8 ± 15.7 |
| SHAM-COC | 7.6 ± 2.7 | 22.6 ± 8.0 | 26.9 ± 4.6 |
| NVHL-COC | 7.4 ± 2.2 | 24.3 ± 8.5 | 34.0 ± 10.7 |
In contrast to post-injection behavior, post-injection %DA efflux did not show main effects of lesion or drug history, though the lesion effect was marginal (F(1, 80) = 3.0, p = 0.09) due to lower %DA efflux (mean across post-injection bins) in NVHLs (N = 44; 249 ± 21%) compared to SHAMs (N = 48; 307 ± 32%). Bins were also strongly significant (F(8, 640) = 31.5, p < 0.001) consistent with the effect of cocaine in causing a pronounced rise then fall of %DA efflux. Similar to the pre-injection %DA data, cannulation region again showed a significant effect (F(2, 80) = 6.8, p < 0.01) and a region × bins interaction (F(15, 640) = 3.8, p < 0.001; Fig. 5). However, the %DA efflux gradient expressed opposite to the baseline DA concentrations; % DA efflux (mean across post-injection bins) in the SHELL (N = 31; 365 ± 45) was marginally higher in comparison to CORE (N = 30; 272 ± 30, p = 0.098) and significantly higher than CAPU (N = 31; 201 ± 12; p < 0.01). Pearson correlation including all rats in the study confirmed a significant inverse relationship between mean baseline DA levels and mean post-injection %DA efflux (R = −0.284, p < 0.01). No other interactions between main effects or bins were noted in post-injection %DA with the exception of a complex significant four-way interaction (lesion × drug history × region × bins: F(16, 640) = 1.68, p < 0.05). Post hoc examinations of %DA efflux performed at each post-injection bin that compared the 12 subgroups (indexed by lesion status, drug history, cannulation region) revealed that SHELL-cannulated SHAM-COC rats showed significantly greater %DA efflux than five of the 11 other subgroups (all four of the CAPU-cannulated groups and the CORE-NVHL-SAL group), across the second, third, and fourth post-injection bins (p < 0.05), while none of the other 11 subgroups were mutually different at any post-injection bin (Fig. 6). While suggestive of a neurochemical sensitization isolated to SHAM-COC rats in the early portion of the %DA curve, this finding was not robust to secondary repeated measures ANOVAs performed on %DA efflux for each region separately (lesion × drug history × bins). Neither lesion nor drug history significantly modulated %DA as main effects or interactions, in the SHELL, CORE, or CAPU, although bins were again robustly significant in all regions (SHELL (F(8, 216) = 11.8, p < 0.001); CORE (F(8, 208) = 18.2, p < 0.001); CAPU (F(8, 216) = 26.1, p < 0.001)). Notably, significant behavioral effects of lesion or drug history would still be detectible given the same ANOVA of post-injection locomotion at each region separately: (SHELL (bins: F(8, 216) = 24.6, p < 001; lesion: F(1, 27) = 7.5, p < 0.01)), (CORE (bins: F(8,208) = 25.6, p < 0.001; drug history: F(1, 26) = 4.6, p < 0.05; drug history × bins: F(8, 208) = 2.1, p < 0.05)), (CAPU (bins: F(8, 216) = 35.2, p < 0.001; lesion F(1, 27) = 8.2, p < 0.01)).
Fig. 5.
%DA efflux across post-injection bins (1–9) according to cannulation region. Significant main effects of region (**p < 0.01) and bins × region (***p < 0.001) were identified. Data depicted as means ± SEM include all rats in the study regardless of drug history or lesion status (SHELL (N = 31); CORE (N = 30); CAPU (N = 31))
Fig. 6.
Locomotor response (upper) and %DA efflux curves (lower) of all rats in the study by cannulation region and lesion/drug history status (SHELL, N = 31; CORE, N = 30; CAPU, N = 31). Significant main effects or their interactions are noted at *p < 0.05, **p < 0.01, ***p < 0.001 levels from the four-way repeated measures ANOVAs on post-injection behavior or %DA efflux conducted separately. Error bars (± SEM) are below only for visual clarity. Behavioral Y-axis scales are the same across regions for behavior but are adjusted lower dorsally for %DA efflux curves for visual clarity
DA efflux as a predictor of behavior
SHAM-SAL (N = 24) and NVHL-COC (N = 23) groups demonstrated the largest mutual differences in post-injection locomotion (Fig. 3) and extreme low and high ranges in behavior among all four treatment groups. Two-way ANOVA (group × region) confirmed a robust difference in mean post-injection locomotion between these two groups (F(1, 41) = 24.6, p < 0.001; Fig. 7). However, two-way ANOVAs applied to %DA efflux measures revealed no effect of group on either mean post-injection %DA efflux or post-injection individual peak %DA efflux. Cannulation region had no significant main effect or interactions with group in any of these analyses. Pearson testing including all rats cannulated in the SHELL (N = 31), irrespective of lesion status or drug history, found no correlation between mean post-injection locomotion and mean post-injection %DA efflux, mean post-injection DA concentrations, or post-injection individual peaks in %DA efflux. The same correlations performed for CORE (N = 30) and CAPU (N = 31) rats also found no significant relationships between behavior and any of these DA measures.
Fig. 7.
Post-injection behavior and DA measures of the two treatment groups (SHAM-SAL (N = 24) vs. NVHL-COC (N = 23)) that represented the widest extremes of post-injection behavior. Mean post-injection locomotion (top panel) of the NVHL-COC group was robustly greater than the SHAM-SAL group (***p < 0.001) but mean post-injection %DA efflux and mean peak %DA efflux (lower panels) were not different between the groups. Data are depicted as means ± SEM
Discussion
In measuring DA efflux across SHELL, CORE, and CAPU compartments in real time with behavior after cocaine injections, significant behavioral differences due to NVHLs, with or without a behavioral sensitizing cocaine history, were not underpinned by differences in cocaine-induced %DA efflux. Pre-injection DA levels were also not different between treatment groups even though mild pre-injection behavioral NVHL effects were observed. Although the no-net flux method would be needed to precisely measure absolute baseline DA levels, the absence of relative differences in baseline DA in this study with large rat numbers, together with no lesion or drug history effects on post-injection %DA efflux, suggests that altered DA transmission is not the key mechanism of increased acute or behaviorally sensitized cocaine-induced locomotion in NVHLs.
Several aspects of our data suggest that our behavioral microdialysis approach was adequate for supporting the conclusions of this study. First, a significant behavioral sensitization effect was demonstrated in cocaine history rats (Figs. 3 and 4), and we observed a close temporal correspondence and similarity of form of locomotor and DA curves with respect to timing of cocaine injections (Fig. 6). Second, irrespective of lesion status or drug history, we identified a trans-striatal anatomical gradient from SHELL to CORE, to CAPU, of both baseline DA levels and %DA efflux and an inverse correlation between the two variables along this gradient. Similar trans-striatal gradients have been shown in numerous studies spanning mice, rats, and monkeys (Becker et al. 2001; Bradberry et al. 2000; Frank et al. 2008; Mattsson et al. 2007; Navailles et al. 2004; Pontieri et al. 1995; Watanabe et al. 2005; Zocchi et al. 2003), including identification of an inverse correlation between baseline DA and %DA efflux (Heidbreder et al. 1996). To our knowledge, this report is the first to document this gradient across all three of these regions in the same rodent study. Thus, our DA detection was more than adequate for detecting differences in baseline and %DA efflux by striatal region at levels comparable to available data. Finally, not only were no lesion/drug history group differences in %DA efflux found overall when including the variance due to region sampled but also there were no differences within regions or other special comparisons. When observing non-significant trends in the DA data, NVHLs showed overall marginal (p = 0.09) decreases in %DA efflux, while limited statistical evidence for a neurochemical sensitization only emerged for SHELL-cannulated SHAM-COC rats in the early phase of the %DA efflux curve. When directly comparing NVHL-COC to SHAM-SAL rats (the comparison that carried the largest behavioral contrast), both mean post-injection %DA efflux and maximum peak% DA efflux in NVHL rats were similar to or trended lower than SHAMS. When including all rats in the study, correlations between individual post-injection behavior and post-injection %DA efflux or peak %DA did not exist.
Cannulation of differential striatal regions did not impact behavior as a main effect or interaction with lesion or cocaine history. When including all rats regardless of cannulation region, we observed behavioral sensitization in SHAM and NVHL rats in statistical patterns consistent with prior studies (Chambers and Taylor 2004; Berg and Chambers 2008; Chambers et al. 2010). However, we did see weakening of behavioral group differences when assessing each cannulation region separately where N’s were only 1/3 as large as the overall group, due to loss of statistical power related to the behavioral microdialysis procedure. Stress from the recent cannulation and probe placements, tethering of animals, and repeated entry of investigators to collect dialysate all likely diluted or suppressed phenotypic behavioral differences. In comparison to a parallel study with six to nine rats per lesion/drug history group that did not incorporate microdialysis (Chambers et al. 2010), results here showed the same statistical outcome but a more narrow range of behavioral differences. Relative increases in the SHAM-SAL group and relative decreases in the NVHL-COC group were consistent with concurrent “raised floor” and “lowered ceiling” effects on the range of locomotion.
Our results are in general agreement with all prior published examinations of DA efflux in the NVHL model, which in using voltammetry or microdialysis/HPLC detection of DA in the NAC, either at baseline or in response to novelty, stress, or amphetamine, also found no change or small decrements in DA efflux (Brake et al. 1999; Corda et al. 2006; Lillrank et al. 1999; Wan et al. 1996). The qualifying exception to this is the observation of Corda et al. that while overall DA efflux in the NAC after acute amphetamine was unchanged in NVHLs (and no baseline lesion differences in NAC SHELL or CORE DA levels were present), there was a relative decrease of %DA efflux in the SHELL and increase in the CORE in NVHLs (Corda et al. 2006). It is unclear why we did not replicate these sub-region specific observations, although pharmacological differences between cocaine and amphetamine doses used may be a possibility (Cadoni et al. 2000). The present study also differs from Corda et al. by examining acute drug injections with and without prior drug history and across a broader anatomy of three striatal regions. Thus, in a more comprehensive examination of anatomy and experimental conditions, the present findings add weight to a general conclusion that NVHLs do not alter psychostimulant-induced sub-cortical DA efflux, and NVHL increases in DA-mediated behavior are not primarily caused by increased DA efflux.
The present results conflict with expectations that might arise from literal interpretations of DA hypotheses of addictions or schizophrenia, e.g., addicted and/or schizophrenic states = increased sub-cortical DA. However, our findings are consistent with several lines of evidence depicting a more nuanced role for DA transmission in these disorders in which DA acts primarily to mediate or express pathological change involving other neural systems, rather than serving itself as a direct biomarker of pathological change. While changes in mesolimbic DA efflux with behavioral sensitization have been documented, the literature is mixed on whether neurochemical sensitization of DA is required for or is the primary mechanism driving sensitized behavior (Chefer and Shippenberg 2002). If and how DA efflux changes with repeated drug exposures appears more closely related to the dosing regimen, time between induction series and challenge, and NAC region being examined, and not whether behavioral sensitization occurs (Heidbreder et al. 1996; Cadoni et al. 2000). In a prior study using a regimen similar to our own (5 days cocaine, 20 mg/kg/day; cocaine challenge 12 days later) behaviorally sensitized Sprague–Dawley showed less% DA efflux than those with only saline histories (Heidbreder et al. 1996). Meanwhile, an emerging body of evidence suggests that long-lasting behavioral and motivational changes due to addictive drug exposure are instantiated by a host of neuroadaptations post-synaptic to mesolimbic DA transmission (Kauer and Malenka 2007). Drug-induced DA efflux facilitates abnormal dendritic and synaptic changes involving glutamatergic synapses within neostriatal networks that mediate habit formation—operating as a mechanism that does not require changes in drug-induced DA efflux over time (Kalivas and O’Brien 2008; Redish 2004; Vanderschuren and Kalivas 2000). Consistent with this view of DA, NVHL investigations have identified lesion-induced changes in frontal cortical–striatal circuits that are projection fields of both ventral hippocampus and mesolimbic DA fibers but little evidence for changes in DA transmission (Tseng et al. 2009). When NVHL vs. SHAM rats are given similar levels of experimentally induced DA stimulation, they show differential activation of post-synaptic glutamatergic and GABAergic neurons within cortical–striatal networks (Goto and O’Donnell 2004; O’Donnell et al. 2002; Tseng et al. 2007, 2008). Thus, dysfunctional cortical–striatal responsiveness to DA, brought on by NVHLs or addictive drug history, may drive their corresponding behavioral phenotypes, without requiring substantial pathological changes in levels of provoked DA efflux. With respect to schizophrenia, this view agrees with a post-synaptic interpretation of the DA hypotheses that is supported by a historical majority of human investigations that have failed to document or substantially replicate direct evidence for increased sub-cortical DA transmission in schizophrenia, untangled from effects of neuroleptics (Laruelle and Abi-Dargham 1999). However, recent neuroimaging, using indirect measures of DA efflux (radio-tracers of DA precursors or DA receptor radioligand binding displacement after amphetamine), has suggested increased striatal DA outflow that may vary by symptom severity or medications (Breier et al. 1997; Laruelle and Abi-Dargham 1999; McGowan et al. 2004). Further studies are needed to understand the extent to which these and the present findings differ based on subject species/illness model, DA detection methodology, or potential confounds of concurrent or recent substance exposures not generally accounted for in human studies.
In terms of understanding mechanisms underlying dual diagnosis, we have shown in a parallel study examining cortical and striatal neuronal activation as measured by expression of c-Fos densities that NVHLs and a behaviorally sensitizing cocaine history produce compounding effects across cortical–striatal compartments (Chambers et al. 2010). Cocaine compared to saline history produces a gradient of increasing striatal activation from SHELL to CORE to CAPU, while NVHLs alone produce a paucity of medial prefrontal cortical activation. When combining these conditions, the striatal effects of cocaine history are quantitatively amplified in proportion to NVHL-based medial prefrontal cortical hypo-activation, corresponding to CAPU c-Fos density levels that directly correlate with locomotor output. By not mirroring these trends across ventral and dorsal striatal compartments in terms of DA efflux and showing no relationship between behavioral output and DA efflux in any striatal region, the present results provide further evidence that altered cortical–striatal network functional and neuroadaptative responses to DA stimulation and not DA efflux per se are the more crucial mechanisms mediating an integrated neurobiology of addictions vulnerability in mental illness.
Acknowledgments
This study was supported by PHS grants NIDA-K08 DA019850 (RAC) and NIAAA-RO1 AA10717 (EAE).
Disclosure/Conflict of interest
The authors, RAC, AMS, EAE, have received no financial support or compensation from any individual or corporate entity for the conceptualization, methods, or conduct of this research. There are no financial holdings or interests associated with any of the authors that could be construed as a conflict of interest with the topic or results of this report.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Becker J, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg SA, Chambers RA. Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology. 2008;54:1201–1207. doi: 10.1016/j.neuropharm.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Barett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, McCallum SE, Glick SD, O’ Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology. 2008;200:205–215. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Sullivan RM, Flores G, Srivastava LK, Gratton A. Neonatal ventral hippocampal lesions attenuate the nucleus accumbens dopamine response to stress: an electrochemical study on the adult rat. Brain Res. 1999;831:25–32. doi: 10.1016/S0006-8993(99)01477-8. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, deBArtolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, EW C, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol. 2000;388:69–76. doi: 10.1016/S0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56:308–316. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JK, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/S0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Jones RM, Brown S, Taylor JR. Natural reward related learning in rats with neonatal ventral hippocampal lesions and prior cocaine exposure. Psychopharmacology. 2005;179:470–478. doi: 10.1007/s00213-004-2042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Sentir AM, Conroy SK, Truitt WA, Shekhar A. Cortical–striatal integration of cocaine history and prefrontal dysfunction in animal modeling of dual diagnosis. Biol Psychiatry. 2010;67:788–792. doi: 10.1016/j.biopsych.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Changes in basal and cocaine-evoked extracellular dopamine reuptake and release in the rat nucleus accumbens during early abstinence from cocaine: quantitative determination under transient conditions. Neuroscience. 2002;112:907–919. doi: 10.1016/S0306-4522(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharm Biochem Behav. 2007;86:386–394. doi: 10.1016/j.pbb.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda MG, Piras G, Giorgi O. Neonatal ventral hippocampal lesions potentiate amphetamine-induced increments in dopamine efflux in the core, but not the shell, of the nucleus accumbens. Biol Psychiatry. 2006;60:1188–1195. doi: 10.1016/j.biopsych.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Drake RE, Wallach MA. Dual diagnosis: 15 years of progress. Psychiatr Serv. 2000;51:1126–1129. doi: 10.1176/appi.ps.51.9.1126. [DOI] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Li T, Lumeng L, Murphy JM. Ethanol drinking experience attenuates (−)sulpiride-induced increases in extracellular dopamine levels in the nucleus accumbens of alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2003;27:424–431. doi: 10.1097/01.ALC.0000056618.57931.A5. [DOI] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Frank ST, Krumm B, Spanagel R. Cocaine-induced dopamine overflow within the nucleus accumbens measures by in vivo microdialysis: a meta-analysis. Synapse. 2008;62:243–252. doi: 10.1002/syn.20489. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Engleman EA, Ingraham CM, McClaren JA, Keith CM, McBride WJ, Murphy JM. A single, moderate ethanol exposure alters extracellular dopamine levels and dopamine D2 receptor function in the nucleus accumbens of Wistar rats. Alcohol Clin Exp Res. 2009;33:1721–1730. doi: 10.1111/j.1530-0277.2009.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Prefrontal lesion reverses abnormal mesoaccumbens response in an animal model of schizophrenia. Biol Psychiatry. 2004;55:172–176. doi: 10.1016/S0006-3223(03)00783-2. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuoropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kohl RR, Katner JS, Chernet E, McBride WJ. Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology. 1998;139:79–85. doi: 10.1007/s002130050692. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Kolachana BS. Attenuated extracellular dopamine levels after stress and amphetamine in the nucleus accumbens of rats with neonatal ventral hippocampal damage. J Neural Transm. 1999;106:183–196. doi: 10.1007/s007020050150. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Mattsson A, Olson L, Svensson TH, Schilstrom B. Cortical cholinergic deficiency enhances amphetamine-induced dopamine release in the accumbens but not the striatum. Exp Neurol. 2007;208:73–79. doi: 10.1016/j.expneurol.2007.07.012. [DOI] [PubMed] [Google Scholar]
- McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61:134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- Navailles S, De Deurwaerdere P, Porras G, Spampinato U. In vivo evidence that 5-HT2c receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2004;29:319–326. doi: 10.1038/sj.npp.1300329. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Charney DS, Lewis L, Cornish JW, Post RM, Woody GE, Zubieta J-K, Anthony JC, Blaine JD, Bowden CL. Priority actions to improve the care of persons with co-occurring substance abuse and other mental disorders: a call to action. Biol Psychiatry. 2004;56:703–713. doi: 10.1016/j.biopsych.2004.10.002. [DOI] [PubMed] [Google Scholar]
- O’Donnell PO, Grace AA. Synaptic interactions among excitatory afferents to the nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska B. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the shell as compared with the core of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. 3. New York: Elsevier; 2004. [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine–glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental animal model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren L, Kalivas P. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vohs JL, Chambers RA, Krishnan GP, O’Donnell BF, Berg SA, Morzorati SL. GABAergic modulation of the 40 Hz auditory steady state response in a rat model of schizophrenia. Int J Neuropsychopharmacol. 2010;13:487–498. doi: 10.1017/S1461145709990307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan RQ, Giovanni A, Kafka SH, Corbett R. Neonatal hippocampal lesions induced hyperresponsiveness to amphetamine: behavioral and in vivo microdialysis studies. Behav Brain Res. 1996;78:211–223. doi: 10.1016/0166-4328(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Fusa K, Takada K, Aono Y, Saigusa T, Koshikawa N, Cools AR. Effects of alpha-methyl-p-tyrosine on extracellular dopamine levels in the nucleus accumbens and dorsal striatum of freely moving rats. J Oral Sci. 2005;47:185–190. doi: 10.2334/josnusd.47.185. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Girlanda E, Varner G, Sartori I, Zanetti L, Wildish GA, Lennon M, Mugnaini M, Heidbreder CA. Dopamine responsiveness to drugs of abuse: a shell–core investigation in the nucleus accumbens of the mouse. Synapse. 2003;50:293–302. doi: 10.1002/syn.10271. [DOI] [PubMed] [Google Scholar]









