Abstract
Purpose
Monoclonal antibodies (mAb) are an important and growing class of cancer therapeutics, but pharmacokinetic analyses have in many cases been constrained by the lack of standard and robust pharmacologic assays. The goal of this project was to develop a general method for the production of immunoassays that can measure the levels of therapeutic monoclonal antibodies in biologic samples at relevant concentrations.
Methods
Alemtuzumab and rituximab are monoclonal approved for the treatment of B-cell malignancies and were used as a model system. Phage-displayed peptide libraries were screened for peptide sequences recognized by alemtuzumab (anti-CD52) or rituximab (anti-CD20). Synthetic biotinylated peptides were used in enzyme-linked immunosorbent assays (ELISA). Peptides directly synthesized on polymer resin beads were used in an immunofluorescent-based assay.
Results
Peptide mimetope sequences were recovered for both mAb and confirmed by competitive staining and kinetic measurements. A peptide-based ELISA method was developed for each. The assay for rituximab had a limit of detection of 4 μg/ml, and the assay for alemtuzumab had a limit of detection of 1 μg/ml. Antibody-specific staining of peptide conjugated beads could be seen in a dose-dependent manner.
Conclusion
Phage-displayed peptide libraries can be a source of highly specific mimetopes for therapeutic mAb. The biotinylated forms of those peptides are compatible with conventional ELISA methods with sensitivities comparable to other assay methods and sufficient for pharmacological studies of those mAb given at high dose. The process outlined here can be applied to any mAb to enable improved pharmacokinetic analysis during the development and clinical use of this class of therapies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00280-009-1240-1) contains supplementary material, which is available to authorized users.
Keywords: Immunoassay, Monoclonal antibody, Peptide, ELISA, Phage display
Introduction
Monoclonal antibodies are used in the treatment of many cancers and proliferative diseases [1]. Dynamic monitoring of monoclonal antibody therapy has the potential to personalize these treatments for the benefit of patients and to lower costs. There are currently eleven approved monoclonal antibody therapies for cancer with an almost equal number approved for other conditions, most notably autoimmunity and transplant rejection. Several hundreds are in development. The pharmacology of mAb presents a particular challenge during clinical development, because they often have very mild acute toxicities at high doses. Traditional paradigms of dose escalation until a maximally tolerated dose is found may not be optimal. As a result, the development of dosing schedules is somewhat arbitrary and can be influenced by the high cost of mAb therapies.
Several approaches have been used to study the pharmacokinetics of these treatments. The target molecule can be produced in a recombinant system for sandwich ELISA [24]. However, it may not always be possible to generate the recognized portion of the target molecule, and it is expensive and cumbersome to generate large amounts of recombinant protein. Peptides designed from the target antigen sequence have been tried in lieu of recombinant antigens, with limited success [4]. An alternate approach is to express the target molecule on a cell line by transfection, using flow cytometry to assess the binding of the desired mAb [22]. This method has been used for alemtuzumab (anti-CD52) but is difficult to develop, requires skilled personnel to execute, and has limited sensitivity [8]. Finally, ELISA have been developed that used antibodies specific for the therapeutic mAb [19]. The antibodies used for this purpose are either anti-idiotypic [13] or specific for residual non-human sequences of the therapeutic mAb, as was the case with alemtuzumab [10]. However, each of these approaches is technically demanding and has limited sensitivity when used in biologic samples because of the background from the high levels of endogenous antibody. The latter approach will not work with fully humanized mAb.
Peptide libraries displayed on bacteriophage are routinely used to identify peptide epitopes, or mimetopes, recognized by antibodies. When short peptides, 7–12 amino acids are screened, the selected peptides almost invariably bind to the antigen-binding site of the antibody and are competed by the natural ligand [17]. This property makes such libraries ideal for the selection of epitope targets that can be used in ELISA or other immunoassays.
Bead-based immunoassays are an alternative to plate-based ELISA and have the several advantages. They can be multiplexed with beads that are distinguishable by fluorescence, size, or other physical parameters [23]. Beads can mix with large volumes of sample and have improved antigen-capture efficiencies compared to the surface of a well. Beads can be made from a variety of materials that can be optimized for the specific application. Automated solid phase peptide synthesis is commonly performed on resin beads [16]. One such resin is TentaGel, a graft copolymer of a crosslinked polystyrene matrix and polyethylene glycol [21]. TentaGel beads have been used extensively in biological assays with combinatorial peptide libraries and show very low non-specific protein adsorption [9, 11]. In this study, we demonstrate a process that combines phage display discovery of mimetope peptides with solid phase immunoassay methods for the quantification of mAb levels in biologic samples. The process used is general and should be applicable to any mAb or other recombinant protein biologic therapy.
Materials and methods
Antibodies
Alemtuzumab (Genzyme, Cambridge, MA) and rituximab (Genentech, San Francisco, CA) were obtained from the UCSD Cancer Center pharmacy. The antibodies were fluorescently labeled using the Zenon R-phycoerythrin human IgG labeling kit, Zenon Alexa fluor 488 human IgG labeling and Alexa fluor 488 protein labeling kits (Invitrogen, Carlsbad, CA). For kinetic studies, Fab fragments of each mAb were prepared by papain digestion using a Fab preparation kit (Thermo Fisher Scientific, Rockford, IL) as per manufacturer’s instructions.
Plasma samples
Blood was collected from consenting patients who satisfied diagnostic and immunophenotypic criteria for B-cell chronic lymphocytic leukemia (CLL) and who presented for evaluation at the referral centers of the CLL research consortium (CRC). Plasma was obtained by standard methods.
Peptide library screening
Three phage-displayed peptide libraries (Ph.D 7, Ph.D. 12, and Ph.D. C7C, New England Biolabs, Ipswich, MA) were combined and screened against the mAbs as previously described [17]. Briefly, the mAb were coated onto ELISA plates at a concentration of 1 μg/ml and incubated at 4°C overnight. The coating solution was removed, and the wells blocked with 2.5% bovine serum albumin (BSA) in tris buffered saline (TBS). After 1 h at 4°C, the blocking solution was removed, the wells washed three times, and the phage libraries added in a final volume of 100 μl in TBS. After 1-h incubation at room temperature, the wells were washed with TBS ten times, and the bound phage eluted for 10 min with 0.2 M Glycine–HCl (pH 2.2). The eluted phage was neutralized with Tris–HCl (pH 9.1) and grown on K91 bacteria overnight. The bacteria were pelleted, the culture supernatant passed through a 0.2-μm syringe tip filter, and the phage precipitated with 2.5 M NaCl–20% polyethylene glycol (PEG). The phage pellet was resuspended in TBS, and the next round of selection done as above. After the final round of selection, individual phage clones were picked and sequenced using the -96gpIII primer provided with the library kits.
Peptides
Peptides were ordered from Sigma-Genosys (St. Louis, MO). The alemtuzumab-binding peptide, pCp-1, had sequence ACGSLSPSSCGGGS. Biotinylated peptides, pCp-1B, had sequence ACGSLSPSSCGGK, and rituximab-binding peptide, pRTX-10B, had sequence ACPYSNPSLCGGK. Both peptides were biotinylated via the C-terminal lysine to maintain the same anchoring orientation as was the case when the peptide was displayed on the phage protein. The peptides were purified to 95 and 82%, respectively, by the manufacturer. Peptide–bead conjugates were obtained from Peptides International (Louisville, KY). The alemtuzumab-binding peptide beads, pCp-1T, had sequence ACGSLSPSSCGGGS, and the rituximab-binding peptide beads, pRTX-10T, had sequence ACPTSNPSLCGGGS. Both were acetylated at the N terminus and coupled to 10 μm TentaGel beads at the C-terminus.
Binding kinetics
Kinetics of antibody–peptide binding was studied using SkiProTM biomolecular interaction technology 1 on a SkiPro interferometer equipped with a 2-channel flow cell and an autosampler (Silicon Kinetics Inc., San Diego, CA). All reagents were purchased from Sigma–Aldrich, St. Louis, MO. All peptides were incubated with oxidizing agent sodium tetrathionate at 10 mM in phosphate buffered saline (PBS) for 1 h immediately before binding experiments. Biotinylated peptides were diluted to 5 μM in PBS/0.05% BSA and immobilized on streptavidin-coated Ski SensorTM Biochips for 10 min at 4 μl/min flow rate. That resulted in optical path difference shift (ΔOPD) of 5–6 nm. Concentration series of antibodies and antibody Fab fragments were prepared as twofold dilutions in PBS/0.05% BSA. Binding was carried out for 10 min followed by 20-min dissociation. For all sensorgrams, reference channel data were subtracted from the sample channel. The resulting multi-concentration series of binding curves were globally fit to a single binding site association/dissociation model.
CLL cell staining
After informed consent was obtained per the declaration of Helsinki, blood samples were collected from patients at the University of California, San Diego (UCSD) Medical Center who satisfied diagnosis and immunophenotypic criteria for common B-cell CLL.
CLL cells (105/per well) were seeded into 96-well plates in 100 μl of X-vivo medium (BioWhittaker, Walkersville, MD) and incubated with Zenon labeled alemtuzumab or rituximab (0.5 μg/well) for 1 h on ice. After, cells were washed two times with 100 μl FACS-wash (PBS, 5% FCS and 0.5% sodium azide) and fixed with 100 μl with 3.7% formaldehyde in PBS.
ELISA
Each well in a 96-well NeutrAvidin coated plate (Pierce, Rockford, IL) was coated with 100 μl of a solution of biotinylated peptide at 30 μg/ml in ddH2O and incubated for 1 h at room temperature. A reference well was left uncoated but filled with buffer. Wells were washed five times with TBS/0.05% Tween-20 (TBST) using an automated plate washer (Columbus Pro, Tecan, Durham, NC). Non-specific binding sites were blocked by incubation with 300 μl of 5% BSA/TBS for 2 h at room temperature. Wells were washed five times with TBST wells and were filled with 100 μl of standard or sample. Plasma samples were diluted in 2.5% BSA/TBST 1:500 for alemtuzumab assays and 1:1000 for rituximab assays. All samples were analyzed in triplicate on both peptide-coated and uncoated wells. Standard curves were assayed in triplicate. Plates were incubated for 1 h at room temperature and then washed with TBST. Hundred microliters of goat anti-human IgG POD antibody (Jackson Immunoresearch, West Grove, PA) was added at dilution 1:5000 in TBST for 30 min at room temperature. Wells were washed ten times with TBST. Hundred microliters/well of turbo TMB (Pierce) substrate was added and incubated for 15–45 min. The reaction was stopped with 100 μl of 1 M sulfuric acid, and the absorbance was measured at 450 nm on an Infinite M200 plate reader (Tecan). For peptide blocking experiments, the antibody was pre-incubated with 12.5 μg/ml of the free peptide.
For all samples and standards, the absorbance reading of each sample on an uncoated well was subtracted from the sample assayed on a peptide-coated well. The differential values for the standard curve were fit with a one-site total binding curve, and the sample values interpolated using Graphpad Prism 5 software (Graphpad Software Inc., La Jolla, CA).
Detection of alemtuzumab and rituximab on beads by flow cytometry
Peptide beads, 6 × 104 beads/sample, were mixed with different concentrations of the respective labeled antibody for 16 h at 4°C in a rotator. The beads were washed twice with PBS by centrifugation at 14,000 rpm for 2 min at room temperature and were resuspended in 100 μl of PBS. Beads were analyzed on a BD FACSCalibur (Becton–Dickinson, Franklin Lakes, NJ), and the data analyzed on FlowJo (Treestar, Ashland, OR) software.
Results
A pool of three phage-displayed peptide libraries was panned against rituximab and alemtuzumab. In each case, significant enrichment in the number of bound and recovered phage was observed after three rounds. We selected individual phage plaques from the final enriched population and determined the sequence of the displayed peptides (Fig. 1). All of the peptides recovered were from the cysteine-containing library. The rituximab-binding peptide sequences were identical to those identified in a previous report [20]. The alemtuzumab-binding sequences shared a motif and were similar in sequence to the extra-cellular domain of CD52 [7]. Phage displaying either the rituximab- or alemtuzumab-binding peptides was specific for the cognate antibody and did not bind the other or normal human IgG (data not shown).
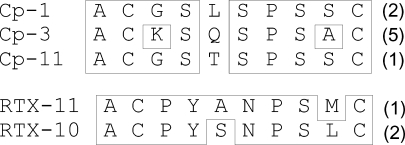
Fig. 1.
Phage-displayed peptide sequences. Phage-displayed libraries were panned against alemtuzumab (top) and rituximab (bottom) for three rounds, after which individual phage clones were picked and sequenced. The deduced sequences of the displayed peptides are aligned, with amino acid identity boxed. The numbers in parenthesis indicate the number of independent clones found to have that sequence. The sequences of Cp-1 and RTX-10 were selected for further analysis as synthetic peptides
Alemtuzumab-binding sequence, Cp-1, and rituximab-binding sequence, RTX-10, were chosen for further analysis. Biotinylated peptides were used for kinetic binding measurements using nano-pore optical interferometry (Fig. 1 in “Supplementary material”). The binding kinetic parameters thus determined are given in Table 1. The K d for both peptide–mAb pairs was 100–200 nM when the intact mAb molecules were used, but when Fab fragments were used, the K d was 2–4 μM. This suggests that surface-immobilized peptides are capable of bivalently interacting with the two Fab regions of the intact molecule, producing a greater binding avidity than the monovalent Fab–peptide interaction.
Table 1.
Binding kinetics
| Whole molecule | Fab | |||
|---|---|---|---|---|
| RTX-10 | Cp-1 | RTX-10 | Cp-1 | |
| Kon, M−1 × s−1 | 7.51E + 03 | 8.13E + 03 | 1.63E + 03 | 2.46E + 03 |
| Koff, s−1 | 9.84E − 04 | 1.32E − 03 | 6.51E − 03 | 5.05E − 03 |
| K d | 131 nM | 163 nM | 3.99 μM | 2.05 μM |
To determine whether the peptides were binding to the antigen-binding region of the mAbs, soluble synthetic peptides were evaluated for their ability to inhibit the binding of alemtuzumab and rituximab to the surface of primary CLL cells (Fig. 2). CLL cells express high levels of CD52 and low levels of CD20. Each peptide inhibited the binding of the respective mAb to the CLL cells. There was no effect on the binding activity of the other mAb (data not shown). This result indicted that the peptides could be used for detection of free, active mAb.
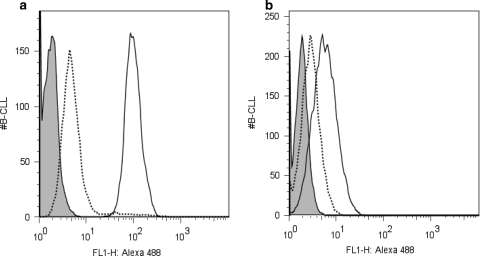
Fig. 2.
Peptide inhibition of CLL cell staining. Fluorescently labeled alemtuzumab (a) or rituximab (b) was incubated with primary CLL cells and evaluated by flow cytometry (solid line). As expected, robust staining with alemtuzumab was seen, while the staining for CD20 with rituximab was weak. When peptides pCp-1B or pRTX-10B were added at a 25,000 M excess (dashed lines), the cell labeling as largely abrogated. The shaded histogram represents CLL cells incubated with fluorescently labeled normal human IgG
Neutravidin plates were coated with the biotinylated peptides for an ELISA. Representative titration curves for each mAb in saline buffer are shown in Fig. 3. The binding of the mAb to the peptide coated plates could be completely inhibited by an excess of soluble peptide (Fig. 2 in “Supplementary material”). Multiple individual serum and plasma samples were used to optimize the dilution factor (data not shown), and it was determined that a sample dilution of 1:1000 for rituximab and 1:500 for alemtuzumab provided the optimal balance between sensitivity and elimination of matrix effects. Furthermore, we found that variations in the level of background signal between samples could be compensated for by testing each sample against both peptide coated and uncoated well and using the difference.
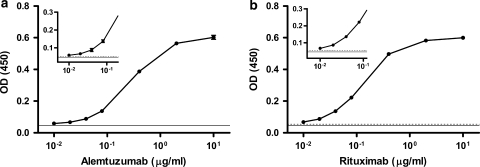
Fig. 3.
Standard curves of alemtuzumab and rituximab by peptide-based ELISA. Biotinylayed peptides were bound onto neutravidin-coated ELISA plates. Both alemtuzumab and rituximab antibodies were diluted in TBST. Each value shows the mean (±SD) of triplicates. The solid line indicates the mean of the buffer control, and the dashed line represents the mean +10 times the SD of the buffer control
The limit of blank was determined by analyzing plasma samples from patients with CLL who had not received mAb therapy. Eighty-five samples from 60 different individuals were analyzed using the rituximab peptide assay and 48 unique samples using the alemtuzumab peptide assay. The limit of blank (the 95th percentile of the negative samples) was 1.76 μg/ml for the rituximab ELISA and 0.53 μg/ml for the alemtuzumab assay. The limit of detection was determined by analyzing six different spiked samples on four different days. The lowest concentration at which the lower 95th percentile was above the limit of blank was 4 μg/ml for rituximab (lower 95th: 1.88 μg/ml) and 1 μg/ml for alemtuzumab (lower 95th: 0.83 μg/ml). The limit of quantitation, defined as the concentration at which the coefficient of variation is <15%, was 32 μg/ml for rituximab and 8 μg/ml for alemtuzumab. The high degree of variation in the rituximab assays may be due in part to varying levels of cell-free CD20 in the plasma of the CLL patients used, a well-known phenomenon [14].
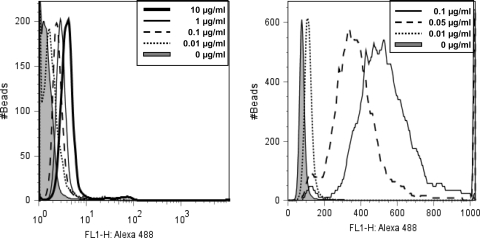
An advantage to using peptides as the primary capture agent in solid phase immunoassays is that they can be directly synthesized on a variety of substrates. To demonstrate this, the rituximab and alemtuzumab peptides were synthesized on 10-μm diameter TentaGel beads, hereafter known as pCp-1T and pRTX-10T. TentaGel, a PEG polystyrene co-polymer, is a common solid phase synthesis support material, and commercially prepared peptides are typically cleaved from the surface of such beads. Specific cognate antibody binding was confirmed by flow cytometry and fluorescent microscopy (Fig. 3 in “Supplementary material”). Alemtuzumab was titrated into normal human serum and incubated with the pCp-1T beads. After washing and addition of a fluorescent secondary anti-human IgG antibody, the fluorescence on the beads was quantitated by flow cytometry. The fluorescent signals correlated with the alemtuzumab concentration, with very little background from serum alone (Fig. 4).
Fig. 4.
Alemtuzumab bead-based detection. Different concentrations of labeled alemtuzumab were analyzed by flow cytometry using pC1-1T conjugated beads
Discussion
The data described here demonstrate the general applicability of peptide phage display to the development of monoclonal antibody-specific immunoassays. Phage-displayed peptide libraries can be used to identify mimetope peptides that bind to the antigen-binding site of antibodies in a specific and selective fashion. While it is theoretically possible that peptides that bind to the constant region of the monoclonal antibody could be enriched, and would thus lack specificity, this is not usually the case. The constant regions of antibodies are generally refractory to short peptide libraries, and even if they are selected, simple competitive blocking schemes can be used [18]. Constant region binding peptides have been selected from longer libraries [5]. The peptides identified in this study were specific and competed with the target cell surface antigen for mAb binding.
ELISA for serum or plasma antibody levels are typically limited by the need to dilute the sample to lower the background due to non-specific binding of endogenous antibodies that react with the secondary antibody. This was the case for our peptide ELISA as well, though the limit of detection achieved is comparable to or better than those used for other therapeutic mAb [15]. In addition, we observed patient-to-patient variation in the background that could be accounted for by analyzing samples against both peptide-coated and uncoated wells. The assays as described here would be sufficient for analysis of mAb in patients treated with high doses of Ab where the expected range of interest is >4 μg/ml. Since the primary goal of this study was to develop the peptide ligands, the assays were not completely optimized, and it is likely further refinements to the assay will lower the limit of detection and improve reproducibility.
While the general pharmacokinetics of monoclonal antibodies is well established, there can be large variation in the achieved pharmacokinetics from one antibody or patient to the next. Some of this variation may be related to the treatment target as larger tumors can absorb more of the mAb and result in lower serum levels. Population pharmacokinetic studies have shown that final drug levels can vary by as much as an order of magnitude among patients given a standard body mass adjusted dose [6]. However, with the exception of radiolabelled antibodies whose doses are personalized based on dosimetry and biodistribution, there is no current practice to monitor mAb levels and tailor the dose to the patient. Several studies with different mAb have suggested correlation between final trough mAb levels and therapeutic outcome [2, 12], indicating that real-time monitoring and patient-specific dosing may improve the success rate achieved with these therapies.
Dosing schedules and routes of administration for mAb are developed with many uncertainties and are often refined through pre-clinical and clinical studies where the need for simple and reliable pharmacokinetic assays is acute. The specific biology of a given mAb target can also lead to additional complications from the conventional dose escalation paradigm. For instance, there have been reports of cell-free CD20 in the plasma of patients with CLL [14], and other reports have suggested that CD20 may be “shaved” from CLL cells consequent to rituximab treatment [25], which may or may not affect the detection of free rituximab [3]. Since peptide mimetopes bind to the antigen-binding site of the mAb and compete with the natural ligand, they intrinsically measure only active mAb. The peptide-based assays described here are ideal for the development of validated clinical assays, because they can be made cheaply at high purity and can be synthesized directly on a variety of substrates, such as the TentaGel beads shown here, that may enable simple, automated point-of-care assay tools for personalized therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants PO1-CA81534 and U54-CA119335 from the National Institutes of Health (US).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 2.Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, Green D, Rosenberg J, McLaughlin P, Shen D. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 3.Beum PV, Kennedy AD, Taylor RP. Three new assays for rituximab based on its immunological activity or antigenic properties: analyses of sera and plasmas of RTX-treated patients with chronic lymphocytic leukemia and other B cell lymphomas. J Immunol Methods. 2004;289:97–109. doi: 10.1016/j.jim.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Blasco H, Lalmanach G, Godat E, Maurel MC, Canepa S, Belghazi M, Paintaud G, Degenne D, Chatelut E, Cartron G, Le Guellec C. Evaluation of a peptide ELISA for the detection of rituximab in serum. J Immunol Methods. 2007;325:127–139. doi: 10.1016/j.jim.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 5.DeLano WL, Ultsch MH, de Vos AM, Wells JA. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- 6.Dirks NL, Nolting A, Kovar A, Meibohm B. Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol. 2008;48:267–278. doi: 10.1177/0091270007313393. [DOI] [PubMed] [Google Scholar]
- 7.Hale G. Synthetic peptide mimotope of the CAMPATH-1 (CD52) antigen, a small glycosylphosphatidylinositol-anchored glycoprotein. Immunotechnology. 1995;1:175–187. doi: 10.1016/1380-2933(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 8.Hale G, Rebello P, Brettman LR, Fegan C, Kennedy B, Kimby E, Leach M, Lundin J, Mellstedt H, Moreton P, Rawstron AC, Waldmann H, Osterborg A, Hillmen P. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104:948–955. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- 9.Hiemstra HS, Benckhuijsen WE, Amons R, Rapp W, Drijfhout JW. A new hybrid resin for stepwise screening of peptide libraries combined with single bead Edman sequencing. J Pept Sci. 1998;4:282–288. doi: 10.1002/(SICI)1099-1387(199806)4:4<282::AID-PSC145>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Jilani I, Keating M, Giles FJ, O’Brien S, Kantarjian HM, Albitar M. Alemtuzumab: validation of a sensitive and simple enzyme-linked immunosorbent assay. Leuk Res. 2004;28:1255–1262. doi: 10.1016/j.leukres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Leon S, Quarrell R, Lowe G. Evaluation of resins for on-bead screening: a study of papain and chymotrypsin specificity using PEGA-bound combinatorial peptide libraries. Bioorg Med Chem Lett. 1998;8:2997–3002. doi: 10.1016/S0960-894X(98)00534-4. [DOI] [PubMed] [Google Scholar]
- 12.Luo FR, Yang Z, Dong H, Camuso A, McGlinchey K, Fager K, Flefleh C, Kan D, Inigo I, Castaneda S, Rose WC, Kramer RA, Wild R, Lee FY. Correlation of pharmacokinetics with the antitumor activity of Cetuximab in nude mice bearing the GEO human colon carcinoma xenograft. Cancer Chemother Pharmacol. 2005;56:455–464. doi: 10.1007/s00280-005-1022-3. [DOI] [PubMed] [Google Scholar]
- 13.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, Wey K, Royston I, Davis T, Levy R. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 14.Manshouri T, Do KA, Wang X, Giles FJ, O’Brien SM, Saffer H, Thomas D, Jilani I, Kantarjian HM, Keating MJ, Albitar M. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood. 2003;101:2507–2513. doi: 10.1182/blood-2002-06-1639. [DOI] [PubMed] [Google Scholar]
- 15.Maple L, Lathrop R, Bozich S, Harman W, Tacey R, Kelley M, Danilkovitch-Miagkova A. Development and validation of ELISA for herceptin detection in human serum. J Immunol Methods. 2004;295:169–182. doi: 10.1016/j.jim.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Merrifield RB, Stewart JM. Automated peptide synthesis. Nature. 1965;207:522–523. doi: 10.1038/207522a0. [DOI] [PubMed] [Google Scholar]
- 17.Messmer BT, Sullivan JJ, Chiorazzi N, Rodman TC, Thaler DS. Two human neonatal igm antibodies encoded by different variable-region genes bind the same linear peptide: evidence for a stereotyped repertoire of epitope recognition. J Immunol. 1999;162:2184–2192. [PubMed] [Google Scholar]
- 18.Messmer BT, Thaler DS. Specific blocking to improve biopanning in biological samples such as serum and hybridoma supernatants. Biotechniques. 2001;30:798–802. doi: 10.2144/01304st06. [DOI] [PubMed] [Google Scholar]
- 19.Montagna M, Avanzini MA, Visai L, Locatelli F, Montillo M, Morra E, Regazzi MB. A new sensitive enzyme-linked immunosorbent assay (ELISA) for Alemtuzumab determination: development, validation and application. Int J Immunopathol Pharmacol. 2007;20:363–371. doi: 10.1177/039463200702000217. [DOI] [PubMed] [Google Scholar]
- 20.Perosa F, Favoino E, Vicenti C, Merchionne F, Dammacco F. Identification of an antigenic and immunogenic motif expressed by two 7-mer rituximab-specific cyclic peptide mimotopes: implication for peptide-based active immunotherapy. J Immunol. 2007;179:7967–7974. doi: 10.4049/jimmunol.179.11.7967. [DOI] [PubMed] [Google Scholar]
- 21.Rapp W, Zhang L, Bayer E (1989) Continuous-flow peptide synthesis on PSPOE-grafi-copolymers. In: Epton R (ed) Innovations and perspectives in solid-phase synthesis. Oxford, Mayflower, Birmingham. pp 205–210
- 22.Rebello P, Hale G. Pharmacokinetics of CAMPATH-1H: assay development and validation. J Immunol Methods. 2002;260:285–302. doi: 10.1016/S0022-1759(01)00556-7. [DOI] [PubMed] [Google Scholar]
- 23.Sachdeva N, Asthana D. Cytokine quantitation: technologies and applications. Front Biosci. 2007;12:4682–4695. doi: 10.2741/2418. [DOI] [PubMed] [Google Scholar]
- 24.Tan AR, Moore DF, Hidalgo M, Doroshow JH, Poplin EA, Goodin S, Mauro D, Rubin EH. Pharmacokinetics of cetuximab after administration of escalating single dosing and weekly fixed dosing in patients with solid tumors. Clin Cancer Res. 2006;12:6517–6522. doi: 10.1158/1078-0432.CCR-06-0705. [DOI] [PubMed] [Google Scholar]
- 25.Williams ME, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, Hamil SH, Eggleton JC, Taylor RP. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol. 2006;177:7435–7443. doi: 10.4049/jimmunol.177.10.7435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.