Abstract
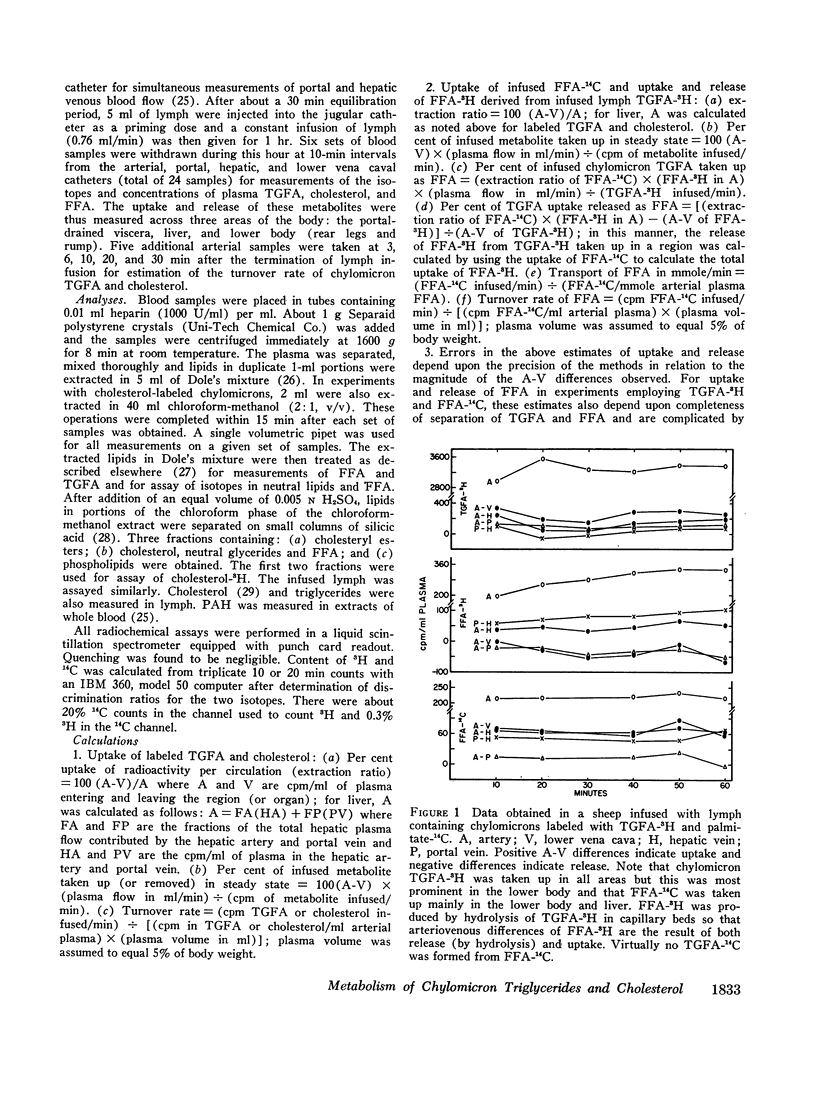
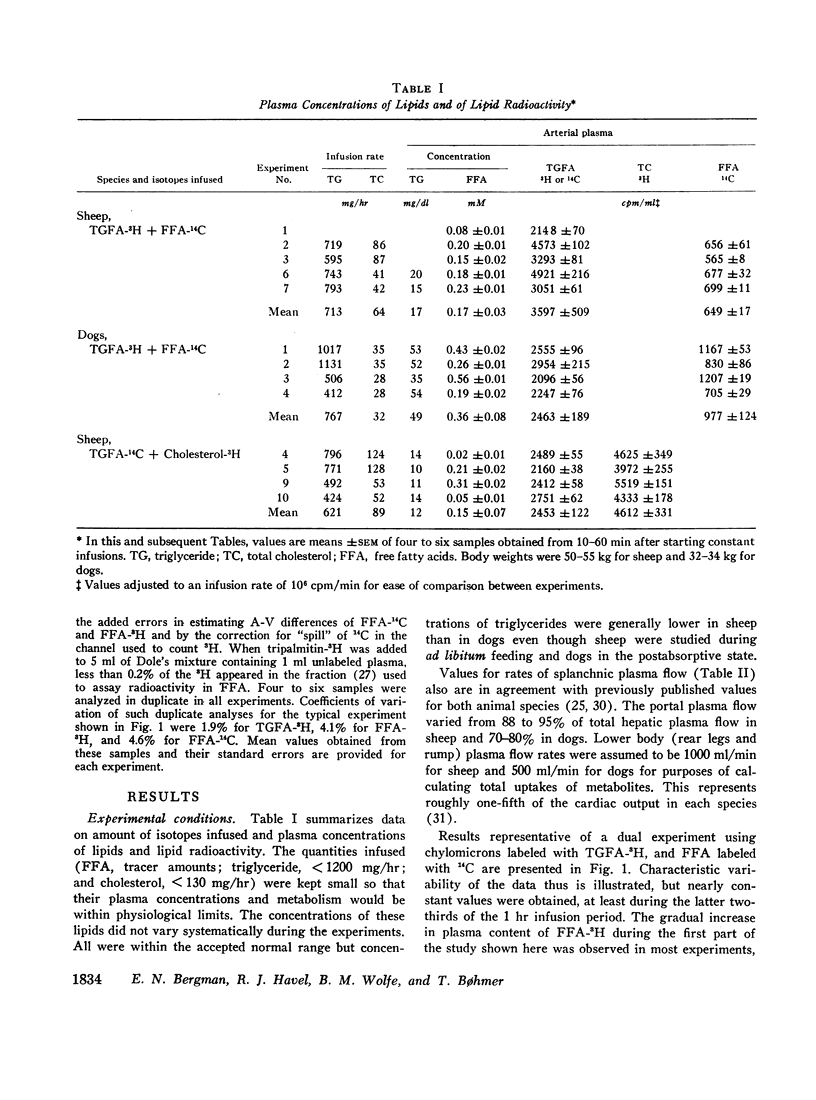
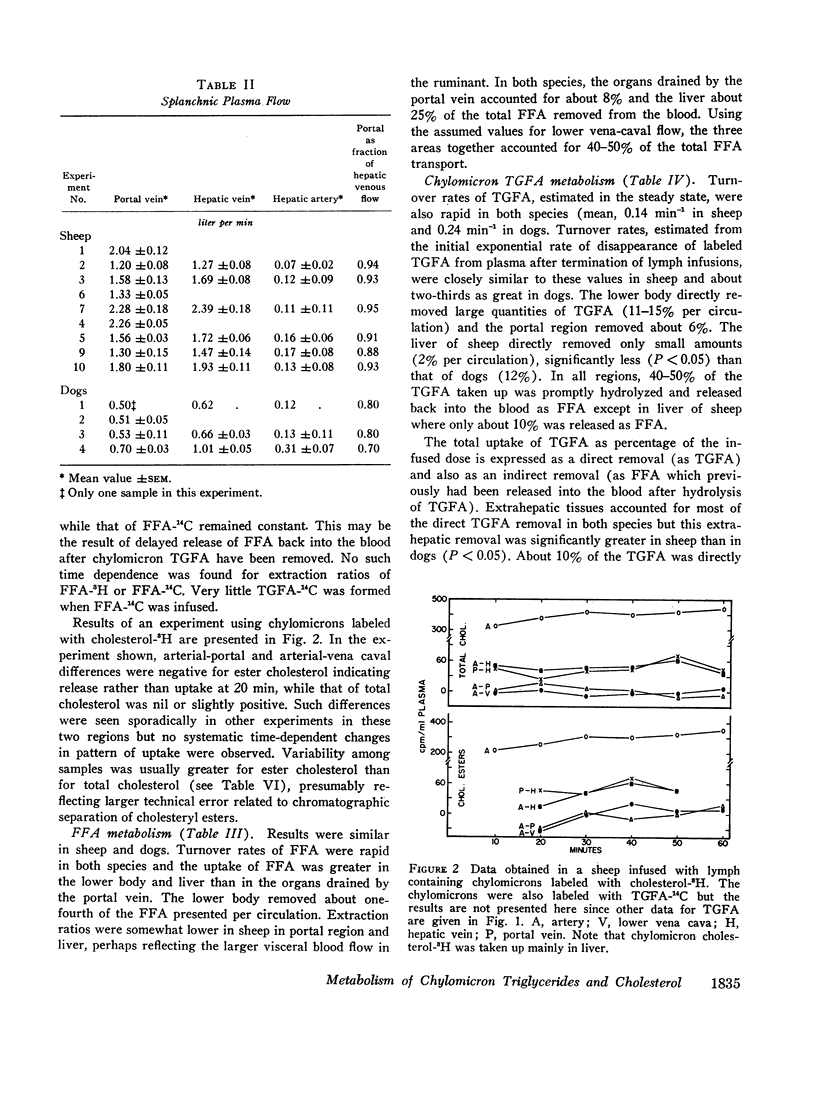
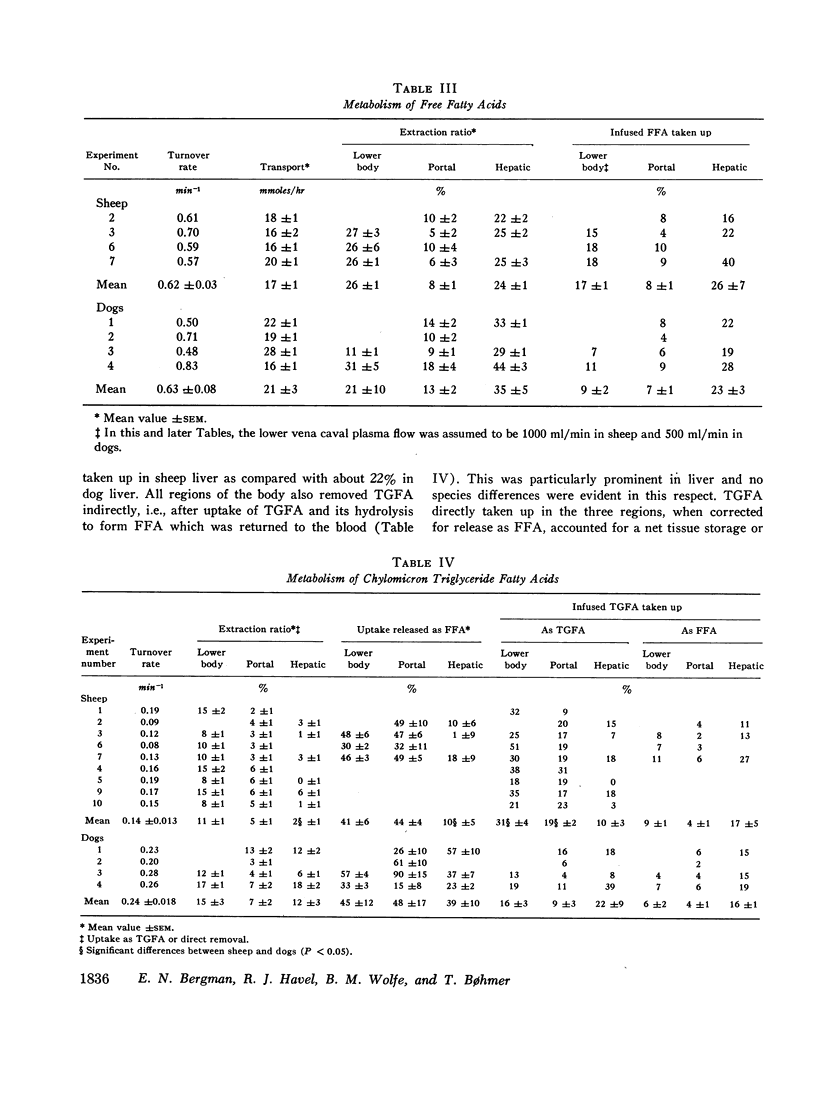
Unanesthetized sheep and dogs, previously fitted with indwelling catheters in the aorta, lower vena cava, mesenteric, portal, left hepatic and jugular veins, were given constant intravenous infusions of lymph in which the chylomicron lipids were variously labeled with 3H or 14C. Para-aminohippuric acid was infused into the mesenteric venous catheter for measurement of portal and hepatic venous blood flow. In some animals, alternately labeled free fatty acids bound to albumin were mixed with the lymph to be infused. In both species, chylomicron triglyceride fatty acids were taken up in the region drained by the lower vena cava and portal vein and free fatty acids derived from hydrolysis of these triglycerides were extensively recycled in the blood. Direct uptake of triglyceride fatty acids also occurred in liver and accounted for about 10% of the total triglyceride fatty acids removed from the blood in sheep and 22% in dogs. In sheep, 10% and, in dogs, about 40% of these triglyceride-fatty acids were released into the blood as free fatty acids. The free fatty acids recycled from various regions accounted for a substantial fraction of the chylomicron fat eventually deposited in each tissue. Uptake of chylomicron cholesterol from the blood of sheep occurred primarily in liver and to a small extent in certain tissues drained by the portal vein. The results obtained, together with other available data, demonstrate that chylomicron triglycerides are removed primarily in extrahepatic tissues of both species, while the liver removes cholesterol contained in chylomicron “skeletons” from which most of the triglycerides have been removed. The quantitative differences between transport of chylomicron lipid in sheep and dogs may be related to known differences in the structure of their hepatic sinusoids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGGS M. W. Studies on exogenous cholesterol metabolism in human atherosclerosis with the aid of isotopes. Adv Biol Med Phys. 1957;5:357–384. doi: 10.1016/b978-1-4832-3111-2.50012-2. [DOI] [PubMed] [Google Scholar]
- BOBERG J., CARLSON L. A., NORMELL L. PRODUCTION OF LIPOLYTIC ACTIVITY BY THE ISOLATED, PERFUSED DOG LIVER IN RESPONSE TO HEPARIN. Life Sci. 1964 Sep;3:1011–1019. doi: 10.1016/0024-3205(64)90113-4. [DOI] [PubMed] [Google Scholar]
- Basso L. V., Havel R. J. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest. 1970 Mar;49(3):537–547. doi: 10.1172/JCI106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfrage P. Metabolism of chyle triglycerides in the liver. I. Studies on the mechanisms for liver uptake of intravenously injected, glycerol- and fatty acid-labeled chyle in the carbohydrate-fed rat. Biochim Biophys Acta. 1966 Dec 7;125(3):474–484. [PubMed] [Google Scholar]
- Bergman E. N., Katz M. L., Kaufman C. F. Quantitative aspects of hepatic and portal glucose metabolism and turnover in sheep. Am J Physiol. 1970 Sep;219(3):785–793. doi: 10.1152/ajplegacy.1970.219.3.785. [DOI] [PubMed] [Google Scholar]
- Brockway J. M., McEwan E. H. Oxygen uptake and cardiac performance in the sheep. J Physiol. 1969 Jun;202(3):661–669. doi: 10.1113/jphysiol.1969.sp008833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOEMLING D. B., STEGGERDA F. R. Chronic thoracic duct-venous shunt preparation in dogs. J Appl Physiol. 1960 Jul;15:745–746. doi: 10.1152/jappl.1960.15.4.745. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts J. M., Mayes P. A. Lack of uptake and oxidation of chylomicron triglyceride to carbon dioxide and ketone bodies by the perfused rat liver. Nature. 1965 Apr 10;206(980):195–196. doi: 10.1038/206195b0. [DOI] [PubMed] [Google Scholar]
- Felts J. M., Mayes P. A. Release of lipoprotein lipase from the perfused liver of the rat. Nature. 1967 May 6;214(5088):620–621. doi: 10.1038/214620a0. [DOI] [PubMed] [Google Scholar]
- Felts J. M. The metabolism of chylomicron triglyceride fatty acids by perfused rat livers and by intact rats. Ann N Y Acad Sci. 1965 Oct 8;131(1):24–33. doi: 10.1111/j.1749-6632.1965.tb34776.x. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S. The metabolism of chylomicron cholesterol ester in the rat. J Clin Invest. 1962 Oct;41:1886–1896. doi: 10.1172/JCI104645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN C., WEBB J. A. THE UPTAKE OF CHYLOMICRON FATTY ACIDS BY ISOLATED LIVER CELLS. Biochim Biophys Acta. 1964 Aug 5;84:404–411. doi: 10.1016/0926-6542(64)90004-6. [DOI] [PubMed] [Google Scholar]
- Grubb D. J., Jones A. L. Ultrastructure of hepatic sinusoids in sheep. Anat Rec. 1971 May;170(1):75–79. doi: 10.1002/ar.1091700106. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J. Transport and metabolism of chylomicra. Am J Clin Nutr. 1958 Nov-Dec;6(6):662–668. doi: 10.1093/ajcn/6.6.662. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. A method for simultaneous cannulation of the major splanchnic blood vessels of the sheep. Am J Vet Res. 1969 Apr;30(4):655–661. [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Simultaneous measurements of hepatic and portal venous blood flow in the sheep and dog. Am J Physiol. 1969 Apr;216(4):946–952. doi: 10.1152/ajplegacy.1969.216.4.946. [DOI] [PubMed] [Google Scholar]
- LASCELLES A. K., MORRIS B. Surgical techniques for the collection of lymph from unanaesthetized sheep. Q J Exp Physiol Cogn Med Sci. 1961 Jul;46:199–205. doi: 10.1113/expphysiol.1961.sp001536. [DOI] [PubMed] [Google Scholar]
- LEQUIRE V. S., HAMILTON R. L., ADAMS R., MERRILL J. M. LIPASE ACTIVITY IN BLOOD FROM THE HEPATIC AND PERIPHERAL VASCULAR BEDS FOLLOWING HEPARIN. Proc Soc Exp Biol Med. 1963 Oct;114:104–107. doi: 10.3181/00379727-114-28598. [DOI] [PubMed] [Google Scholar]
- MORRIS B. THE METABOLISM OF FREE FATTY ACIDS AND CHYLOMICRON TRIGLYCERIDES BY THE ISOLATED PERFUSED LIVER OF THE RAT. J Physiol. 1963 Oct;168:564–583. doi: 10.1113/jphysiol.1963.sp007208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. The functional status of lipoprotein lipase in rat liver. Biochem J. 1968 Jul;108(3):483–487. doi: 10.1042/bj1080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTEL P. J., HAVEL R. J., BEZMAN A. METABOLISM OF CONSTITUENT LIPIDS OF DOG CHYLOMICRONS. J Clin Invest. 1963 Aug;42:1313–1321. doi: 10.1172/JCI104815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Havel R. J., Bezman A. SITES OF INITIAL REMOVAL OF CHYLOMICRON TRIGLYCERIDE FATTY ACIDS FROM THE BLOOD. J Clin Invest. 1962 Oct;41(10):1915–1921. doi: 10.1172/JCI104648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontko J. A., Zilversmit D. B. Metabolism of chylomicrons by the isolated rat liver. J Lipid Res. 1967 Mar;8(2):90–96. [PubMed] [Google Scholar]
- Quarfordt S. H., Goodman D. S. Heterogeneity in the rate of plasma clearance of chylomicrons of different size. Biochim Biophys Acta. 1966 Apr 4;116(2):382–385. doi: 10.1016/0005-2760(66)90019-1. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970 Mar;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOEMAKER W. C., WALKER W. F., VAN ITALLIE T. B., MOORE F. D. A method for simultaneous catheterization of major hepatic vessels in a chronic canine preparation. Am J Physiol. 1959 Feb;196(2):311–314. doi: 10.1152/ajplegacy.1959.196.2.311. [DOI] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Schotz M. C., Arnesjö B., Olivecrona T. The role of the liver in the uptake of plasma and chyle triglycerides in the rat. Biochim Biophys Acta. 1966 Dec 7;125(3):485–495. doi: 10.1016/0005-2760(66)90037-3. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y., Goodman D. S., Fidge N. H. The metabolism of chylomicron cholesteryl ester in rat liver. A combined radioautographic-electron microscopic and biochemical study. J Cell Biol. 1969 Dec;43(3):410–431. doi: 10.1083/jcb.43.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., Stein Y. The role of the liver in the metabolism of chylomicrons, studied by electron microscopic autoradiography. Lab Invest. 1967 Oct;17(4):436–446. [PubMed] [Google Scholar]
- WOOD R. L. Evidence of species differences in the ultrastructure of the hepatic sinusoid. Z Zellforsch Mikrosk Anat. 1963;58:679–692. doi: 10.1007/BF00410656. [DOI] [PubMed] [Google Scholar]
- Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970 Apr;31(1):125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]


