Abstract
AIM: To investigate the number of intestinal immunoglobulin A (IgA+) plasma cells and expression of intestinal IgA in mice with acute liver necrosis.
METHODS: A model of acute liver necrosis was established by intraperitoneal injection of galactosamine (GalN) and lipopolysaccharide (LPS). Sixty mice were randomly divided into one of 4 equal groups: normal control, acute liver necrosis, LPS, or GalN. Hematoxylin and eosin staining, immunohistochemistry, and an enzyme-linked immunosorbent assay were employed to assess liver and intestinal injury, count intestinal IgA+ plasma cells, and measure the expression level of IgA and interferon γ (IFN-γ) in the small intestinal mucosa of mice.
RESULTS: Injured intestinal mucosa was observed in the acute liver necrosis group but not in the normal, LPS or GalN groups. Compared with the normal group, intestinal IgA+ plasma cells were slightly decreased in the LPS and GalN groups [429 ± 20 per high power field (HPF), 406 ± 18/HPF, respectively], whereas they were markedly decreased in the acute liver necrosis group (282 ± 17/HPF vs 495 ± 26/HPF in normal group, P < 0.05). The expression of intestinal IgA was also slightly decreased in LPS and GalN groups, but was markedly reduced in the acute liver necrosis group as determined by enzyme-linked immunosorbent assay (P < 0.05). In contrast, the level of IFN-γ was slightly increased in LPS, GalN and acute liver necrosis groups, but with no statistical significance (P > 0.05).
CONCLUSION: Intestinal IgA+ plasma cells and IgA expression levels indicating that mucosal immune barrier dysfunction, does exist in acute liver necrosis.
Keywords: Acute liver necrosis, Intestinal mucosa, Immunity, Immunoglobulin A
INTRODUCTION
Patients with acute liver necrosis are at high risk for enterogenic infections. Enterogenic infections are an important cause of death in patients with acute liver necrosis associated with intestinal barrier injury, including immunological barrier injury[1,2]. Immunoglobulin A (IgA) is an important component of the intestinal immunological barrier and is the most abundant immunoglobulin at mucosal surfaces where it plays crucial roles in mucosal protection[3]. The protective barrier of the gastrointestinal system is impaired in IgA deficiencies, and IgA-deficient individuals have a tendency to develop gastrointestinal infections[4]. Previous studies have shown decreased levels of secretory IgA and decreased numbers of IgA+ plasma cells in the intestinal tract during stress and thermal injury suggesting that the humoral immune function was dramatically inhibited in these situations[5,6]. Intestinal IgA was also decreased in endotoxemia and intra-abdominal sepsis models[7,8].
Previous studies have primarily focused on mechanical barrier interruption in acute liver necrosis models[9]. So far, no studies have shown a role for the intestinal immunological barrier in acute liver necrosis. It has been reported that an increase in levels of interferon γ (IFN-γ), a TH1 cytokine, was related to tissue injury[10] and led to a decreased expression of IgA[11].
This study set out to determine whether the number of intestinal IgA+ plasma cells and the expression of IgA were modified in mice with acute liver necrosis, in an attempt to establish whether dysfunction of the intestinal immunological barrier occurs during acute liver necrosis. In addition, IFN-γ levels in the intestinal mucosa were also evaluated.
MATERIALS AND METHODS
Animals
Sixty male BALB/c mice 6-8 wk of age (provided by the Laboratory Animal Center of the China Medical University) were housed under constant room temperature and humidity, and subjected to a 12 h light/dark cycle. Experiments were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals. Mice were equally and randomly divided into one of 4 groups: normal control, acute liver necrosis, lipopolysaccharide (LPS), or galactosamine (GalN). GalN (800 mg/kg body weight, Sigma, USA) and LPS (10 μg/kg body weight, Sigma, USA) were injected intraperitoneally to induce acute liver necrosis as previously described[12,13]. Serum, liver and proximal small intestinal tissues samples were obtained 9 h after GalN/LPS injection.
Blood biochemistry assay
Serum alanine transaminase (ALT) levels were determined using an automatic analyzer (Hitachi 7250; Hitachi, Japan).
Histological testing
The liver and proximal small intestinal tissue were separately stored in formalin, and embedded by paraffin. The liver and intestinal sections were cut at a thickness of 5 μm and stained with hematoxylin and eosin (HE) to explore the histopathological changes in the liver and intestinal mucosa.
Immunohistochemistry for intestinal IgA+ plasma cells
Intestinal IgA+ plasma cells were investigated by immunohistochemistry (IHC). Sections of proximal small intestine were deparaffined, and antigen retrieval was performed by pressure cooker boiling for 2 min in 10 mmol/L citrate buffer (pH 6.0). IHC analysis was performed using goat anti-mouse IgA (Zymed, USA, diluted 1:50) for 12 h at 4°C, and the secondary antibody (rabbit anti-goat IgG) was applied for 2 h at 37°C. Fresh peroxidase reaction mixture containing equal amounts of 0.02% hydrogen peroxide in H2O and 0.1% diaminobenzidine in H2O were prepared. Sections were mounted on Uvinert mountant (BDH, UK). Five fields of small intestinal mucosa lamina propria were examined in each section at high magnification (200 ×), and the number of IgA+ cells were counted (i.e. lymphocytes that stained a brownish-yellow color). The average number of IgA+ was calculated.
Enzyme-linked immunosorbent assay for IgA expression
The levels of intestinal IgA were examined by enzyme-linked immunosorbent assay (ELISA). Intestinal tissue (50 μg) immersed in 1 mL (10 volumes, w/v) of phosphate-buffered saline (PBS) was incubated at room temperature for 15 min. Samples were vortexed, left to settle for 15 min, revortexed until all material was suspended, then centrifuged at 12 000 r/min for 10 min. The supernatant was collected and tested on an ELISA kit for IgA (Bethyl Laboratories, Montgomery, TX, USA). Briefly, 96-well microtiter plates were coated with goat anti-mouse IgA affinity purified antibody and incubated for 60 min, then washed with PBS, and each well was incubated with 1% bovine serum albumin in PBS to block any nonspecific binding. After washing with PBS containing 0.1% Tween-20, 100 μL test samples and 100 mol/L standards were added into each well and incubated for 60 min followed by incubation with peroxidase-labeled goat anti-mouse specific IgA antibody for 30 min. Then 0.1 mol/L acetate buffer containing 1 mg/mL ortho-phenylenediamine was prepared and 3 μL of the prepared solution in 10 mL of H2O2 was added to each well. The reactions were stopped by adding 25 μL of 2 mol/L sulfuric acid. The absorbance of each solution was determined at a wavelength of 450 nm.
Detection of IFN-γ in the intestinal mucosa
The small intestinal mucosa homogenate was prepared as described previously[8]. The levels of IFN-γ in the homogenate were measured by sandwich ELISA (Quantikine ELISA Kits, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Statistical analysis
Software SPSS 11.0 was used for statistical analysis. Each value was expressed as the mean ± SE, and compared by using one-way ANOVA, followed by the Tukey test. P < 0.05 was considered statistically significant.
RESULTS
Mortality rate of mice and serum ALT levels
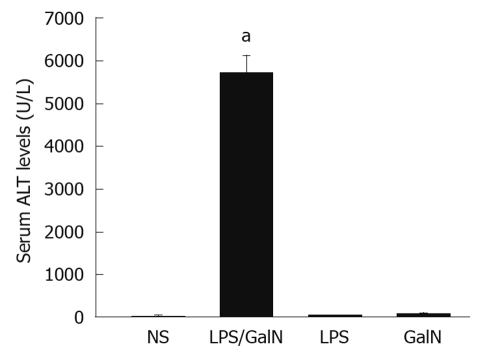
In the acute liver necrosis group, the mortality rate was 53.3% (8/15), compared to 0% in the other control groups. The serum ALT levels in LPS, GalN and normal groups were almost at the same level (44.3 ± 12.1, 74.2 ± 14.3, and 24.8 ± 14.7 U/L, respectively), but increased significantly in the acute liver necrosis group (5730.1 ± 383.5 U/L vs 24.8 ± 14.7 U/L, P < 0.05) (Figure 1).
Figure 1.
Serum alanine transaminase levels. Each value was expressed as mean ± SE. aP < 0.05 vs normal saline (NS). ALT: Alanine transaminase; GalN: Galactosamine; LPS: Lipopolysaccharide.
Assessment of liver and proximal small intestinal injury with HE staining
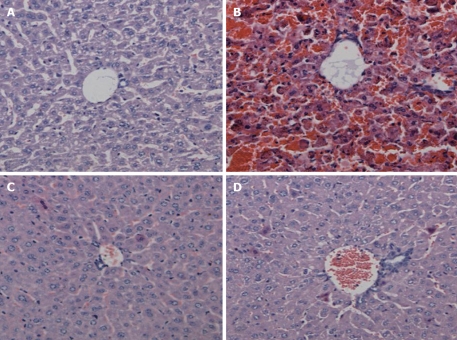
In the normal group, the liver clearly showed normal structure of both the hepatic lobuli and hepatic cords. In contrast, the livers from the acute necrosis group had severe hemorrhage, hepatic necrosis, acidophilic degeneration in some residual hepatocytes, disappearance of hepatic cords, and deranged structure of hepatic lobules. Acidophilic degeneration and swelling were observed in the LPS group and edematous and spotty necrosis, as well as a few hepatic cells with acidophilic changes were found in the GalN group (Figure 2).
Figure 2.
Hematoxylin and eosin staining of liver tissue (100 ×). A: Normal group; B: Acute liver necrosis group; C: Lipopolysaccharide group; D: Galactosamine group.
In normal, LPS and GalN groups, the intestinal mucosa was complete and the intestinal cells appeared ordered. In contrast, the intestinal mucosa of mice with acute liver necrosis were loosened and some of epithelial cells were edematous and necrotic (Figure 3).
Figure 3.
Morphology of intestinal samples stained with hematoxylin and eosin (200 ×). A: Normal group; B: Acute liver necrosis group; C: Lipopolysaccharide group; D: Galactosamine group.
IHC for IgA+ plasma cells
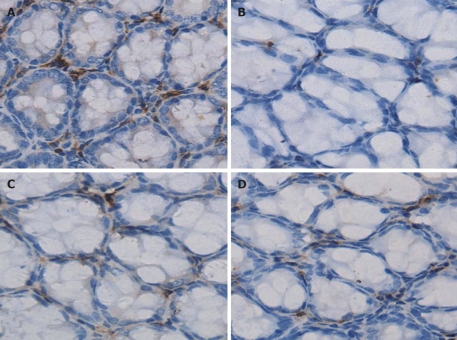
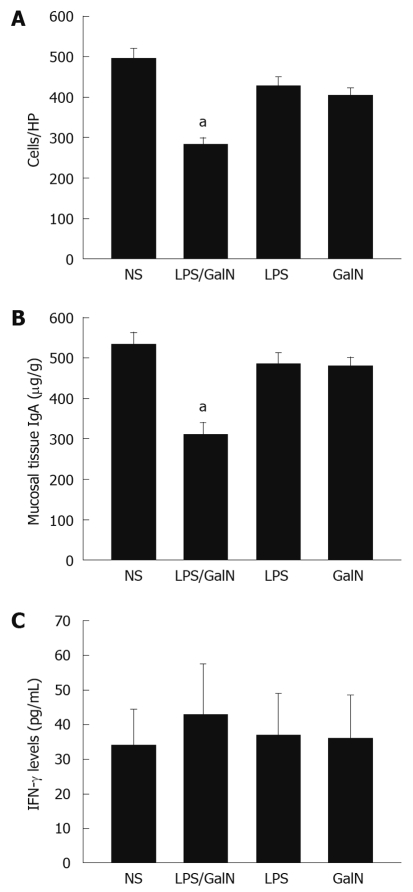
As shown in Figure 4, the number of IgA+ plasma cells within the lamina propria (as determined by IHC) were 495 ± 26/high power field (HPF), 282 ± 17/HPF, 429 ± 20/HPF and 406 ± 18/HPF in the normal, acute liver necrosis, LPS and GalN groups, respectively. The LPS group and GalN group had slightly lower numbers of IgA+ plasma cells than the normal group; however, the acute liver necrosis group had significantly lower numbers of IgA+ plasma cells compared to the other groups (P < 0.05, Figure 5A).
Figure 4.
Immunoglobulin A+ cells determined by immunohistochemistry (400 ×). Immunohistochemistry demonstrated that Immunoglobulin A in the plasma cells within the lamina propria were stained brown. A: Normal group; B: Acute liver necrosis group; C: Lipopolysaccharide group; D: Galactosamine group.
Figure 5.
Immunoglobulin A+ plasma cells (A), the immunoglobulin A expression levels (B) and interferon-γ expression (C) in intestinal mucosal tissue. aP < 0.05 vs normal saline (NS). HP: High power field; GalN: Galactosamine; LPS: Lipopolysaccharide.
ELISA measurement of IgA
There was a slight decrease in the expression of IgA in LPS and GalN groups whereas no difference was noted in IgA expression in the small intestine compared with normal control. IgA expression in the small intestines from mice in the acute liver necrosis group was markedly reduced compared with the normal group (P < 0.05, Figure 5B).
IFN-γ in small intestinal mucosa
There were a slight increase in IFN-γ levels in LPS, GalN and acute liver necrosis groups, but no significant difference was noted compared with the normal group (P > 0.05, Figure 5C).
DISCUSSION
Acute liver necrosis is associated with a high mortality rate[14]. Infection is a common serious complication of acute liver necrosis and is a major cause of death[15]. A myriad of researchers have noted that secondary infections primarily originate from intestinal bacterial translocation. While the intestinal barrier has to be permeable for nutrients and macromolecules which are indispensable for growth and development, at the same time it also has to provide an effective barrier against harmful macromolecules and microorganisms to ensure local homeostasis[16]. The intestinal barrier consists of a mechanical barrier, immunological barrier, microorganism barrier, and a chemical barrier. The immunological barrier is considered the first line of antigen-specific immune defense against pathogenic microorganisms[17,18]. Recent reports indicated that immunosuppression, involving the local intestinal immunological barrier, is a major cause of intestinal bacterial translocation[19].
In agreement with previous reports[20,21], we found that injection of GalN/LPS induced increases in serum ALT and the development of severe hepatocyte necrosis. As the mortality of mice with acute liver necrosis was 53.3%, these findings indicate that the model employed to study IgA and IFN-γ was successful. In addition to the observed liver injury, loosened intestinal mucosa and some edematous and necrotic intestinal epithelial cells were also noted in the mice with acute liver necrosis. These features were not found in any of the other groups. These histological findings of intestinal mucosal injury in acute liver necrosis were consistent with other studies[14].
IgA is the most abundant immunoglobulin present on all mucosal surfaces, where it plays crucial roles in mucosal protection[3]. IgA is produced and released as a J chain-linked dimer by resident IgA+ plasma cells in mucosal tissues, including the extensive lamina propria of the intestine[22]. For decades, it has already been known that IgA plays a protective role in mucosal immunity. IgA exerts its protective effects via 3 primary mechanisms. First, IgA serves as an immunologic barrier which inhibits binding of organisms to mucosal surfaces. Next, the normal movement of IgA from the basilar to apical region of epithelial cells suggests that it may be effective in neutralizing intracellular pathogens. Finally, pathogens bound to IgA are taken up by macrophages via phagocytosis[23]. An additional property of IgA is its inability to trigger the release of inflammatory mediators through receptors specific to its Fc domain[24-26].
IgA-deficient individuals have a tendency to develop infections and disorders of the gastrointestinal tract[27-29]. Zinneman et al[30] reported that the protective barrier of the gastrointestinal system was impaired in IgA deficiency and that protozoa such as Giardia lamblia can adhere to the epithelium, proliferate, and cause infection.
In the experiment presented herein, the number of IgA+ plasma cells and the IgA levels in intestinal mucosa in LPS and GalN groups showed a slight decrease but no significant difference was noted compared to the normal group. The number of IgA+ plasma cells and the IgA levels in the intestinal mucosa in the acute liver necrosis group were the lowest among all 4 study groups. The IgA+ plasma cells and the IgA levels were significantly different between the acute liver necrosis group and normal controls (Figure 5A and B). The decrease in the number of IgA+ cells and the IgA levels in the GalN/LPS group were significantly greater than the sum of the decrease in the LPS and GalN groups. It was thought that the decreased IgA+ plasma cells and decreased IgA levels in the intestinal mucosa were not the result of GalN or LPS injection, but rather of acute liver necrosis. These findings suggest that intestinal immunological barrier injury, which is a component of intestinal barrier injury, does occur in acute liver necrosis.
The mechanism of reduction in IgA+ plasma cells in acute liver necrosis is complicated and likely multifactorial. First, an increased rate of apoptosis in the subpopulation in Peyer’s patches secondary to acute liver necrosis could negatively impact IgA+ plasma cell numbers[9,31]. Second, multiple organ damage, particularly the bone marrow, spleen, and mesenteric lymph nodes, in concert with mucosal edema and injury caused by acute liver necrosis, could affect the production and proliferation of IgA+ plasma cells. Third, the structural damage to the intestinal mucosa could interfere with recirculation of plasma cell precursors[32]. An accurate mechanism of IgA+ plasma cell reduction in acute liver necrosis clearly requires further study.
In this study, it was also found that the decreased IgA expression in acute liver necrosis coincided with a decline in IgA+ plasma cells. One explanation for this could be that the decrease in the number and function of IgA+ plasma cells leading to a simultaneous reduction in IgA secretion. At present, the specific pathogenesis and progression of acute liver necrosis remains unclear.
Inflammatory mediators are thought to be involved in the development and progression of acute liver necrosis. Previous studies reported that serum levels of a number of inflammatory factors, such as IFN-γ, are elevated in patients with severe liver injury[14,33]. In addition, IFN-γ is known to downregulate IgA expression[34].
In this study, IFN-γ levels in the small intestinal mucosa were slightly increased in LPS, GalN and acute liver necrosis groups, but no significant difference in IFN-γ expression was identified between the acute liver necrosis and normal control group. IFN-γ expression does not seem to explain the decrease in IgA secretion from the intestinal mucosa. Other factors involved in the reduction in IgA expression in acute liver necrosis warrant further attention.
In conclusion, this study found that mice with acute liver necrosis had a reduced number of intestinal IgA+ plasma cells and IgA expression levels indicating that mucosal immune barrier dysfunction does exist in acute liver necrosis. IFN-γ expression does not seem to explain the decrease in IgA secretion from the intestinal mucosa. Further research regarding the mechanism(s) of intestinal immune barrier injury and ways to prevent this type of injury in acute liver necrosis is warranted.
COMMENTS
Background
Enterogenic infection is an important cause of death in patients with acute liver necrosis. Intestinal immunological barrier injury plays a vital role in the pathophysiology of enterogenic infection. Immunoglobulin A (IgA) is an important component of the intestinal immunological barrier and is the most abundant immunoglobulin present on mucosal surfaces, where it plays crucial roles in mucosal protection.
Research frontiers
IgA is considered a first line antigen-specific immune defense against pathogenic microorganisms and plays an important role in intestinal mucosal immunity. This study found significant changes in the number of IgA+ plasma cells and IgA expression levels during acute liver necrosis.
Innovations and breakthroughs
Previous studies have mainly focused on mechanical barrier interruption in acute liver necrosis models. So far, no studies have shown the indispensable nature of the intestinal immunological barrier in acute liver necrosis. In this study, the number of IgA+ plasma cells and IgA expression in mice with acute liver necrosis were determined to investigate whether dysfunction of the immunological barrier occurred during acute liver necrosis.
Applications
IgA is an important component of mucosal immune system and is significantly reduced during acute liver necrosis. Thus, a protective or an immunoregulative treatment of intestinal immune function could be beneficial in patients with acute liver necrosis.
Peer review
The manuscript by Fu et al describes studies examining in mice the change of intestinal IgA+ plasma cells and the expression of intestinal IgA in acute liver necrosis. Although the underlying mechanisms that contribute to the decreased expression of intestinal IgA was not thoroughly investigated, the study addresses an issue of topical interest, providing important data.
Acknowledgments
We are grateful to Professor Ya-Luo Dong and Professor Dong-Yan Liu for their technical guidance and help.
Footnotes
Supported by National Science Foundation of China, Grant No. 30670947; and National Ministry of Health, China, Grant No. 97100252
Peer reviewers: Damiao Carlos Moraes Santos, DCM, PhD, Department of Quality Control (deque), Bio-Manguinhos - FIOCRUZ, Avenida Brazil, 4365, Manguinhos - Rio de Janeiro, 21045-900, Brazil; Mara Massimi, PhD, Department of Basic and Applied Biology, University of L’Aquila, Via Vetoio, Coppito, 67010, Italy
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Deitch EA, Berg R. Bacterial translocation from the gut: a mechanism of infection. J Burn Care Rehabil. 1987;8:475–482. [PubMed] [Google Scholar]
- 2.Chiva M, Guarner C, Peralta C, Llovet T, Gómez G, Soriano G, Balanzó J. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. Eur J Gastroenterol Hepatol. 2003;15:145–150. doi: 10.1097/00042737-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J Immunol. 2007;179:7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 4.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Leeuwen PA, Boermeester MA, Houdijk AP, Meyer S, Cuesta MA, Wesdorp RI, Rodrick ML, Wilmore DW. Pretreatment with enteral cholestyramine prevents suppression of the cellular immune system after partial hepatectomy. Ann Surg. 1995;221:282–290. doi: 10.1097/00000658-199503000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmatz PR, Carter EA, Sullivan D, Hatz RA, Baker R, Breazeale E, Grant K, Bloch KJ. Effect of thermal injury in the rat on transfer of IgA protein into bile. Ann Surg. 1989;210:203–207. doi: 10.1097/00000658-198908000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho HB, Robalinho TI, Coutinho VB, Amorim AM, Furtado AF, Ferraz A, Ferraz E, Walker F, King G, Sewell HF, et al. Intra-abdominal sepsis: an immunocytochemical study of the small intestine mucosa. J Clin Pathol. 1997;50:294–298. doi: 10.1136/jcp.50.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Li A, Weng YB, Duan ML, Wang BE, Zhang SW. Changes in intestinal mucosal immune barrier in rats with endotoxemia. World J Gastroenterol. 2009;15:5843–5850. doi: 10.3748/wjg.15.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song HL, Lu S, Liu P. Tumor necrosis factor-alpha induces apoptosis of enterocytes in mice with fulminant hepatic failure. World J Gastroenterol. 2005;11:3701–3709. doi: 10.3748/wjg.v11.i24.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 11.Kramer DR, Sutherland RM, Bao S, Husband AJ. Cytokine mediated effects in mucosal immunity. Immunol Cell Biol. 1995;73:389–396. doi: 10.1038/icb.1995.61. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Abe K, Ikeda T, Matsushita T, Wake K, Sato T, Sato T, Inoue H. Inhibitory effect of glycyrrhizin on lipopolysaccharide and d-galactosamine-induced mouse liver injury. Eur J Pharmacol. 2007;576:136–142. doi: 10.1016/j.ejphar.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Li Y. Protective effect of bicyclol on acute hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. Eur J Pharmacol. 2006;534:194–201. doi: 10.1016/j.ejphar.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 14.Song HL, Lv S, Liu P. The roles of tumor necrosis factor-alpha in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol. 2009;9:70. doi: 10.1186/1471-230X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castaldo ET, Chari RS. Liver transplantation for acute hepatic failure. HPB (Oxford) 2006;8:29–34. doi: 10.1080/13651820500465741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davids BJ, Palm JE, Housley MP, Smith JR, Andersen YS, Martin MG, Hendrickson BA, Johansen FE, Svärd SG, Gillin FD, et al. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J Immunol. 2006;177:6281–6290. doi: 10.4049/jimmunol.177.9.6281. [DOI] [PubMed] [Google Scholar]
- 18.Corthésy B. Recombinant secretory immunoglobulin A in passive immunotherapy: linking immunology and biotechnology. Curr Pharm Biotechnol. 2003;4:51–67. doi: 10.2174/1389201033378020. [DOI] [PubMed] [Google Scholar]
- 19.Ancel D, Barraud H, Peyrin-Biroulet L, Bronowicki JP. [Intestinal permeability and cirrhosis] Gastroenterol Clin Biol. 2006;30:460–468. doi: 10.1016/s0399-8320(06)73203-1. [DOI] [PubMed] [Google Scholar]
- 20.Eipel C, Kidess E, Abshagen K, Leminh K, Menger MD, Burkhardt H, Vollmar B. Antileukoproteinase protects against hepatic inflammation, but not apoptosis in the response of D-galactosamine-sensitized mice to lipopolysaccharide. Br J Pharmacol. 2007;151:406–413. doi: 10.1038/sj.bjp.0707230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyanaga K, Yoshioka T, Nakagawa H, Kitahara T, To H, Ichikawa N, Nakashima M, Nishida K, Nakamura J, Sasaki H. Influence of murine hepatitis induced by D-(+)-galactosamine hydrochloride and lipopolysaccharide on gene expression of polyethylenimine/plasmid DNA polyplex. Biol Pharm Bull. 2008;31:1585–1589. doi: 10.1248/bpb.31.1585. [DOI] [PubMed] [Google Scholar]
- 22.Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer's patches. J Virol. 2001;75:10870–10879. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazanec MB, Nedrud JG, Kaetzel CS, Lamm ME. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993;14:430–435. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- 24.Russell MW, Sibley DA, Nikolova EB, Tomana M, Mestecky J. IgA antibody as a non-inflammatory regulator of immunity. Biochem Soc Trans. 1997;25:466–470. doi: 10.1042/bst0250466. [DOI] [PubMed] [Google Scholar]
- 25.Mestecky J, Russell MW, Elson CO. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut. 1999;44:2–5. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffié C, Hénin D, Benhamou M, Pretolani M, Blank U, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J Clin Immunol. 2001;21:303–309. doi: 10.1023/a:1012241117984. [DOI] [PubMed] [Google Scholar]
- 28.Hammarström L, Vorechovsky I, Webster D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID) Clin Exp Immunol. 2000;120:225–231. doi: 10.1046/j.1365-2249.2000.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards E, Razvi S, Cunningham-Rundles C. IgA deficiency: clinical correlates and responses to pneumococcal vaccine. Clin Immunol. 2004;111:93–97. doi: 10.1016/j.clim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Zinneman HH, Kaplan AP. The association of giardiasis with reduced intestinal secretory immunoglobulin A. Am J Dig Dis. 1972;17:793–797. doi: 10.1007/BF02231148. [DOI] [PubMed] [Google Scholar]
- 31.Fan J, Xie Y, Li X, Guo G, Meng Q, Xiu Y, Li T, Feng W, Ma L. The influence of Peyer's patch apoptosis on intestinal mucosal immunity in burned mice. Burns. 2009;35:687–694. doi: 10.1016/j.burns.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Scott BB, Goodall A, Stephenson P, Jenkins D. Duodenal bulb plasma cells in duodenitis and duodenal ulceration. Gut. 1985;26:1032–1037. doi: 10.1136/gut.26.10.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Q, Batey R, Pang G, Russell A, Clancy R. IL-6, IFN-gamma and TNF-alpha production by liver-associated T cells and acute liver injury in rats administered concanavalin A. Immunol Cell Biol. 1998;76:542–549. doi: 10.1046/j.1440-1711.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 34.Park SR, Jung MH, Jeon SH, Park MH, Park KH, Lee MR, Kim PH. IFN-gamma down-regulates TGF-beta1-induced IgA expression through Stat1 and p300 signaling. Mol Cells. 2010;29:57–62. doi: 10.1007/s10059-010-0004-4. [DOI] [PubMed] [Google Scholar]