Abstract
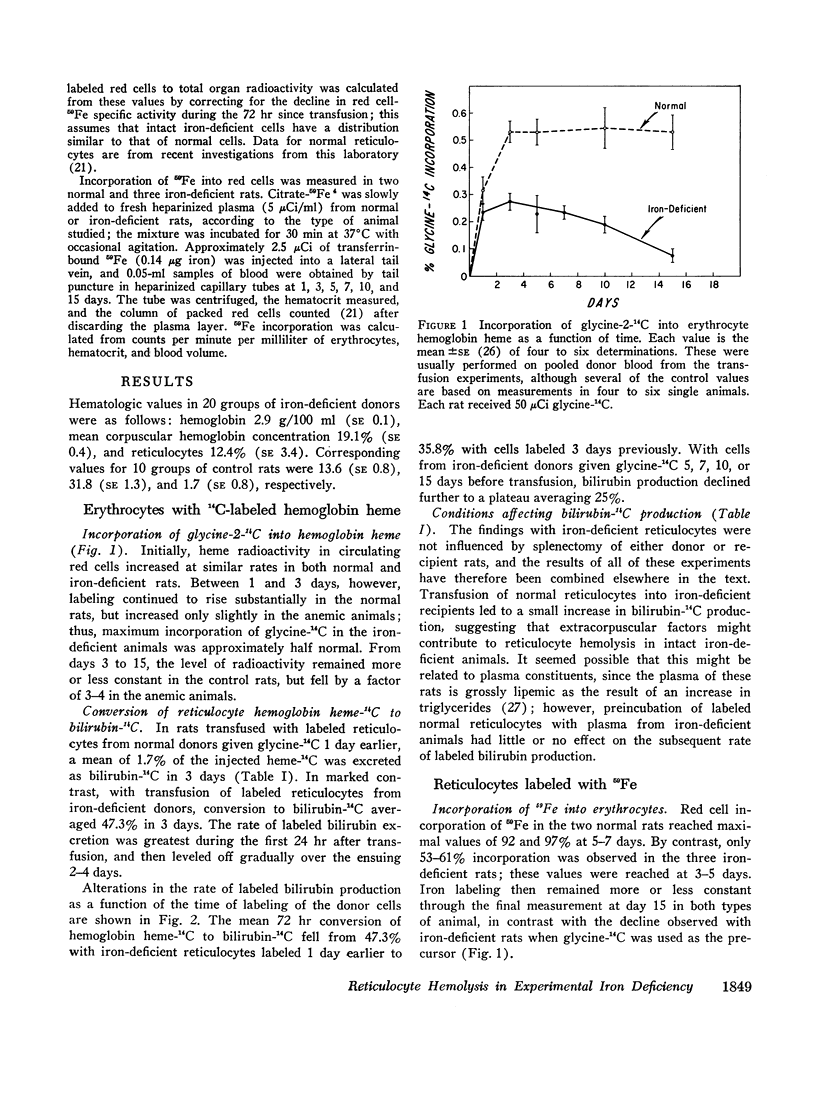
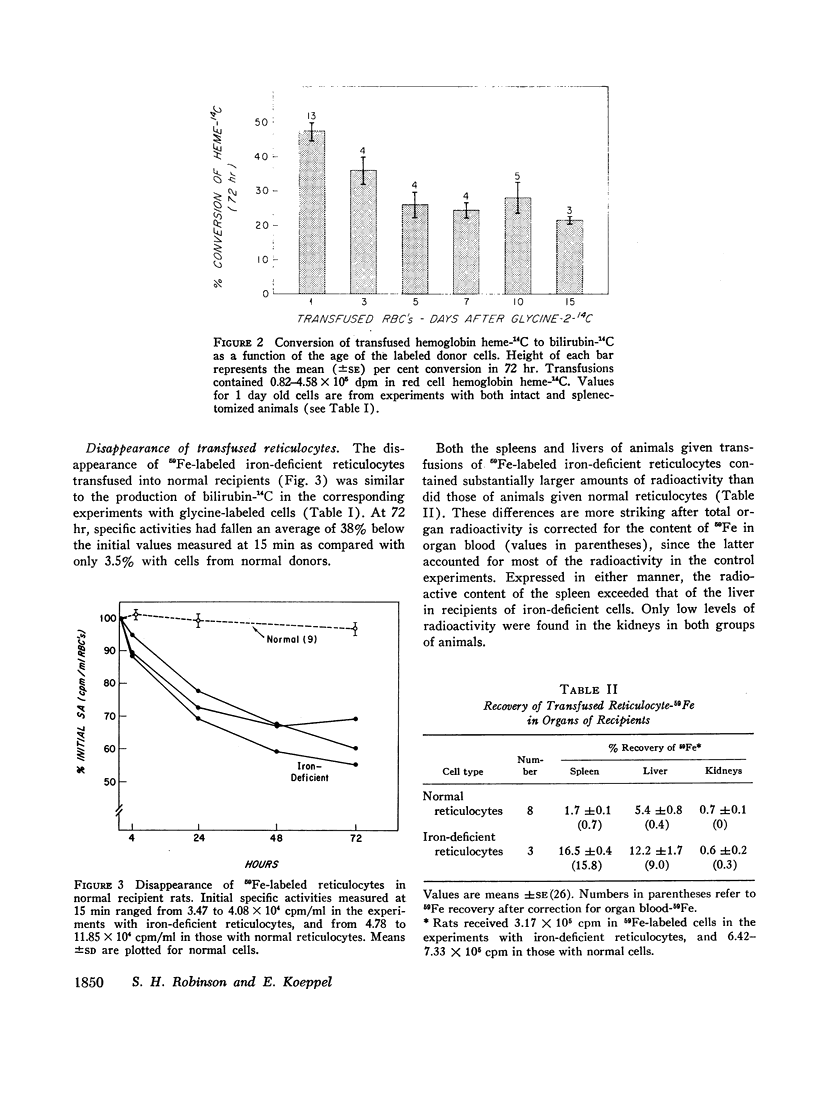
Bilirubin-14C production was measured in rats transfused with labeled erythrocytes from animals with iron deficiency anemia, a condition associated with ineffective erythropoiesis. With labeled reticulocytes harvested 1 day after the administration of glycine-2-14C, conversion of hemoglobin-14C to bilirubin averaged 47.3% over the 3 days of observation; the corresponding value for reticulocytes from normal rats was only 1.7%. Findings were not altered by splenectomy. Bilirubin-14C production fell to 35.8% with iron-deficient cells harvested 3 days after glycine-14C administration, and declined further to a plateau averaging 25% with cells labeled 5, 7, 10, or 15 days earlier. The latter values still far exceed those for mature erythrocytes from normal animals.
The findings indicate that experimental iron deficiency anemia is associated with hemolysis of red cells of various ages, but with preferential destruction of the youngest cells. Degradation of hemoglobin from reticulocytes is sufficient to account for a major fraction of the increase in erythropoietic bilirubin production found in this disorder, as has also been shown for physiologically regulated erythroid hyperplasia. However, the defect is quantitatively much more striking in experimental iron deficiency, and this and perhaps a similar defect in bone marrow cells appear to explain the decrease in net hemoglobin production that is characteristic of pathologic ineffective erythropoiesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERENDES M. The proportion of reticulocytes in the erythrocytes of the spleen as compared with those of circulating blood, with special reference to hemolytic states. Blood. 1959 May;14(5):558–563. [PubMed] [Google Scholar]
- BERLIN N. I., HEWITT C., LOTZ C. Hippuric acid synthesis in man after the administration of [alpha-14C]Glycine. Biochem J. 1954 Nov;58(3):498–503. doi: 10.1042/bj0580498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunström G. M., Fielding J., Karabus C. effect of small doses of iron on desferrioxamine chelation in iron-deficiency anaemia: evidence for ineffective erythropoiesis. Br J Haematol. 1968 May;14(5):525–531. doi: 10.1111/j.1365-2141.1968.tb07004.x. [DOI] [PubMed] [Google Scholar]
- GIBLETT E. R., COLEMAN D. H., PIRZIOBIROLI G., DONOHUE D. M., MOTULSKY A. G., FINCH C. A. Erythrokinetics: quantitative measurements of red cell production and destruction in normal subjects and patients with anemia. Blood. 1956 Apr;11(4):291–309. [PubMed] [Google Scholar]
- GRAY C. H., NEUBERGER A., SNEATH P. H. A. Studies in congenital porphyria. 2. Incorporation of 15N in the stercobilin in the normal and in the porphyric. Biochem J. 1950 Jun-Jul;47(1):87–92. doi: 10.1042/bj0470087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY C. H., SCOTT J. J. The effect of haemorrhage on the incorporation of [alpha-14C]Glycine into stercobilin. Biochem J. 1959 Jan;71(1):38–42. doi: 10.1042/bj0710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRINSTEIN M., BANNERMAN R. M., VAVRA J. D., MOORE C. V. Hemoglobin metabolism in thalassemia. In vivo studies. Am J Med. 1960 Jul;29:18–32. doi: 10.1016/0002-9343(60)90004-8. [DOI] [PubMed] [Google Scholar]
- Ganzoni A., Hillman R. S., Finch C. A. Maturation of the macroreticulocyte. Br J Haematol. 1969 Jan-Feb;16(1):119–135. doi: 10.1111/j.1365-2141.1969.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Huser H. J., Rieber E. E., Berman A. R. Experimental evidence of excess hemolysis in the course of chronic iron deficiency anemia. J Lab Clin Med. 1967 Mar;69(3):405–414. [PubMed] [Google Scholar]
- ISRAELS L. G., YAMAMOTO T., SKANDERBEG J., ZIPURSKY A. Shunt bilirubin: evidence for two components. Science. 1963 Mar 15;139(3559):1054–1055. doi: 10.1126/science.139.3559.1054. [DOI] [PubMed] [Google Scholar]
- Ibrahim G. W., Schwartz S., Watson C. J. Early labeling of bilirubin from glycine and delta-aminolevulinic acid in bile fistula dogs, with special reference to stimulated versus suppressed erythropoiesis. Metabolism. 1966 Dec;15(12):1129–1139. doi: 10.1016/0026-0495(66)90103-x. [DOI] [PubMed] [Google Scholar]
- Ibrahim G. W., Schwartz S., Watson C. J. The conversion of protoporphyrin-C14 to heme compounds and bilirubin in dogs. Metabolism. 1966 Dec;15(12):1120–1128. doi: 10.1016/0026-0495(66)90102-8. [DOI] [PubMed] [Google Scholar]
- JANDL J. H. The agglutination and sequestration of immature red cells. J Lab Clin Med. 1960 May;55:663–681. [PubMed] [Google Scholar]
- LAYRISSE M., LINARES J., ROCHE M. EXCESS HEMOLYSIS IN SUBJECTS WITH SEVERE IRON DEFICIENCY ANEMIA ASSOCIATED AND NONASSOCIATED WITH HOOKWORM INFECTION. Blood. 1965 Jan;25:73–91. [PubMed] [Google Scholar]
- LONDON I. M., BRUNS G. P., KARIBIAN D. THE REGULATION OF HEMOGLOBIN SYNTHESIS AND THE PATHOGENESIS OF SOME HYPOCHROMIC ANEMIAS. Medicine (Baltimore) 1964 Nov;43:789–802. doi: 10.1097/00005792-196411000-00024. [DOI] [PubMed] [Google Scholar]
- LONDON I. M., WEST R., SHEMIN D., RITTENBERG D. On the origin of bile pigment in normal man. J Biol Chem. 1950 May;184(1):351–358. [PubMed] [Google Scholar]
- LONDON I. M., WEST R. The formation of bile pigment in pernicious anemia. J Biol Chem. 1950 May;184(1):359–364. [PubMed] [Google Scholar]
- Levitt M., Schacter B. A., Zipursky A., Israels L. G. The nonerythropoietic component of early bilirubin. J Clin Invest. 1968 Jun;47(6):1281–1294. doi: 10.1172/JCI105820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loría A., Sánchez-Medal L., Lisker R., De Rodríguez E., Labardini J. Red cell life span in iron deficiency anaemia. Br J Haematol. 1967 May;13(3):294–302. doi: 10.1111/j.1365-2141.1967.tb08743.x. [DOI] [PubMed] [Google Scholar]
- MENDEL G. A., WEILER R. J., MANGALIK A. STUDIES ON IRON ABSORPTION. II. THE ABSORPTION OF IRON IN EXPERIMENTAL ANEMIAS OF DIVERSE ETIOLOGY. Blood. 1963 Oct;22:450–458. [PubMed] [Google Scholar]
- McKee L. C., Wasson M., Heyssel R. M. Experimental iron deficiency in the rat. The use of 51Cr, DF32P and 59Fe to detect haemolysis of iron-deficient cells. Br J Haematol. 1968 Jan;14(1):87–94. doi: 10.1111/j.1365-2141.1968.tb01476.x. [DOI] [PubMed] [Google Scholar]
- OSTROW J. D., HAMMAKER L., SCHMID R. The preparation of crystalline bilirubin-C14. J Clin Invest. 1961 Aug;40:1442–1452. doi: 10.1172/JCI104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollycove M. Iron metabolism and kinetics. Semin Hematol. 1966 Oct;3(4):235–298. [PubMed] [Google Scholar]
- ROBINSON S., VANIER T., DESFORGES J. F., SCHMID R. Jaundice in thalassemia minor: a consequence of "ineffective erythropoiesis". N Engl J Med. 1962 Sep 13;267:523–529. doi: 10.1056/NEJM196209132671101. [DOI] [PubMed] [Google Scholar]
- Robinson S. H. Increased bilirubin formation from nonhemoglobin sources in rats with disorders of the liver. J Lab Clin Med. 1969 Apr;73(4):668–676. [PubMed] [Google Scholar]
- Robinson S. H. Increased formation of early-labeled bilirubin in rats with iron deficiency anemia: evidence for ineffective erythropoiesis. Blood. 1969 Jun;33(6):909–917. [PubMed] [Google Scholar]
- Robinson S. H., Owen C. A., Jr, Flock E. V., Schmid R. Bilirubin formation in the liver from nonhemoglobin sources. Experiments with isolated, perfused rat liver. Blood. 1965 Dec;26(6):823–829. [PubMed] [Google Scholar]
- Robinson S. H. The origins of bilirubin. N Engl J Med. 1968 Jul 18;279(3):143–149. doi: 10.1056/NEJM196807182790306. [DOI] [PubMed] [Google Scholar]
- Robinson S. H., Tsong M., Brown B. W., Schmid R. The sources of bile pigment in the rat: studies of the "early labeled" fraction. J Clin Invest. 1966 Oct;45(10):1569–1586. doi: 10.1172/JCI105463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. H., Tsong M. Hemolysis of "stress" reticulocytes: a source of erythropoietic bilirubin formation. J Clin Invest. 1970 May;49(5):1025–1034. doi: 10.1172/JCI106302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMPERLEY I. J., SHARP A. A. The life span of erythrocytes in iron-deficiency anaemia. J Clin Pathol. 1962 Jul;15:346–349. doi: 10.1136/jcp.15.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERLOOP M. C., van der WOLK, HEIER A. J. Radioactive iron studies in patients with iron deficiency anemia with concurrent abnormal hemolysis. Blood. 1960 May;15:791–806. [PubMed] [Google Scholar]
- WATSON C. J. The erythrocyte coproporphyrin; variation in respect to erythrocyte protoporphyrin and reticulocytes in certain of the anemias. AMA Arch Intern Med. 1950 Dec;86(6):797–809. doi: 10.1001/archinte.1950.00230180002001. [DOI] [PubMed] [Google Scholar]