Abstract
A method has been developed for measuring the adhesion of platelets to purified collagen fibers obtained from bovine tendon. This method differs from others in that: (a) platelet adhesion is measured in the absence of platelet aggregation; (b) platelet-rich plasma collected in ACD (acid citrate dextrose) or EDTA, or washed platelets can be employed; (c) adherent platelets are enumerated directly; (d) erythrocytes and leukocytes do not adhere.
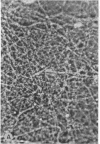
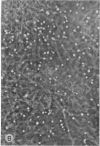
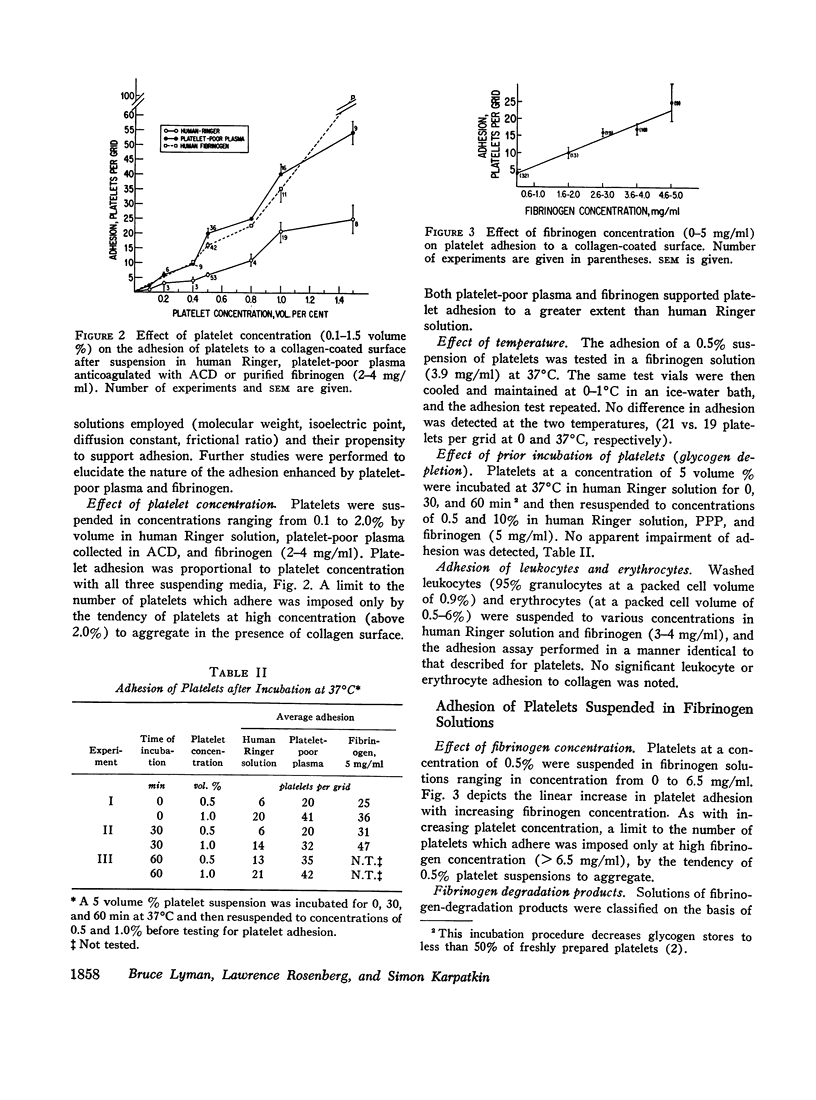
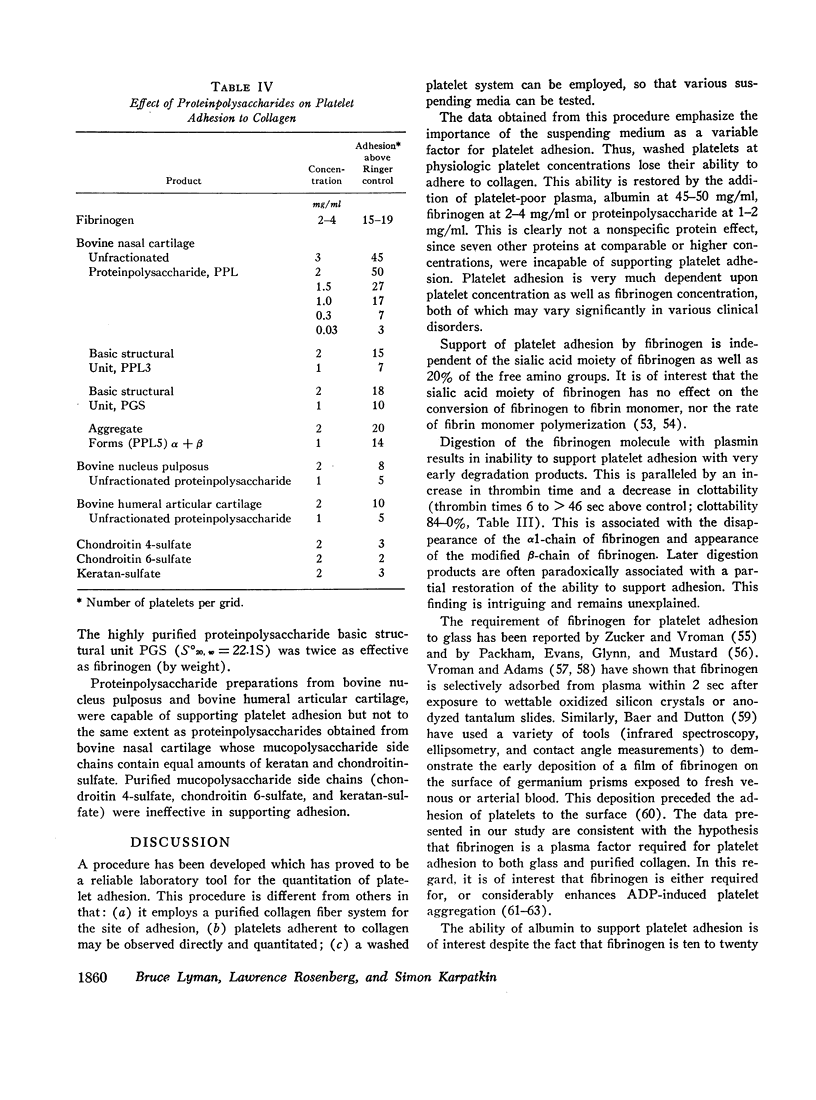
Washed platelets suspended in human Ringer solution exhibit negligible adhesion (at the platelet concentrations employed) in contrast to washed platelets suspended in plasma. Addition of purified human fibrinogen (95% clottable, 2-4 mg/ml) to human Ringer solution completely restores the ability of washed platelets to adhere to collagen fibers. Albumin (fatty acid free, 50 mg/ml) is also capable of restoring adhesion. Albumin and seven other proteins at concentrations of 5-10 mg/ml, with varying molecular weights, isoelectric points, and frictional coefficients are incapable of supporting the adhesion of washed platelets. The proteins tested were human globulin, hexokinase, hemoglobin, cytochrome-C, insulin, thyroglobulin, and muramidase. Platelet adhesion is proportional to both platelet concentration and fibrinogen concentration, but is independent of temperature or glycogen stores.
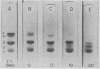
Modification of fibrinogen by acylation of amino groups or removal of sialic acid has no effect on its ability to support platelet adhesion. Degradation of fibrinogen with purified plasmin results in decreased support of platelet adhesion. This accompanied formation of early breakdown products with clottability ranging from 84-0%. Formation of fibrinogen degradation products was monitored by SDS-polyacrylamide gel electrophoresis of the corresponding fibrins after reduction of disulfide bonds (a method capable of distinguishing α-, β- and gamma-chains). Decreased support of platelet adhesion is associated with the disappearance of intact α- chains and early modification of the β-chains.
Purified proteinpolysaccharide macromolecules obtained from bovine nasal and humeral cartilage, and from nucleosus pulposus are as effective as fibrinogen on a weight basis and ten to thirty times more effective on a molar basis in supporting platelet adhesion. The purified mucopolysaccharide side chains: chondroitin-4-sulfate, chondroitin-6-sulfate, and keratan-sulfate are incapable of supporting platelet adhesion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aledort L. M., Troup S. B., Weed R. I. Inhibition of sulfhydryl-dependent platelet functions by penetrating and non-penetrating analogues of parachloromercuribenzene. Blood. 1968 Apr;31(4):471–479. [PubMed] [Google Scholar]
- BOUNAMEAUX Y. [The adherence of blood platelets to subendothelial fibers]. Thromb Diath Haemorrh. 1961 Dec 15;6:504–516. [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier R. E., Dutton R. C. Initial events in interactions of blood with a foreign surface. J Biomed Mater Res. 1969 Mar;3(1):191–206. doi: 10.1002/jbm.820030115. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R., Tranzer J. P., Studer A. An electron microscopic study of platelet thrombus formation in the rabbit with particular regard to 5-hydroxytryptamine release. Thromb Diath Haemorrh. 1967 Dec 31;18(3-4):592–604. [PubMed] [Google Scholar]
- Bańkowski E., Niewiarowski S., Rogowski W. Decrease of platelet aggregating activity of soluble human collagen fractions during ageing. Thromb Diath Haemorrh. 1969 Jun 15;21(3):441–449. [PubMed] [Google Scholar]
- Brinkhous K. M., Read M. S., Rodman N. F., Mason R. G. Need of fibrinogen for thrombin-induced aggregation of porcine platelets. J Lab Clin Med. 1969 Jun;73(6):1000–1010. [PubMed] [Google Scholar]
- Caen J. P., Legrand Y. L'analyse des fonctions plaquettaires. Adhésion et agrégation des plaquettes au collagène purifié. Rev Fr Etud Clin Biol. 1968 Dec;13(10):1028–1030. [PubMed] [Google Scholar]
- Deykin D., Pritzker C. R., Scolnick E. M. Plasma co-factors in adenosine diphosphate-induced aggregation of human platelets. Nature. 1965 Oct 16;208(5007):296–298. doi: 10.1038/208296b0. [DOI] [PubMed] [Google Scholar]
- Dutton R. C., Webber A. J., Johnson S. A., Baier R. E. Microstructure of initial thrombus formation on foreign materials. J Biomed Mater Res. 1969 Mar;3(1):13–23. doi: 10.1002/jbm.820030104. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- HASLAM R. J. ROLE OF ADENOSINE DIPHOSPHATE IN THE AGGREGATION OF HUMAN BLOOD-PLATELETS BY THROMBIN AND BY FATTY ACIDS. Nature. 1964 May 23;202:765–768. doi: 10.1038/202765a0. [DOI] [PubMed] [Google Scholar]
- HOVIG T. AGGREGATION OF RABBIT BLOOD PLATELETS PRODUCED IN VITRO BY SALINE "EXTRACT" OF TENDONS. Thromb Diath Haemorrh. 1963 Jul 15;143:248–263. [PubMed] [Google Scholar]
- HUGUES J. [Binding of platelets to collagen]. C R Seances Soc Biol Fil. 1960;154:866–868. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hirsh J., Glynn M. F., Mustard J. F. The effect of platelet age on platelet adherence to collagen. J Clin Invest. 1968 Mar;47(3):466–473. doi: 10.1172/JCI105743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovig T., Jorgensen L., Packham M. A., Mustard J. F. Platelet adherence to fibrin and collagen. J Lab Clin Med. 1968 Jan;71(1):29–40. [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969 Jun;48(6):1083–1087. doi: 10.1172/JCI106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S., Langer R. M. Biochemical energetics of simulated platelet plug formation. Effect of thrombin, adenosine diphosphate, and epinephrine on intra- and extracellular adenine nucleotide kinetics. J Clin Invest. 1968 Sep;47(9):2158–2168. doi: 10.1172/JCI105902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S., Siskind G. W. In vitro detection of platelet antibody in patients with idiopathic thrombocytopenic purpura and systemic lupus erythematosus. Blood. 1969 Jun;33(6):795–812. [PubMed] [Google Scholar]
- Kefalides N. A., Denduchis B. Structural components of epithelial and endothelial basement membranes. Biochemistry. 1969 Nov;8(11):4613–4621. doi: 10.1021/bi00839a057. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation and characterization of the collagen from glomerular basement membrane. Biochemistry. 1968 Sep;7(9):3103–3112. doi: 10.1021/bi00849a012. [DOI] [PubMed] [Google Scholar]
- Lewis N., Majerus P. W. Lipid metabolism in human platelets. II. De novo phospholipid synthesis and the effect of thrombin on the pattern of synthesis. J Clin Invest. 1969 Nov;48(11):2114–2123. doi: 10.1172/JCI106178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder P. B., Hirsh J., De Gruchy G. C. The effect of collagen on platelet glycolysis and nucleotide metabolism. Br J Haematol. 1968 Jun;14(6):563–573. doi: 10.1111/j.1365-2141.1968.tb00363.x. [DOI] [PubMed] [Google Scholar]
- MILLS D. A., COYNE R., POLLARA B., VONKORFF R. W. THE ACTION OF PLASMIN ON FIBRINOGEN ADN FIBRIN. I. CHANGES IN THE N-TERMINAL RESIDUES. Biochim Biophys Acta. 1964 Jun 8;86:527–534. doi: 10.1016/0304-4165(64)90092-3. [DOI] [PubMed] [Google Scholar]
- Mahadevan V., Singh H., Lundberg W. O. Effects of saturated and unsaturated fatty acids on blood platelet aggregation in vitro. Proc Soc Exp Biol Med. 1966 Jan;121(1):82–85. doi: 10.3181/00379727-121-30702. [DOI] [PubMed] [Google Scholar]
- Mester L., Moczar E., Vass G., Szabados L. Structure et role des fractions glucidiques du fibrinogène. Pathol Biol. 1965 May;13(9):540–545. [PubMed] [Google Scholar]
- Mills D., Karpatkin S. Heterogeneity of human fibrinogen: possible relation to proteolysis by thrombin and plasmin as studies by SDS-polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1970 Jul 13;40(1):206–211. doi: 10.1016/0006-291x(70)91067-3. [DOI] [PubMed] [Google Scholar]
- Mürer E. H. Clot retraction and energy metabolism of platelets. Effect and mechanism of inhibitors. Biochim Biophys Acta. 1969 Feb 25;172(2):266–276. doi: 10.1016/0005-2728(69)90069-3. [DOI] [PubMed] [Google Scholar]
- O'BRIEN J. R. The adhesiveness of native platelets and its prevention. J Clin Pathol. 1961 Mar;14:140–149. doi: 10.1136/jcp.14.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham M. A., Evans G., Glynn M. F., Mustard J. F. The effect of plasma proteins on the interaction of platelets with glass surfaces. J Lab Clin Med. 1969 Apr;73(4):686–697. [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- Previero A., Coletti-Previero M. A., Cavadore J. C. A reversible chemical modification of the tryptophan residue. Biochim Biophys Acta. 1967 Dec 12;147(3):453–461. doi: 10.1016/0005-2795(67)90005-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- Rosenberg L., Pal S., Beale R., Schubert M. A comparison of proteinpolysaccharides of bovine nasal cartilage isolated and fractionated by different methods. J Biol Chem. 1970 Aug 25;245(16):4112–4122. [PubMed] [Google Scholar]
- Rosenberg L., Schubert M. The proteinpolysaccharides of bovine nucleus pulposus. J Biol Chem. 1967 Oct 25;242(20):4691–4701. [PubMed] [Google Scholar]
- Rozenberg M. C., Holmsen H. Adenine nucleotide metabolism of blood platelets. IV. Platelet aggregation response to exogenous ATP and ADP. Biochim Biophys Acta. 1968 Apr 22;157(2):280–288. [PubMed] [Google Scholar]
- SALZMAN E. W. MEASUREMENT OF PLATELET ADHESIVENESS. A SIMPLE IN VITRO TECHNIQUE DEMONSTRATING AN ABNORMALITY IN VON WILLEBRAND'S DISEASE. J Lab Clin Med. 1963 Nov;62:724–735. [PubMed] [Google Scholar]
- SPAET T. H., ZUCKER M. B. MECHANISM OF PLATELET PLUG FORMATION AND ROLE OF ADENOSINE DIPHOSPHATE. Am J Physiol. 1964 Jun;206:1267–1274. doi: 10.1152/ajplegacy.1964.206.6.1267. [DOI] [PubMed] [Google Scholar]
- Salzman E. W., Levine L. Cyclic 3',5'-adenosine monophosphate in human blood platelets. II. Effect of N6-2'-o-dibutyryl cyclic 3',5'-adenosine monophosphate on platelet function. J Clin Invest. 1971 Jan;50(1):131–141. doi: 10.1172/JCI106467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoffone E., Fontana A., Rocchi R. Sulfenyl halides as modifying reagents for polypeptides and proteins. I. Modification of tryptophan residues. Biochemistry. 1968 Mar;7(3):971–979. doi: 10.1021/bi00843a014. [DOI] [PubMed] [Google Scholar]
- Shulman N. R., Watkins S. P., Jr, Itscoitz S. B., Students A. B. Evidence that the spleen retains the youngest and hemostatically most effective platelets. Trans Assoc Am Physicians. 1968;81:302–313. [PubMed] [Google Scholar]
- Sjögren A., Böttiger L. E., Biörck G. Platelet adhesion: a comparison of four methods. Acta Med Scand. 1969 Jan-Feb;185(1-2):127–131. doi: 10.1111/j.0954-6820.1969.tb07308.x. [DOI] [PubMed] [Google Scholar]
- Spaet T. H., Lejnieks I. A technique for estimation of platelet-collagen adhesion. Proc Soc Exp Biol Med. 1969 Dec;132(3):1038–1041. doi: 10.3181/00379727-132-34362. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Baumgartner H. R., Spaet T. H. The subendothelial microfibril and platelet adhesion. Lab Invest. 1971 Mar;24(3):179–186. [PubMed] [Google Scholar]
- Tranzer J. P., Baumgartner H. R. Filling gaps in the vascular endothelium with blood platelets. Nature. 1967 Dec 16;216(5120):1126–1128. doi: 10.1038/2161126a0. [DOI] [PubMed] [Google Scholar]
- Vroman L., Adams A. L. Identification of rapid changes at plasma-solid interfaces. J Biomed Mater Res. 1969 Mar;3(1):43–67. doi: 10.1002/jbm.820030106. [DOI] [PubMed] [Google Scholar]
- Vroman L., Adams A. L. Possible involvement of fibrinogen and proteolysis in surface activation. A study with the recording ellipsometer. Thromb Diath Haemorrh. 1967 Dec 31;18(3-4):510–524. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., LeRoy E. C. Aggregation of platelets by collagen. J Clin Invest. 1968 Dec;47(12):2616–2621. doi: 10.1172/JCI105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. B., Vroman L. Platelet adhesion induced by fibrinogen adsorbed onto glass. Proc Soc Exp Biol Med. 1969 Jun;131(2):318–320. doi: 10.3181/00379727-131-33866. [DOI] [PubMed] [Google Scholar]