Summary
Background
Atypical genital naevi (AGN) are naevi of special sites with atypical histological features that overlap with those of malignant melanoma. Activating BRAF mutations, identified in the majority of banal melanocytic naevi and cutaneous melanomas, are reportedly uncommon in naevomelanocytic proliferations in nonsun-exposed sites. We have recently shown that constitutive activation of the BRAF-MEK-ERK signalling pathway in oncogenic BRAF-positive naevi increases expression and secretion of IGFBP7, which induces senescence and apoptosis.
Objectives
To ascertain the frequency of BRAF V600E mutations in AGN compared with banal naevi without atypia. An additional aim was to assess the expression of IGFBP7 in oncogenic BRAF-positive AGN.
Methods
Genomic DNA was isolated per protocol from seven genital naevi without atypia and 13 AGN for BRAF genotyping. Immunohistochemical staining for IGFBP7 was performed on all cases.
Results
The BRAF V600E mutation was identified in 43% of genital naevi without atypia and 23% of AGN (P = 0.61). In both groups, IGFBP7 expression was maintained in 67% of BRAF V600E-positive cases.
Conclusions
The prevalence of BRAF V600E in AGN suggests that ultraviolet exposure is not essential for generating the mutation. The BRAF V600E mutational status appears to be of limited diagnostic utility in distinguishing genital naevi that exhibit atypia from those that do not. Similar to oncogenic BRAF-positive common naevi without atypia, enhanced expression of the tumour suppressor IGFBP7 in oncogenic BRAF-positive AGN supports that they are biologically inert.
Keywords: atypical genital naevi, BRAF V600E, IGFBP7
First described by Clark et al., atypical genital naevi (AGN) are a relatively uncommon subset of clinically benign naevi of the genitalia or perineum that exhibit atypical histological features.1,2 Similar lesions have subsequently been described along the anatomical milk line including the groin area.3 Unifying histological features of AGN include variably sized nests with retraction artefact, consumption of the epidermis, cellular dyshesion, dermal fibrosis and cytological atypia ranging from mild to moderate severity.1 As these features are concerning for melanoma, overdiagnosis of AGN is not uncommon and may lead to unnecessary surgical intervention and sentinel lymph node biopsy.1 This is of particular concern given that these naevi typically occur in women of childbearing age.1,2
No study to date has sought to elucidate the genomic profile of AGN as compared with benign genital naevi without atypia. We recently found that in oncogenic BRAF-positive naevi, constitutive activation of the BRAF-MEK-ERK pathway increases expression and secretion of IGFBP7,4 a protein that suppresses the growth of a range of cancer cell types.5,6 We also noted that IGFBP7 expression is maintained or enhanced in BRAF wild-type (WT) melanomas but is not detectable in oncogenic BRAF-positive melanomas. Based on these findings, we concluded that IGFBP7 inhibits BRAF-MEK-ERK signalling and activates senescence via an autocrine/paracrine pathway.
Therefore, the primary goal of this study was to assess the frequency of BRAF V600E mutations in AGN and to compare with that in banal naevi of genital skin. Given our recent experience with IGFBP7, an additional goal was to assess IGFBP7 expression in oncogenic BRAF-positive AGN.
Materials and methods
This study was approved by Boston University School of Medicine institutional review board (IRB docket H27806). Archival materials between 2006 and 2008 with a histological diagnosis of ‘naevus’ (n = 7) and ‘naevus with atypia’ (n = 13) located in the genital area [mons, pubic and suprapubic (n = 11), labia (n = 4), perineum (n = 1), groin (n = 3) and scrotum (n = 1)] were retrieved from the pathology files of the Skin Pathology Laboratory (Boston University School of Medicine, Boston, MA, U.S.A.). All patient data were de-identified. All histological sections were re-reviewed and diagnoses confirmed by the dermatopathologist (M.M.). Five of seven (71%) cases of genital naevi without atypia and 12 of 13 (92%) cases of AGN were female. Median age was 33.5 years (range 11.5–76.6) and 33.4 years (range 14.7–50.1) for each group, respectively.
Microscopic examination of the 13 AGN revealed compound naevi exhibiting varying degrees of one or more of the following: variably sized nests with retraction artefact and/or consumption of the epidermis, cellular dyshesion, and dermal fibrosis. Severity of cytological atypia ranged from mild (n = 2) to moderate (n = 8) and severe (n = 3). All seven cases of genital naevi without atypia were compound naevi with features of a congenital naevus.
Mutational analysis
DNA was extracted by proteinase K digestion of microdissected samples per protocol. Briefly, 5- to 7-μm thick sections of formalin-fixed paraffin-embedded archival tissue were deparaffinized and rehydrated prior to microdissection. Direct DNA sequencing was performed on exon 15 of the BRAF gene (forward strand) spanning codon 600 using an ABI BigDye TerV3.1 cycle sequencing terminator ready reaction kit (Applied Biosystems, Inc., Foster City, CA, U.S.A.). The sequence of the forward primer was 5′-TCATAATGCTTGCTCTGATAGGA-3′. Sequencing reactions were performed on an ABI 9700 thermocycler utilizing the ABI recommended protocol and analysed with the Genetic Analyzer 3100-avant (ABI). The sequencing results were analysed with ABI DNA Sequencing Analysis Software version 3.7. A positive and/or negative control was included.
IGFBP7 immunohistochemistry
Immunohistochemical studies were performed on 5-μm thick sections using standard peroxidase immunohistochemistry techniques, heat-induced epitope retrieval buffer and a primary antibody against IGFBP7 (C-16; 1:20; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A.). Appropriate positive and negative controls were used. Positive expression of IGFBP7 in the cytoplasm was scored as 1+ (< 10% of tumour cells), 2+ (10–50%) and 3+ (> 50%). Cases with an immunohistochemical score of 1+ were considered negative while those with a score of 2+ or 3+ were considered positive. Significant nuclear staining was not observed. All stained slides were initially reviewed and scored by the first author (L.P.N.) and were re-reviewed by the dermatopathologist (M.M.) in a blinded fashion with respect to genotype. Disagreements were reviewed together to achieve a consensus score.
Statistical analysis
Fisher's exact test was used to compare categorical variables. An alpha of 0.05 was used as the level of significance.
Results
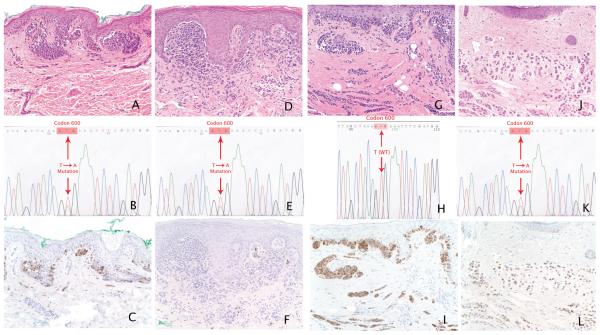
Data from genotyping and expression of tumour suppressor IGFBP7 are summarized in Table 1, and representative cases are illustrated in Figure 1.
Table 1.
Summary of BRAF mutational status and IGFBP7 positivity in genital naevi
| Atypical genital naevi | ||
| No. of cases/total (%) | BRAF WT (n = 10) | BRAF V600E (n = 3) |
| IGFBP7 positive | 8/10 (80) | 2/3 (67) |
| IGFBP7 negative | 2/10 (20) | 1/3 (33) |
| Genital naevi without atypia | ||
| No. of cases/total (%) | BRAF WT (n = 4) | BRAF V600E (n = 3) |
| IGFBP7 positive | 4/4 (100) | 2/3 (67) |
| IGFBP7 negative | 0/4 (0) | 1/3 (33) |
WT, wild-type.
Fig 1.
Composite of genomic and immunohistochemical staining with representative cases with/without BRAF V600E mutation and positive/negative IGFBP7 expression. (a) Atypical genital naevus with moderate cytological atypia, with (b) BRAF V600E mutation and (c) positive IGFBP7 expression. (d) Atypical genital naevus with moderate cytological atypia, with (e) BRAF V600E mutation and (f) negative IGFBP7 expression. (g) Atypical genital compound melanocytic neoplasm with moderate to severe atypia, with (h) BRAF wild-type (WT) and (i) positive IGFBP7 expression. (j) Compound melanocytic naevus with features suggestive of congenital onset, with (k) BRAF V600E mutation and (l) positive IGFBP7 expression. (a, d, g, j) Haematoxylin and eosin; original magnification × 20.
BRAF mutational status
The BRAF V600E allele was detected in three of 13 (23%) AGN and three of seven (43%) genital naevi without atypia (P = 0.61). All three AGN expressing the BRAF V600E mutation demonstrated a moderate degree of cytological atypia.
IGFBP7
Of the cases of AGN, cytoplasmic staining (3+) was observed in two of three cases (67%) that were BRAF V600E-positive and in eight of 10 cases (80%) that were BRAF WT. Of the cases of genital naevi without atypia, cytoplasmic staining (3+) was observed in two of three cases (67%) that were BRAF V600E-positive and in four of four cases (100%) that were BRAF WT. No significant association was found between BRAF mutational status and IGFBP7 expression in AGN or genital naevi without atypia (P = 0.58 and P = 0.43, respectively).
Discussion
Oncogenic BRAF mutations are known to predispose to melanoma with a reported attributable risk of 1.6% and have been found in both melanocytic naevi and melanoma.7,8 However, the mutation is not present in all types of melanoma, and differences in incidence have been attributed to aetiological factors including sun exposure.9 In keeping with this, BRAF mutations have been documented in 62% of melanomas arising in sun-exposed sites compared with 0% of vulval melanomas, suggesting that distinct molecular pathways may be involved depending on the presence or absence of sun exposure.9 However, the BRAF V600E mutation was identified in 23% and 43% of AGN and genital naevi without atypia, respectively, in the current study. Ichii-Nakato et al. also found the presence of the BRAF V600E mutation in up to 81% of acquired naevi from glabrous skin.10 The higher proportion may be attributable to different methodologies used in mutation analysis (Mutector assay vs. direct sequencing in the current study) and the cohort studied (acquired naevi without atypia vs. congenital naevi and naevi with atypia in the current study). None the less, both studies support the concept that ultraviolet (UV) radiation is not essential for acquisition of the BRAF mutation. While the clinical relevance of BRAF mutations in naevomelanocytic proliferations in non-sunexposed skin remains unclear, somatic mutations of BRAF have previously been identified as the most common early genetic event causally associated with development of papillary thyroid cancer in patients without a history of radiation exposure.11 This implicates other environmental or intrinsic biological factors as potential causative agents for BRAF mutations.
AGN pose a significant diagnostic conundrum. Exemplifying this, one of the cases in the current study was that of a ‘moderate to severely atypical compound neoplasm’ in a 14-year-old patient. The report noted that ‘although the lesion exhibited maturation, given the degree of cytological atypia, florid junctional melanocytic proliferation and presence of dyscohesive nests, malignant melanoma, level III could not be excluded’ by five experienced dermatopathologists. No study to date has sought to elucidate the genomic profile of AGN as compared with benign genital naevi without atypia. We found no significant difference in the frequency of oncogenic BRAF in AGN compared with naevi without atypia from the same anatomical location. Thus, the utility of oncogenic BRAF as a diagnostic adjunct appears to be limited. However, a larger study is needed before a definitive conclusion can be reached.
We have previously found that normal melanocytes express detectable, albeit low, levels of IGFBP7.4 In vitro evidence indicates that IGFBP7 blocks BRAF-MEK-ERK signalling to activate the apoptotic pathway, suggesting that it is an inducer of senescence and apoptosis. Consistent with this, we also found IGFBP7 expression to be upregulated in BRAF WT melanomas and oncogenic BRAF V600E-positive naevi, but absent in BRAF V600E-positive melanoma. In the current study, the enhanced expression of IGFBP7 found in 67% of oncogenic BRAF-positive AGN suggests that these lesions are biologically inert. While the significance of our findings is limited by the small number of cases studied, oncogenic BRAF-positive AGN cases with loss of IGFBP7 expression may warrant complete excision and close clinical follow-up.
In conclusion, the prevalence of BRAF V600E in AGN suggests that UV exposure is not essential for generating the BRAF V600E mutation. Furthermore, AGN appear not to differ from genital naevi without atypia with respect to BRAF mutational status. Enhanced expression of the tumour suppressor IGFBP7 in oncogenic BRAF-positive AGN suggests that these lesions behave similarly to oncogenic BRAF-positive naevi. Larger studies and long-term follow-up of AGN is required to confirm their putative banal biological behaviour.
Footnotes
Conflicts of interest
None declared.
References
- 1.Clark WH, Jr, Hood AF, Tucker MA, et al. Atypical melanocytic nevi of the genital type with a discussion of reciprocal parenchymal-stromal interactions in the biology of neoplasia. Hum Pathol. 1998;29:S1–24. doi: 10.1016/s0046-8177(98)80028-2. [DOI] [PubMed] [Google Scholar]
- 2.Gleason BC, Hirsch MS, Nucci MR, et al. Atypical genital nevi. A clinicopathologic analysis of 56 cases. Am J Surg Pathol. 2008;32:51–7. doi: 10.1097/PAS.0b013e318068420c. [DOI] [PubMed] [Google Scholar]
- 3.Massi G, LeBoit PE, editors. Nevi on Genital Skin. SteinKopf Verlag; Darmstadt: 2004. [Google Scholar]
- 4.Wajapeyee N, Serra RW, Zhu X, et al. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–74. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan WJ, Lin J, Xu EP, et al. IGFBP7 plays a potential tumor suppressor role against colorectal carcinogenesis with its expression associated with DNA hypomethylation of exon 1. J Zhejiang Univ Sci B. 2006;7:929–32. doi: 10.1631/jzus.2006.B0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato MV. A secreted tumor-suppressor, mac25, with activin-binding activity. Mol Med. 2000;6:126–35. doi: 10.1007/s0089400060126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James MR, Roth RB, Shi MM, et al. BRAF polymorphisms and risk of melanocytic neoplasia. J Invest Dermatol. 2005;125:1252–8. doi: 10.1111/j.0022-202X.2005.23937.x. [DOI] [PubMed] [Google Scholar]
- 8.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 9.Cohen Y, Rosenbaum E, Begum S, et al. Exon 15 BRAF mutations are uncommon in melanomas arising in nonsun-exposed sites. Clin Cancer Res. 2004;10:3444–7. doi: 10.1158/1078-0432.CCR-03-0562. [DOI] [PubMed] [Google Scholar]
- 10.Ichii-Nakato N, Takata M, Takayanagi S, et al. High frequency of BRAFV600E mutation in acquired nevi and small congenital nevi, but low frequency of mutation in medium-sized congenital nevi. J Invest Dermatol. 2006;126:2111–18. doi: 10.1038/sj.jid.5700366. [DOI] [PubMed] [Google Scholar]
- 11.Vasko V, Hu S, Wu G, et al. High prevalence and possible de novo formation of BRAF mutation in metastasized papillary thyroid cancer in lymph nodes. J Clin Endocrinol Metab. 2005;90:5265–9. doi: 10.1210/jc.2004-2353. [DOI] [PubMed] [Google Scholar]