Abstract
Ultraviolet (UV) can be highly beneficial in the treatment of skin disorders such as psoriasis. It is thought to cause immunosuppression by depleting or altering the function of epidermal Langerhans cells (LC). Our previous studies identified a novel langerin+ dendritic cell in the dermis, distinct from LC in phenotype, circulation, and function. In this study, we determined the role of LC and dermal langerin+ cells in UV suppression. UV suppressed the CD8 T cell response to both CHS and epicutaneous protein immunization, and resulted in a dramatically altered phenotype of LC. UV did not alter early CD8 T cell activation in the lymph nodes, but rather reduced CD8 T cell expansion at later time points. We found that dermal langerin+ cells, but not LC, were essential for the CD8 T cell response. Furthermore, in the selective absence of LC, ultraviolet light still caused suppression of both CD8 T cell expansion and CHS.
Keywords: Skin, Dendritic cells, Tolerance/Suppression
INTRODUCTION
Ultraviolet (UV) light therapy is commonly used to treat skin disorders such as psoriasis. This effect is thought to be due to UV induced immunosuppression (1, 2). UV suppression is most commonly studied in mice using the contact hypersensitivity (CHS) model to hapten. Epidermal Langerhans cells (LC) are the predominant dendritic cell (3) subset in the epidermis and thus are thought to be an important antigen presenting cell (APC) affected by UV in this model (4). UV irradiation was first reported to decrease the abundance of epidermal LC in the skin in 1980 (5), and this was corroborated and extended in other studies, which showed that UV enhances the migration of epidermal LC from skin to subcutaneous lymph nodes (6). Furthermore, LC that were UV treated in isolation and transferred back into animals were able to suppress CHS (7). Since then it has been generally accepted that LC are critical for UV induced immunosuppression.
In our previous research, we identified a novel subset of langerin+ CD103+ DC in the skin, which are distinct from epidermal LC in terms of phenotype, microanatomic localization, and circulation (8). This was confirmed by two other independent studies (9, 10). Unlike the conventional concept that epidermal LC were the only langerin+ DC in skin and that they could be found in the dermis when migrating to the lymph nodes, this newly characterized bone marrow derived dermal langerin+ CD103+ DC constitutively resides in the dermis. Furthermore, two thirds of langerin+ DC in the subcutaneous lymph nodes are derived from dermal langerin+ DC, and one third are derived from epidermal Langerhans cells (8). Importantly, we showed that dermal langerin+ DC have distinct biological functions from epidermal Langerhans cells, and were able to promote a CD8 T cell response to OVA protein through epicutaneous immunization, and the contact hypersensitivity response to hapten, even in the absence of epidermal LC (8, 11). In contrast, evidence is emerging that epidermal Langerhans cells may also regulate or suppress immune responses (12, 13). Mice that lack epidermal Langerhans cells, but still retain dermal Langerin+ DC, show an enhanced contact hypersensitive response to hapten (14) and skin grafts from these mice are more efficiently rejected (12).
Thus, in order to better understand the therapeutic and pathogenic effects of UV irradiation, we wanted to clarify the roles of both epidermal LC and dermal langerin+ DC in promoting skin immune responses and in UV induced immunosuppression. To examine this question we first investigated the UV induced alteration of epidermal LC using the Lang-EGFP mice, a knock-in mouse with the enhanced GFP (EGFP) expressed in the langerin locus (15). In addition, we conditionally depleted either epidermal LC or dermal langerin+ DC, and investigated the ability of UV irradiation to suppress contact hypersensitivity or the CD8 T cell response to OVA protein applied on the skin. We found that dermal langerin+ DC are required for both responses. In contrast, epidermal Langerhans cells were not required for either response, or for UV suppression of the response.
MATERIALS AND METHODS
Animals
C57BL/6 (B6) mice were purchased from the National Cancer Institute and bm1 mice were purchased from Jackson Laboratory. Lang-EGFP and Lang-DTR knock-in mice were previously described (15). OT-I TCR transgenic mice (16) were crossed to B6.PL-Thy1a mice to generate Thy1.1 congenic OT-I.PL. All mice were treated in accordance with federal guidelines, and protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee.
UVB irradiation
UVB irradiation was provided by a bank of two TL 20W/12RS lamps (Philips, Eindhoven, and Holland). Mice were anaesthetized with ketamine and xylazine and the shaved area (the sides of the flanks in epicuteneous immunization and the backs in the CHS experiment) was exposed to 45 mJ/cm2 UVB daily for 3 consecutive days. The ears and eyes of the mice were protected from UVB irradiation.
T cell adoptive transfer and epicutaneous immunization
These procedures were conducted as previously described (11). CD44lo (naïve phenotype) CD8+ OT-I.PL T cells were purified by negative depletion using magnetic cell sorting MACS (Miltenyi Biotec) and labeled with CFSE (Molecular Probes). A total of 2.5 × 105, or 2 ×106 purified cells were intravenously injected into recipient mice. On the day of immunization mice were anesthetized and the flanks were hydrated for 15 min with water. The hydrated areas were painted with 10 μg OVAp or 469 μg OVA in 25 μl PBS (Sigma-Aldrich). These mice were then covered with an occlusive patch (DuoDERM Extra Thin, ConvaTec).
Preparation of epidermal suspension
Epidermal cell suspensions from flank skin were prepared by limited trypsinization and dissociation of epidermal sheets by pipetting in DNase, as previously described (8). Epidermal Langerin+ cells were identified after this digestion by expression of EGFP.
Radiation bone marrow chimeras
Single-cell suspensions of bone marrow were prepared and depleted of mature T cells by complement-mediated cytotoxicity with 30H12 (anti-Thy1.2; American Type Culture Collection), as previously described (8). Bone marrow cells were injected i.v. (5 × 106 cells/recipient) into lethally irradiated recipient mice (1000 rads).
In vivo depletion of Langerin+ cells
In all experiments, Langerin+ cells were ablated by injection of 1 μg diphtheria toxin (DT) i.p. either on day −4 and −1 or on day −7, with day 0 defined as the day of immunization.
CHS
All mice were shaved at least 1 day before immunization. 25 μl of 0.3% DNFB (Sigma-Aldrich) in a mixture of acetone and olive oil (4:1) was painted on the backs of the mice. On day 5, all mice were challenged with 5 μl of 0.15% DNFB on both sides of one ear. Ear thickness was measured before and 24 h after challenge, with a spring-loaded micrometer (Mitutoyo).
RESULTS
Ultraviolet irradiation suppresses the CD8 T cell response to epicutaneous immunization with OVA protein
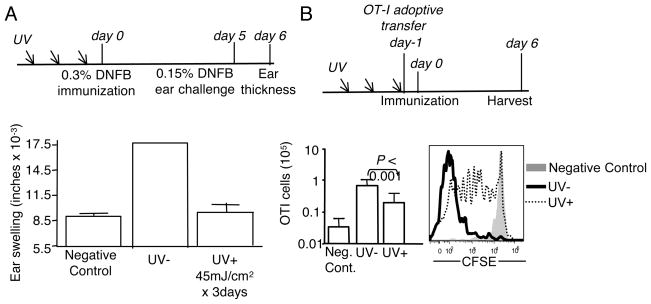
UV suppression is commonly studied in animal models of CHS to hapten. However, because it is difficult to track and study antigen specific T cells in this model, we wanted to determine if UV could suppress the CD8 T cells response to epicutaneously applied protein. We employed a hapten contact hypersensitivity model to determine the optimal dose of UVB to use for suppression of CD8 T cell dependent immune responses. C57BL/6 mice were primed and challenged with hapten as shown (Fig. 1A). The immune response to DNFB measured by ear swelling was routinely decreased between >85% by UV at this dose (Fig. 1A). We split the administration of UV over 3 days to avoid the occasional skin lesions observed with a single dose. This UV irradiation dose and timing was then applied for all of the experiments (Fig. 1B). Epicutaneous ovalbumin (OVA) protein elicits a modest CD8 T cell proliferative response that can be tracked by adoptive transfer of CD8 T cells from ovalbumin-specific TCR transgenic mice (OT-I) (11). Thus, mice were adoptively transferred with 2.5 × 105 OT-I CD8 T cells immediately after the third UV irradiation dose, and then immunized with OVA protein on the skin surface. The immune response was checked 6 days after immunization (Fig. 1B). UV irradiation inhibited the CD8 T cell response to this protein immunogen. In the UV treated group, about 15–20% of transferred OT-I cells remained undivided, and the expansion was reduced about 5 fold in comparison to those in the un-treated group (Fig. 1B).
Figure 1. UV reduces the CD8 T cell response to epicutaneous OVA protein immunization.
(A) Groups of mice were irradiated consecutively for 3 days with 45 mJ/cm2 UV at each time point, then immunized and challenged according to the experimental scheme shown. DNFB immunization was performed on the flank, while challenge was on the ear. Ear thickness was measured 24 hours later. Error bars show the standard deviation, n=3, and is representative of at least 4 independent experiments. (B) As in (A), but mice were intravenously injected with 2.5 × 105 CFSE labeled CD44lo (naïve) OT-I.PL CD8 T cells and 24 hours later epicutaneously immunized with OVA protein. Mice were then harvested 6 days after immunization. Total OT-I CD8 T cell numbers from spleen and subcutaneous lymph nodes were determined using flow cytometric analysis. Data is expressed as the mean ± SD. n=18–24 mice/group from 8 independent experiments. The CFSE histogram indicated the division of OT-I CD8 T cells.
UV irradiation did not deplete epidermal Langerhans cells, or prevent T cell activation in the lymph nodes, but altered Langerhans cell phenotype
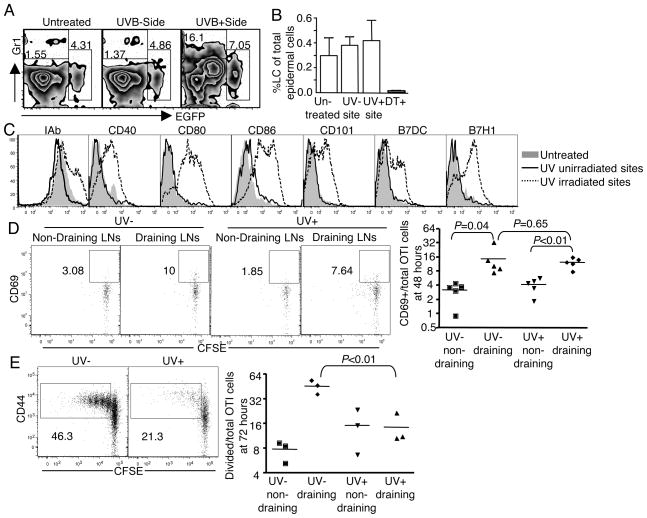
The association of CHS suppression with the disappearance of epidermal LC induced by UV irradiation was first shown in 1980 (5). Since then epidermal LC have been generally accepted to be one of the mediators in UV induced immunosuppression. Further studies suggested that the epidermal LC disappearance might result from UV induced DNA damage (17) and impaired migration from epidermis to subcutaneous lymph nodes as well (6). In addition, UV irradiation was observed to alter the phenotype of epidermal LC including the reduction of ATPase and Class II (18), inhibition of ICAM-1 (19) and reduced expression of the costimulatory molecules B7-1 and B7-2 (20). In this study, we used the lang-EGFP mice created by Kissenpfenning and his colleagues (15) to investigate the effect of UV on epidermal LC in vivo. In these mice, enhanced GFP (EGFP) was expressed under the control of langerin promoter and EGFP expression accurately reflects langerin expression. One side of the lang-EGFP mouse was irradiated, while the other side protected from UV as control. Epidermal suspensions from both sides were prepared and stained separately 24 hours after the last UV irradiation. Unlike previous reports that UV depleted epidermal LC, UV irradiation under these conditions did not reduce the abundance of epidermal LC, comparing the percentage of EGFP+ cells to those from the un-irradiated side or an un-irradiated control animal (Fig. 2, A and B). However, UV irradiation did upregulate the expression of Class II MHC and other costimulatory molecules (Fig. 2C). The absence of LC depletion is likely due to the UV dose employed, as LC depletion is highly sensitive to dose in this range (18), and we sought to use the lowest possible dose that still gave suppression, in order to avoid direct damage to the skin. It is possible that LC depletion could have occurred after 24 hours, but we chose 24 hours for the analysis, as that was the time point at which animals were epicutaneously immunized.
Figure 2. UV does not deplete epidermal LC, or prevent T cell activation in the lymph nodes, but alters LC phenotype.
(A) Lang-EGFP transgenic mice were used to identify epidermal LC, in which all of epidermal LC were EGFP+ cells. One side of the mouse was irradiated with UV as described in Figure 1, with the other side protected from irradiation. 24 hours after the last irradiation, epidermal suspension from both sides was prepared separately and EGFP+ epidermal LC and Gr1+ cells were gated in the CD45+ hematopoietic cells gate. (B) The bar graph shows the calculated percentage of epidermal LC for 4 mice/group (mean ± SD). The “DT+” control was the Lang-EGFP-DTR mice treated with two dose of 1 μg diphtheria toxin i.p. at 96 and 24 hours before harvest. The data are representative of 2 experiments. (C) Representative histogram from 3 experiments is shown for the phenotype of epidermal LC. (D, E) Mice were transferred with 2 × 106 CFSE labeled CD44 low OT-I CD8 T cells flowed by OVA protein immunization on the UV irradiated side of the flank. OT-I cells both from the draining lymph nodes and non-draining nodes were collected 48 (D) and 72 hours (E) after immunization. The plot shows CFSE dilution by CD69 at the early time point (D), or CD44 at the later time point (E). Graphs show the calculated percentage of activated OT-I CD8 T cells.
The alteration of phenotype indicated that UV irradiation skewed epidermal LC toward an activated or mature state. We therefore tested whether these UV conditioned epidermal LC resulted in greater CD8 T cell activation in the lymph node. In epicutaneously immunized mice, CD69 on antigen specific CD8 T cells was upregulated in the draining lymph nodes 48 hours after immunization, while in the same mouse, CD69 from the non-draining lymph nodes remained at the background level (Fig. 2D), as did CD69 on the non-antigen specific CD8 T cells (data not shown). However, there was no significant difference between UV irradiated and unirradiated animals in terms of CD69 upregulation in lymph nodes at this time point (Fig. 2D left). This suggests that the tempo of DC migration and initiation of T cell activation are not grossly altered by UV irradiation. However, 72 hours after immunization, only about 14±3.5 % of CD8 T cell in the draining nodes had divided (CFSE low) in UV irradiated animals in comparison to 45±5% divided in unirradiated animal, indicating a significant reduction caused by UV (Fig. 2E). This selective effect on cell division, in the face of relatively normal initial activation is consistent with a potential role for regulatory T cells, as suggested by other studies (21, 22).
Bone marrow derived (dermal) langerin+ cells are essential for epicutaneous immunization to OVA protein
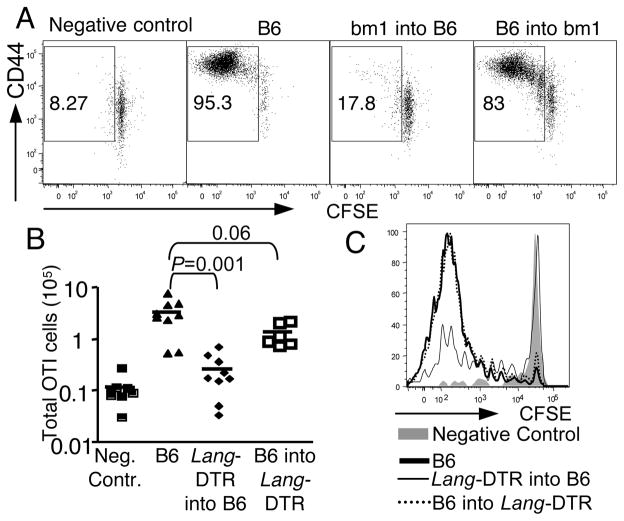
Epidermal LC had been thought to be the principal APC in the skin to promote both innate and adaptive immune responses (23, 24). However, this has been challenged recently by analysis of CHS in mice where epidermal LC were either conditionally depleted (15, 25) or permanently ablated (14). The latter mice lack epidermal LC from birth, and CHS was actually increased (14), suggesting that LC suppress and not promote CHS. In the conditional deficiency models, one group found CHS was decreased (25), while the other group did not observe any change (15). We and others subsequently identified a novel population of dermal langerin+ cells and showed that this population was affected in the conditional deficient strain (8–10). Furthermore, we found that the dermal langerin+ DC were sufficient to promote CHS (8) and the response to OVA protein when epicutaneously applied (11). However, all of the studies had been done under conditions where epidermal LC were also absent. There was no data examining the exclusive role of dermal langerin+ cells. To directly characterize the role of epidermal LC versus dermal langerin+ DC, we took advantage of the fact that dermal langerin+ DC are radiosensitive while epidermal LC are radioresistant (8–10, 26). We reconstituted bone marrow ablated C57BL/6 mice with bone marrow from bm1 mice, a C57BL/6 mouse strain carrying a mutant Kb molecule that cannot not present SIINFEKL peptide to OT-I CD8 T cells. In this bone marrow chimera, the radio-resistant epidermal LC were the only dendritic cells capable of presenting the OVA protein. After epicutaneous immunization with OVA protein, we observed almost no OT-I proliferation in bm1→B6 chimeras (20±3% OT-I CD8 T cells were divided) compared to the reverse chimeras B6→bm1, where the extent of division (83±3%) was similar to the positive control (93%) (Fig. 3A). These data suggest that the CD8 T cell response to epicutaneous ovalbumin requires recognition of antigen presented by a bone marrow derived cell.
Figure 3. Bone marrow derived (dermal) langerin+ DC are essential for epicutaneous immunization.
(A) 2.5 × 105 CFSE labeled CD44lo OT-I CD8 T cells were adoptively transferred into bone marrow chimeric mice whose ability to present OVA protein was limited either to radioresistant epidermal LC (bm1 into B6) or to bone marrow derived DC (B6 into bm1). Representative plot from 3 experiments shows CFSE dilution 6 days after immunization. (B) As in (A) except that bone marrow chimeras of Lang-DTR into B6 mice and B6 into Lang-DTR mice were treated with two dose of 1 μg diphtheria toxin i.p. at 96 and 24 hours before immunization. The bar graph shows OT-I CD8 T cell expansion. (C) Histogram shows the OT-I CD8 T cell division 6 days after immunization.
To directly test the contribution of langerin + cells, we reconstituted lethally irradiated B6 mice with bone marrow from Lang-DTR mice, a mouse where the diphtheria toxin receptor (DTR) was introduced into the endogenous langerin locus, and in which both epidermal LC and dermal langerin+ DC can be acutely depleted by administration of diphtheria toxin (15). In this bone marrow chimeric setting, either epidermal LC (in the case of B6→LangDTR) or dermal langerin+ DC (in the case of LangDTR→B6) could be selectively eliminated after diphtheria toxin treatment, while with the other potential antigen presenting DC network remained unchanged. In the absence of dermal langerin+ DC, OT-I CD8 T cells showed minimal expansion (Fig. 3B) and proliferation (Fig. 3C) following epicutaneous OVA immunization. However, a nearly equivalent CD8 T cell response was primed in the absence of epidermal LC, with full cell division. Collectively, our findings indicated that dermal langerin+ DC, but not epidermal LC or any other DC population, are essential for the CD8 response to epicutaneous immunization with OVA protein.
UV suppresses the CD8 T cell response to epicutaneous antigen even in the absence of epidermal Langerhans cells
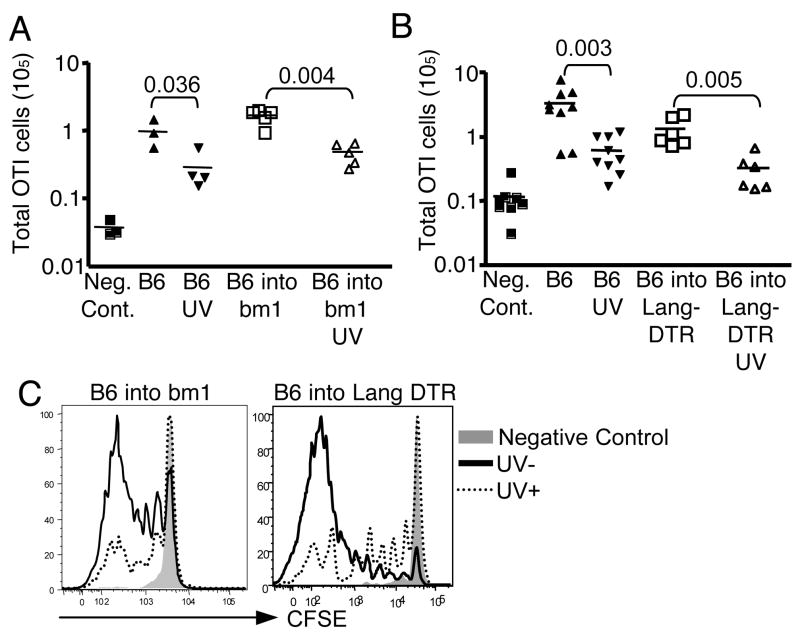
Given that epidermal LC were not required to prime a CD8 T cell response to epicutaneous OVA (Fig. 3B), we were able to evaluate whether these cells are required for UV suppression of the response. We tested this hypothesis using chimeric mice. Bone marrow chimeric mice were UV irradiated on 3 consecutive days as described in Fig. 1B, followed by cell transfer and OVA protein immunization. CD8 T cell expansion and division were measured 6 days later after immunization. UV suppressed the response to OVA to a similar extent in both B6→bm1 and B6→Lang-DTR chimeras as in control mice (Fig. 4A, B, and C). The inhibition was indicated both by the CD8 T cell expansion (Fig. 4A and B) and cell division (Fig. 4C). This result suggests that neither LC antigen presentation nor epidermal LC themselves are required to mediate UV suppression.
Figure 4. UV suppresses the CD8 response to epicutaneous antigen even in the absence of epidermal LC.
(A) The individual mice and chimeras were irradiated with UV, transferred with 2.5 × 105 CFSE labeled CD44lo OT-I CD8 T cells and then immunized with OVA protein. OT-I CD8 T cell expansion was calculated 6 days later. (B) As in (A) but all of the bone marrow chimeric mice were treated with diphtheria toxin 96 and 24 hours before immunization. One group of mice was irradiated with UV and followed with OT-I CD8 T cells transfer and immunization. The graph shows OT-I CD8 T cell expansion 6 days after immunization. (C) Representative histograms show CFSE dilution of CD8 T cells from 3 experiments. n=3 mice/group
UV suppressed contact hypersensitivity in the absence of epidermal Langerhans cells
UV induced immunosuppression has been generally tested using the CHS model, thus we wished to test the role of epidermal Langerhans cells in that system. Our previous data demonstrated that dermal langerin+ DC are required for promoting the CHS to hapten, similar to that shown above for ovalbumin (8). Furthermore, previous data showed that after the depletion of all langerin+ cells with diphtheria toxin, dermal langerin+ DC started returning to the dermis after three days, continually increasing and by day 7 repopulated about 50% (8). In contrast, epidermal LC remained >95% depleted at day 7. Replenishment was not observed until at least 2 weeks (8, 15), and did not reach 50% even 28 days later (data not shown). Thus, we herein took advantage of the time window of repopulation in the skin to test the hypothesis that UV suppression of CHS requires epidermal LC. Lang-DTR mice were treated with 1 μg diphtheria toxin i.p. 7 days before immunization to create conditions where epidermal LC were absent, but the dermal langerin+ cell compartment was partially recovered. Mice were UV irradiated, immunized with DNFB and then challenged with low dose DNFB. The immune response measured by ear swelling was significantly reduced in comparison to the UV unirradiated group in both the diphtheria toxin treated mice, as well as controls (Fig. 5). Thus, epidermal LC are not required for UV induced immunosuppression of CHS either.
Figure 5. UV suppresses contact hypersensitivity even in the absence of epidermal Langerhans cells.
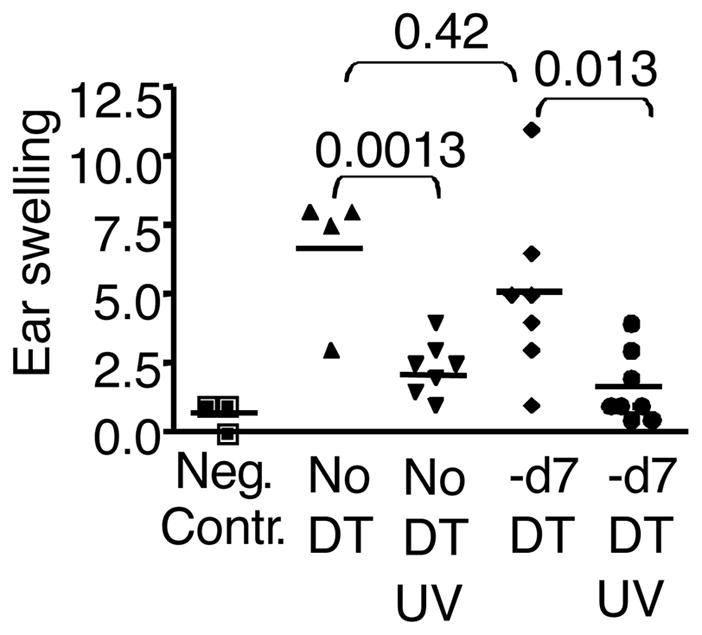
Lang-DTR mice were untreated or treated with diphtheria toxin 7 days prior to treatment to selectively deplete epidermal LC, while allowing dermal DC to repopulate. Mice were then irradiated with UV for 3 consecutive days, and 24 hours after the last UV treatment, immunized with 25 μl of 0.3% DNFB on the shaved back. 5 days after immunization, mice were challenged with 5 μl of 0.15% DNFB on the ear. Ear thickness was measured before and 24 hour after challenge. The immune response= ear swelling (after challenge-before challenge).
DISCUSSION
The finding that UV suppression still occurs in the absence of LC is somewhat surprising, as LC have been suggested to be an important target of UV suppression. Their numbers (5, 27), migration (3, 28–30), phenotype (18–20), and antigen presenting properties (19, 20) have all been shown to be altered by UV treatment. These are correlative findings, of course. However, a gain of function approach, where LC were UV irradiated and loaded with hapten in vitro, resulted in suppression of immune responses upon reintroduction into animals (7). Furthermore, other gain of function approaches suggested that LC can influence the migration of suppressive Treg cells (22). In contrast, our loss of function experiments clearly showed that LC are not required for UV suppression. One possibility is that there is more than one mechanism by which UV causes immune suppression. Indeed, several soluble mediators have been shown to contribute to UV suppression, including IL-10 (31–33), TNFα (31), calcitonin gene-related peptide (31), cis-urocanic acid (34), prostaglandins (35), and others (4). Although epidermal LC can produce some of these, other cell types may as well, including keratinocytes. Furthermore, the induction of Treg has been suggested to play a role in UV suppression (36, 37) and the induction of a Treg response could occur independently of LC. Thus although LC may contribute to UV suppression, they are not required for it, at least in this model.
In this study we also report that dermal langerin+ cells are essential for epicutaneous immunization to OVA protein. This is consistent with previous results from our lab showing that dermal langerin+ CD103+ DC were sufficient to promote CHS (8) and the response to OVA protein when epicutaneously applied (11). But we here further show that dermal langerin+ DC, but not epidermal LC or any other DC population, are essential for the CD8 response to epicutaneous immunization with OVA protein. A profound requirement for dermal langerin+ DC is somewhat surprising given that only about half of the dendritic cells in the dermis are langerin+. However, we and others have noted a slight difference in the localization of langerin+ DC in the dermis, compared to other DC (generally more peri-follicular) (8, 10). Langerin+ DC were recently shown to be required for the CD8 T cell response to influenza in the lung (38), despite the presence of several other dendritic cell types, and dermal langerin+ DC were required for optimal production of beta-galactosidase-specific IgG2a/c and IgG2b after gene gun immunization (39). Finally, Heath and colleagues recently showed that langerin+ CD103(+) dermal DCs are the main migratory subtype able to cross-present viral and self antigens (40). Thus, the langerin+ CD103+ interstitial DC population may be specialized for carrying antigens to local lymph nodes and initiating CD8 T cell responses.
Because of the unique functions ascribed to the langerin+ CD103+ dermal DC subset in the mouse, it will be important to determine if human skin bears a population that is functionally equivalent. Three prominent DC populations have been described in human skin, epidermal Langerhans cells, which are CD1a+ langerin+, and two dermal DC populations: one that is CD1a+ CD14−, the other that is CD14+CD1a− (41). Human dermal CD1a+ DC might be equivalent to dermal langerin+ DC in the mouse, as they have been shown to be distinct from migrating epidermal LC (42). However, the expression of Langerin is not prominent on either dermal DC subset and the expression of CD103 has not been reported. Further analysis is clearly needed on this subject.
In summary, we show here that UV irradiation with the dose used in this study did not reduce the abundance of epidermal LC, but did alter their phenotype and presumed activation status. However, this alteration did not affect the initiation of a CD8 T cell response in the lymph node. This is likely due to the fact that dermal Langerin+ DC, but not epidermal LC are essential for initiating the CD8 T cell response to protein and hapten applied to the skin. Finally, UV was fully effective in inhibiting the CD8 T cell response to protein and hapten, even in the absence of epidermal LC. These findings are important in the context of attempts to develop epicutaneous vaccine strategies, and they improve our understanding of the therapeutic and pathogenic aspects of UV induced immunosuppression.
Acknowledgments
The authors wish to thank Dan Kaplan and Laura Bursch for critical input and advice on the manuscript, and Xiao Jie Ding and Brian Goudy for technical support. We are grateful to Bernard Malissen for developing and making the Lang-DTR and Lang-EGFP mice available to us.
GRANT SUPPORT
This work was supported by NIH grants P01 AI 35296 to KAH and U01 AI 70380 to SCJ.
Abbreviations used in this paper
- CHS
contact hypersensitivity
- UV
ultraviolet
- LC
langerhans cell
- DC
dendritic cell
- EGFP
enhanced green fluorescent protein
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
Footnotes
This work was supported by NIH grants P01 AI 35296 to KAH and U01 AI 70380 to SCJ.
DISCLOSURES
The authors have no financial conflict of interest.
References
- 1.Erkin G, Ugur Y, Gurer CK, Asan E, Korkusuz P, Sahin S, Kolemen F. Effect of PUVA, narrow-band UVB and cyclosporin on inflammatory cells of the psoriatic plaque. J Cutan Pathol. 2007;34:213–219. doi: 10.1111/j.1600-0560.2006.00591.x. [DOI] [PubMed] [Google Scholar]
- 2.Stern RS. Psoralen and ultraviolet a light therapy for psoriasis. N Engl J Med. 2007;357:682–690. doi: 10.1056/NEJMct072317. [DOI] [PubMed] [Google Scholar]
- 3.Dandie GW, Clydesdale GJ, Radcliff FJ, Muller HK. Migration of Langerhans cells and gammadelta dendritic cells from UV-B-irradiated sheep skin. Immunol Cell Biol. 2001;79:41–48. doi: 10.1046/j.1440-1711.2001.00975.x. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutation research. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 5.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 6.Kolgen W, Both H, van Weelden H, Guikers KL, Bruijnzeel-Koomen CA, Knol EF, van Vloten WA, De Gruijl FR. Epidermal langerhans cell depletion after artificial ultraviolet B irradiation of human skin in vivo: apoptosis versus migration. The Journal of investigative dermatology. 2002;118:812–817. doi: 10.1046/j.1523-1747.2002.01742.x. [DOI] [PubMed] [Google Scholar]
- 7.Cruz PD, Jr, Tigelaar RE, Bergstresser PR. Langerhans cells that migrate to skin after intravenous infusion regulate the induction of contact hypersensitivity. J Immunol. 1990;144:2486–2492. [PubMed] [Google Scholar]
- 8.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. The Journal of experimental medicine. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. The Journal of experimental medicine. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. The Journal of experimental medicine. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 12.Obhrai JS, Oberbarnscheidt M, Zhang N, Mueller DL, Shlomchik WD, Lakkis FG, Shlomchik MJ, Kaplan DH. Langerhans Cells Are Not Required for Efficient Skin Graft Rejection. The Journal of investigative dermatology. 2008;128:1950–1955. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. European journal of immunology. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. The Journal of experimental medicine. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aberer W, Schuler G, Stingl G, Honigsmann H, Wolff K. Ultraviolet light depletes surface markers of Langerhans cells. The Journal of investigative dermatology. 1981;76:202–210. doi: 10.1111/1523-1747.ep12525745. [DOI] [PubMed] [Google Scholar]
- 19.Tang A, Udey MC. Inhibition of epidermal Langerhans cell function by low dose ultraviolet B radiation. Ultraviolet B radiation selectively modulates ICAM-1 (CD54) expression by murine Langerhans cells. J Immunol. 1991;146:3347–3355. [PubMed] [Google Scholar]
- 20.Weiss JM, Renkl AC, Denfeld RW, de Roche R, Spitzlei M, Schopf E, Simon JC. Low-dose UVB radiation perturbs the functional expression of B7.1 and B7.2 co-stimulatory molecules on human Langerhans cells. Eur J Immunol. 1995;25:2858–2862. doi: 10.1002/eji.1830251022. [DOI] [PubMed] [Google Scholar]
- 21.Ghoreishi M, Dutz JP. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4+CD25+ T regulatory cells and is dependent on host-derived IL-10. J Immunol. 2006;176:2635–2644. doi: 10.4049/jimmunol.176.4.2635. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz A, Maeda A, Schwarz T. Alteration of the migratory behavior of UV-induced regulatory T cells by tissue-specific dendritic cells. J Immunol. 2007;178:877–886. doi: 10.4049/jimmunol.178.2.877. [DOI] [PubMed] [Google Scholar]
- 23.Stingl G, Tamaki K, Katz SI. Origin and function of epidermal Langerhans cells. Immunol Rev. 1980;53:149–174. doi: 10.1111/j.1600-065x.1980.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 24.Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells - dendritic cells of the epidermis. Apmis. 2003;111:725–740. doi: 10.1034/j.1600-0463.2003.11107805.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shklovskaya E, Roediger B, Fazekas de St Groth B. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J Immunol. 2008;181:418–430. doi: 10.4049/jimmunol.181.1.418. [DOI] [PubMed] [Google Scholar]
- 27.Obata M, Tagami H. Alteration in murine epidermal Langerhans cell population by various UV irradiations: quantitative and morphologic studies on the effects of various wavelengths of monochromatic radiation on Ia-bearing cells. The Journal of investigative dermatology. 1985;84:139–145. doi: 10.1111/1523-1747.ep12275379. [DOI] [PubMed] [Google Scholar]
- 28.Moodycliffe AM, Kimber I, Norval M. The effect of ultraviolet B irradiation and urocanic acid isomers on dendritic cell migration. Immunology. 1992;77:394–399. [PMC free article] [PubMed] [Google Scholar]
- 29.Sontag Y, Guikers CL, Vink AA, de Gruijl FR, van Loveren H, Garssen J, Roza L, Kripke ML, van der Leun JC, van Vloten WA. Cells with UV-specific DNA damage are present in murine lymph nodes after in vivo UV irradiation. The Journal of investigative dermatology. 1995;104:734–738. doi: 10.1111/1523-1747.ep12606971. [DOI] [PubMed] [Google Scholar]
- 30.McLoone P, Woods GM, Norval M. Decrease in langerhans cells and increase in lymph node dendritic cells following chronic exposure of mice to suberythemal doses of solar simulated radiation. Photochemistry and photobiology. 2005;81:1168–1173. doi: 10.1562/2005-04-10-RA-484. [DOI] [PubMed] [Google Scholar]
- 31.Rivas JM, Ullrich SE. The role of IL-4, IL-10, and TNF-alpha in the immune suppression induced by ultraviolet radiation. Journal of leukocyte biology. 1994;56:769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 32.Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, Kripke ML. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ullrich SE. The role of epidermal cytokines in the generation of cutaneous immune reactions and ultraviolet radiation-induced immune suppression. Photochemistry and photobiology. 1995;62:389–401. doi: 10.1111/j.1751-1097.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 34.De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. The Journal of experimental medicine. 1983;158:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shreedhar V, Giese T, Sung VW, Ullrich SE. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]
- 36.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochemistry and photobiology. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 37.Schade N, Esser C, Krutmann J. Ultraviolet B radiation-induced immunosuppression: molecular mechanisms and cellular alterations. Photochem Photobiol Sci. 2005;4:699–708. doi: 10.1039/b418378a. [DOI] [PubMed] [Google Scholar]
- 38.Geurtsvan Kessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. The Journal of experimental medicine. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, Merad M, Udey MC. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature immunology. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 41.Haniffa M, Ginhoux F, Wang XN, Bigley V, Abel M, Dimmick I, Bullock S, Grisotto M, Booth T, Taub P, Hilkens C, Merad M, Collin M. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. The Journal of experimental medicine. 2009;206:371–385. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angel CE, George E, Brooks AE, Ostrovsky LL, Brown TL, Dunbar PR. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol. 2006;176:5730–5734. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]