Abstract
Background
While much is known about the role of prefrontal cortex (PFC) in working memory (WM) deficits of schizophrenia, the nature of the relationship between cognitive components of WM and brain activation patterns remains unclear. We aimed to elucidate the neural correlates of the maintenance component of verbal WM by examining correct and error trials with event-related fMRI.
Methodology/Findings
Twelve schizophrenia patients (SZ) and thirteen healthy control participants (CO) performed a phonological delayed-matching-to-sample-task in which a memory set of three nonsense words was presented, followed by a 6-seconds delay after which a probe nonsense word appeared. Participants decided whether the probe matched one of the targets, and rated the confidence of their decision. Blood-oxygen-level-dependent (BOLD) activity during WM maintenance was analyzed in relation to performance (correct/error) and confidence ratings. Frontal and parietal regions exhibited increased activation on correct trials for both groups. Correct and error trials were further segregated into true memory, false memory, guess, and true error trials. True memory trials were associated with increased bilateral activation of frontal and parietal regions in both groups but only CO showed deactivation in PFC. There was very little maintenance-related cortical activity during guess trials. False memory was associated with increased left frontal and parietal activation in both groups.
Conclusion
These findings suggest that a wider network of frontal and parietal regions support WM maintenance in correct trials compared with error trials in both groups. Furthermore, a more extensive and dynamic pattern of recruitment of the frontal and parietal networks for true memory was observed in healthy controls compared with schizophrenia patients. These results underscore the value of parsing the sources of memory errors in fMRI studies because of the non-linear nature of the brain-behavior relationship, and suggest that group comparisons need to be interpreted in more specific behavioral contexts.
Introduction
Working memory (WM) deficit in schizophrenia is a cardinal feature of the disorder and is a potential candidate for an endophenotypic marker [1]. WM is a limited-capacity, active short-term memory system that guides and controls behavior in context [2], [3]. A majority of patients with schizophrenia show stable WM deficits [4] across diverse paradigms, modalities and methods [5]. Impaired verbal WM predicts poor functional outcome [6] and WM deficits have become a major therapeutic target for pharmacological treatments. Therefore it has become increasingly important to understand and specify the reasons for this deficit.
Clear evidence exists for the central role of the dorsolateral prefrontal cortex (DLPFC) in WM and its regulation of higher cognitive functions in non-human primates [7]. Past studies using single cell recording revealed that maintenance of WM representations is coded by increased firing rate of cells in the principal sulcus (PS, Area 46) and this robust increase of prefrontal activity during WM maintenance is correlated with accuracy of the task performance [8]–[10]. Similarly, WM accuracy is correlated with increased DLPFC activation in healthy humans in neuroimaging studies [11]–[13]. However, numerous neuroimaging studies of WM have demonstrated task-related hypofrontality in schizophrenia patients [14], [15]. On the other hand, some studies have also observed hyperfrontality in schizophrenia [16], [17]. This discrepancy may arise from different WM loads across studies [18]. In healthy people DLPFC activity increases with WM load until the capacity of WM is exceeded at which point, it decreases [19], [20]. This relationship between WM load and DLPFC activity, often described as an inverted U, appears to be shifted in schizophrenia patients such that peak DLPFC activation is reached at a lower memory load compared with healthy controls. This hypothesis is supported by studies that demonstrate increased DLPFC activation in individuals with schizophrenia relative to controls for lower WM load [14], [16], [17] but reduced DLPFC activation with higher WM load [21], [22]. These findings have been interpreted as evidence for an inefficient WM system in schizophrenia such that they must “work harder” to maintain accuracy as WM load increases [14], [18], [22], [23].
One difficulty in interpreting these discrepant results is that very few studies have examined neural activity yoked to behavior on a trial-by-trial basis using an event-related design; the majority of fMRI studies of WM in schizophrenia have utilized block designed tasks that do not allow analyses of neural activation linked with specific type of responses.
Recently, Lee and colleagues [13] conducted an event-related fMRI and near-infrared spectroscopy (NIRS) study of spatial WM in schizophrenia to investigate prefrontal activation associated with correct and incorrect memory trials during WM maintenance. The rationale of this study follows from the known neural correlates of success and failure during WM tasks in non-human primates; the increased firing rates of PS cells are correlated with WM maintenance on the trials that the targets are remembered correctly but not on error trials [8]–[10]. Lee et al [13] observed increased prefrontal activation during WM maintenance on correct trials in both controls and patients. However, healthy controls recruited right frontal and parietal regions, consistent with a right hemisphere specialization for spatial processing [24]. On the other hand, schizophrenia patients showed a more bilateral frontoparietal activation pattern. Furthermore, they found that schizophrenia patients produced a large proportion of “false memory” errors (i.e. incorrect response with high confidence). Frontoparietal regions were recruited equally for false and correct memory trials, suggesting active maintenance of internal representation during the delay whether that representation was correctly or incorrectly encoded. This finding suggests that hyper or hypofrontality in schizophrenia may need to be re-interpreted. For example, hyperfrontality coupled with increased verbal WM errors in schizophrenia patients [16] is often interpreted in the context of general “inefficiency”. The concept of inefficiency could be further refined by distinguishing the case where there is unspecific increased neural activity versus the case where there is a specific increase in activity due to the maintenance of incorrectly encoded material. The former case would signify a true case of general inefficiency but the latter represents appropriate maintenance of incorrectly encoded stimulus. Both cases would look similar on the surface (i.e., hyperactivity coupled with WM errors). The crucial difference is that in the latter case, although the participant had an encoding error, the maintenance process itself is intact. It is possible that many WM errors made by persons with schizophrenia could arise because they maintain incorrectly encoded target representations. In this case, the problem would lie in the encoding process and not in the maintenance, and a general inefficiency hypothesis would not provide an optimal model.
The major goal of the present study was to elucidate the neural correlates of success and failure during verbal WM performance using an event-related design. Lee et al. [13] focused on spatial WM. In addition, they observed that schizophrenic patients tended to show both left and right frontal activity during spatial WM maintenance compared with the control participants, who showed a more right-lateralized network of activity during spatial WM maintenance. It would be important to ascertain if these findings generalize to the verbal domain.
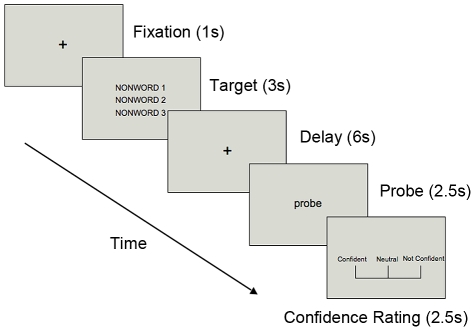
In the present experiment, we compared cortical activation in schizophrenia patients and healthy controls on a phonological delayed-matching-to-sample task (see Fig. 1) using an event-related design. We were specifically interested in examining neural activity associated with correct vs. error trials. In the delayed-matching-to-sample task, participants were asked to encode three nonsense words, followed by a 6-seconds delay period. Then a probe nonsense word was presented. Participants were asked to decide whether the probe word matched one of the three nonsense words from the encoding phase. Immediately after the recognition task, subjects were asked to rate the confidence of their recognition response. This procedure allowed us to separate correct and error trials based on the accuracy of their response, and to further divide correct and error trials according to the confidence ratings in order to examine hypothesized true memory vs. false memory trials. Considering the results from Lee et al. [13], we hypothesized that patients would show reduced frontal asymmetry corresponding to correct trials during the verbal WM task. Moreover, we hypothesized that neural activity corresponding to true correct and false memory trials would be very similar in SZ as well as in controls if during the delay period, the maintenance process is intact.
Figure 1. Procedure of the phonological verbal WM task.
Results
Behavioral data
All significant tests are 2-tailed unless otherwise noted. We excluded trials with missing responses or missing confidence ratings. Mean number of excluded trials was 17.5 (SD = 19.7) in CO and 26.3 (SD = 16.4) in SZ. This difference was not statistically significant (t(23) = 1.20, p = 0.24).
Difference in mean overall % correct (82.6 (SD = 10.5) in CO; 76.8 (SD = 12.1) in SZ) was not statistically significant (t(23) = 1.27, p = 0.22, Cohen's d = 0.53), suggesting that this group of SZ did not show a significant overall deficit in verbal WM overall.
Correct trials were further segregated into “confident” and “not confident” trials. We categorized correct-and-confident trials as ‘true correct memory’ trials in which correct encoding and adequate maintenance are assumed to have taken place. The number of true correct memory trials was greater in CO than in SZ, with a large effect size (t(23) = 1.91, p = 0.03, 1-tailed, Cohen's d = 0.79). This suggests that SZ may be impaired in phonological verbal WM.
Correct-but-not-confident trials were hypothesized to be guess trials because the participants produced correct responses but had no idea if they were correct (i.e., they were guessing). The two groups did not differ in the number of guess trials (t(23) = −1.1, p = 0.28, Cohen's d = 0.46).
Among error trials, we examined ‘false memory’ trials in which subjects were wrong but nevertheless were highly confident of that they were right. In these trials, subjects were likely to have encoded incorrect stimuli and maintaining them in WM during the delay. Therefore, they are expected to be confident of their responses since they did remember, albeit incorrect items. Although SZ made more false memory errors than did controls but this difference was not statistically significant (t(23) = −1.30, p = 0.22, Cohen's d = 0.54).
The number of error trials with low confidence (true error) was miniscule and almost identical between the two groups. Behavioral results are summarized in Table 1.
Table 1. Summary of behavioral performance.
| SZ (n = 12) | CO (n = 13) | t | p | Effect size (Cohen's d) | |
| Number of excluded trials | 26.25 (16.43)a | 17.46 (19.67) | −1.21 | 0.24 | 0.50 |
| % Correct trials | 76.84 (12.10) | 82.56 (10.48) | 1.27 | 0.11b | 0.53 |
| % True memory | 56.29 (21.33) | 69.55 (12.76) | 1.91 | 0.03b | 0.79 |
| % Correct guess | 20.55 (23.56) | 13.01 (7.13) | −1.10 | 0.14b | 0.46 |
| % False memory | 16.14 (12.88) | 10.37 (9.07) | −1.30 | 0.11b | 0.54 |
| % True error | 7.01 (6.61) | 7.05 (6.61) | 0.013 | 0.49b | 0.005 |
| % Confident trials | 72.42 (27.87) | 79.93 (11.42) | 0.89 | 0.38 | 0.37 |
Mean (standard deviation).
1-tailed.
fMRI data
Correct trials
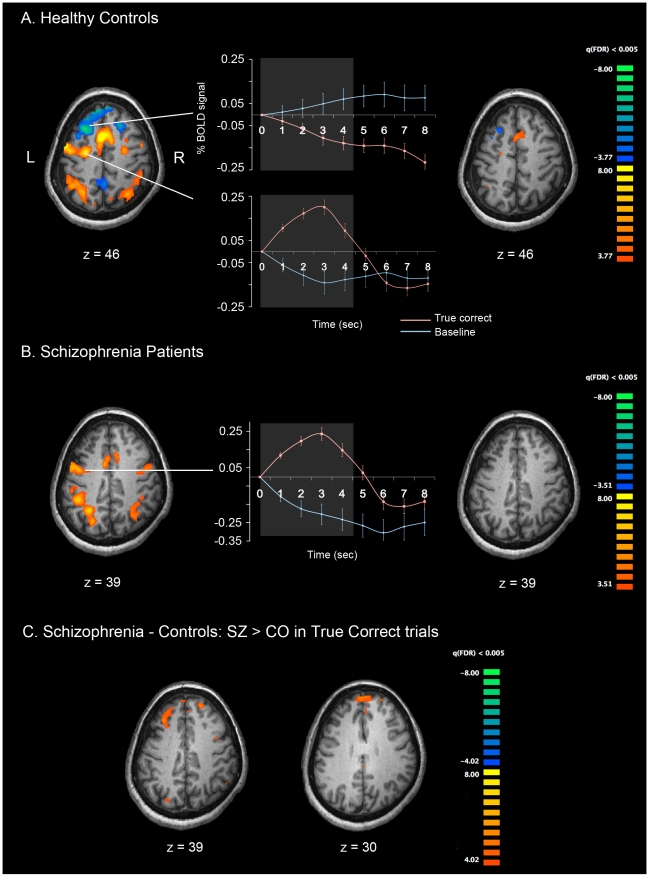
To identify brain regions that were associated with phonological WM maintenance, we contrasted brain activity associated with true memory with baseline for each group, and then compared the activity between the two groups. Figures 2a and b (left) represent the activation patterns during the delay period for true correct memory trials in each group. Figure 2c represents the areas significantly different between the two groups.
Figure 2. Cortical activation patterns during verbal WM maintenance for the two groups.
Healthy controls (A), patients with schizophrenia (B), and significantly different activation between groups (subtraction of SZ-CO) (C) are shown. The time series plots in the middle column show activation associated with true memory maintenance (red lines) relative to the baseline activities (blue line). Bright parts in the middle of each plot represent 1-volume (1.5 s) after onset, and offset of the maintenance phase (4.5 secs). All p-values are corrected with false discovery rate of q<0.005.
In true correct memory trials, CO showed increased bilateral activation in frontal regions including medial frontal (BA6), left superior frontal (BA6), middle frontal (BA6/10), precentral gyri, right middle frontal (BA6/9) and inferior frontal gyri (BA9). SZ showed bilateral activation in medial, middle frontal and precentral gyri (BA6). Parietal activation in superior and inferior parietal lobule (BA 7/40) that is involved in sensory processing was observed bilaterally in both groups.
Interestingly, CO also showed “deactivated (less activation than baseline)” frontal and posterior regions, which was not observed in SZ. This deactivation was greater in the left superior frontal gyrus (Fig. 2a left), resulting in relatively greater activity in SZ within this region (Fig. 2c). The regions activated during the delay for true memory trials are listed in Table 2.
Table 2. Activated areas during verbal WM maintenance on true correct trials.
| L/R | x | Y | z | t | p | q(FDR) | BA | |
| CO | > Baseline | 0.000856 | 0.005 | |||||
| Superior Frontal Gyrus | L | −2 | 8 | 54 | 10.2 | 6 | ||
| Middle Frontal Gyrus | L | −26 | −7 | 47 | 6.98 | 6 | ||
| L | −32 | 43 | 21 | 6.51 | 10 | |||
| Precentral Gyrus | L | −44 | −4 | 46 | 6.89 | 6 | ||
| Middle Frontal Gyrus | R | 26 | −5 | 46 | 5.6 | 6 | ||
| R | 39 | 31 | 32 | 4.9 | 9 | |||
| Inferior Frontal Gyrus | R | 40 | 6 | 27 | 5.87 | 9 | ||
| Inferior Parietal Lobule | L | −33 | −49 | 37 | 8.89 | 40 | ||
| Parietal Angular Gyrus | R | 29 | −56 | 37 | 8.62 | 39 | ||
| Insula | L | −29 | 21 | 9 | 3.36 | 13 | ||
| R | 30 | 23 | 8 | 7.56 | 13 | |||
| CO | < Baseline | 0.000856 | 0.005 | |||||
| Superior Frontal Gyrus | L | −26 | 24 | 49 | −7.31 | 8 | ||
| L | −13 | 48 | 38 | −6.05 | 9 | |||
| L | −3 | 59 | 28 | −8.06 | 9 | |||
| R | 21 | 26 | 45 | −6.06 | 8 | |||
| Inferior Frontal Gyrus | R | 45 | 34 | 9 | −4.75 | 46 | ||
| Parietal Precuneus | L | −10 | −45 | 31 | −6.66 | 31 | ||
| L | −2 | −49 | 51 | −4.8 | 7 | |||
| Limbic Cingulate Gyrus | R | 2 | −45 | 30 | −7.53 | 31 | ||
| SZ | > Baseline | 0.000401 | 0.005 | |||||
| Medial Frontal Gyrus | L | −6 | 4 | 51 | 8.97 | 6 | ||
| L | −44 | 2 | 38 | 7.10 | 6 | |||
| R | 47 | 4 | 41 | 4.55 | 6 | |||
| Frontal Precentral Gyrus | L | −38 | 3 | 25 | 5.46 | 6 | ||
| Inferior Parietal Lobule | L | −41 | −39 | 38 | 6.91 | 40 | ||
| Superior Parietal Lobule | L | −29 | −54 | 38 | 7.02 | 7 | ||
| Parietal Supramarginal Gyrus | R | 39 | −37 | 34 | 5.74 | 40 | ||
| Parietal Angular Gyrus | R | 27 | −56 | 33 | 5.81 | 39 | ||
| Group Difference | SZ > CO | 0.000058 | 0.005 | |||||
| Middle Frontal Gyrus | L | −31 | 30 | 41 | 5.2 | 8 | ||
| Superior Frontal Gyrus | L | −6 | 57 | 31 | 4.7 | 9 | ||
| R | 18 | 47 | 38 | 4.5 | 8 | |||
*Brodmann Area. x,y,z are the Talairach stereotaxic coordinates.
There were a large number of correct but not confident trials (guesses), therefore we looked at the activation patterns for these trials (Fig. 2a, right panel). With the same level of threshold (q(FDR)<0.005), both groups showed very little activity compared with the baseline. CO still had greater activation than baseline in the superior frontal gyrus (medial BA8) and deactivation in left BA8/9. Comparison between CO and SZ did not reveal significant activation difference overall.
Error trials
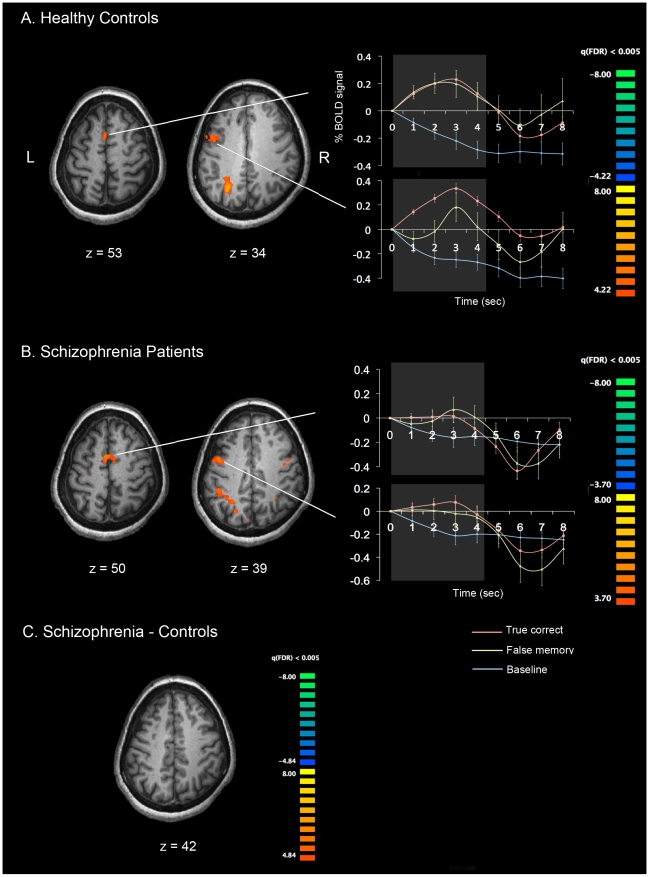
BOLD activity during error trials with high confidence ratings were examined (see [13]). On false memory trials, both CO and SZ recruited only a subset of the regions that were activated in true memory trials, and significantly greater activation than baseline was observed mostly in left hemisphere (see Fig. 3). Unlike the true correct memory, CO did not exhibit “deactivated” regions on these false memory trials (Fig. 3a). We did not observe a significant group difference of frontal activation in false memory trials (Fig. 3c). The regions activated during the delay for false memory trials are listed in Table 3.
Figure 3. Cortical activation patterns during false memory trials.
(A) False memory – Baseline in CO. (B) False memory – Baseline in SZ. (C) SZ – CO. All p-values are corrected with FDR of q<0.005. The time course plots show false memory related activities (yellow) and true memory related activities (red) relative to the baseline (blue).
Table 3. Activated areas during verbal WM maintenance on confident but incorrect trials (false memory trials).
| L/R | x | y | z | t | p | q(FDR) | BA | |
| CO | False Memory > Baseline | 0.000025 | 0.005 | |||||
| Superior Frontal Gyrus | L | −3 | 7 | 53 | 4.69 | 6 | ||
| Middle Frontal Gyrus | L | −25−48 | −95 | 4434 | 4.764.34 | 66 | ||
| Insula | L | −30 | 19 | 8 | 4.93 | 13 | ||
| R | 29 | 24 | 3 | 4.64 | 13 | |||
| Parietal Angular Gyrus | L | −26 | −57 | 34 | 6.33 | 39 | ||
| Superior Parietal Lobule | R | 29 | −60 | 42 | 4.81 | 7 | ||
| SZ | False Memory > Baseline | 0.000069 | 0.005 | |||||
| Medial Frontal Gyrus | L | −5 | 3 | 50 | 5.11 | 6 | ||
| Middle Frontal Gyrus | L | −43 | 1 | 37 | 4.14 | 6 | ||
| Inferior Parietal Lobule | L | −36 | −48 | 41 | 4.31 | 40 | ||
We also asked whether greater activation is associated with the maintenance of incorrectly encoded internal representations (i.e., false memory) than simple guesses, we compared false memory trials with correct guess trials (figures are not shown). In SZ, greater activation for false memory was observed in right superior frontal gyrus (BA 9 (28, 43, 28), t = 4.71) and bilateral parietal regions (BA 7 (L: −14, −51, 50; R: 10, −57, 48), BA 40 (37, −35, 55), t = 4.05) at the q(FDR)<0.05. When we applied higher threshold (<0.005) used for the other analyses, this difference disappeared. In CO, there was no significant activation difference between these two trial types.
As for the true error trials (error trials with no confidence), CO had greater activation than baseline in the same area of the superior frontal gyrus (medial BA 8) that was activated for correct guesses (see Fig. 2a right). SZ showed no significantly activated regions at q(FDR)<0.005. As shown in Table 2, there were not many true error trials in both groups.
Discussion
The present study investigated the brain activation pattern during WM maintenance associated with correct and error trials of phonological WM in healthy individuals and patients with schizophrenia.
Overall accuracy, when confidence ratings are not taken into account, indicated that our group of schizophrenic patients did not show a significant verbal WM deficit compared with CO. However, when we further examined how correct and error trials arose by analyzing different trial types, interesting differences emerged. Correct trials with low confidence ratings are likely to be guesses. We had hypothesized that guess trials would not correspond to changes in cortical activity above baseline because no WM maintenance is expected to have occurred. Overall accuracy score, when confidence ratings are not taken into account, included both true correct trials and guess trials. Therefore, it does not accurately reflect true accuracy of memory especially if there are many lucky guesses. When only ‘true memory’ trials (i.e. correct and confident) were considered, the group difference emerged, which suggests that SZ may be impaired in the phonological verbal WM task.
On the other hand, behavioral performance was not different in false memory trials between the two groups and this was also true for brain activation during these trials (Fig. 3). This finding diverges from the previous study of spatial WM by Lee et al. [13], in which they found increased rate of false memory trials in schizophrenia. This difference may be due to differences in task difficulty and available strategies for spatial and verbal WM tasks.
We had also hypothesized a reduced hemispheric asymmetry associated with correct trials in SZ based on previous studies [13], [33], [34]. In the present study, we did not observe reduced asymmetry in SZ compared with CO. CO showed bilateral activation in frontal regions, including the left superior and the middle, precentral gyri, the right inferior frontal gyri, and bilateral parietal regions on true correct memory trials. CO also showed regions of deactivation (relative to baseline activity) in the frontal cortex, including the superior and inferior gyri, the precuneus, and the cingulate gyrus in the left hemisphere. SZ also showed activation in regions of medial frontal, middle frontal, precentral gyri, and bilateral parietal areas. In error trials with high confidence (false memory), both CO and SZ showed more left-hemisphere lateralized activation pattern. Those activated regions overlapped with the regions activated in true memory trials. However, there was one important difference between the SZ and CO; CO did not show regions of deactivation on false memory trials that were observed on true memory trials.
Overall, the results of the present study suggest that verbal WM impairment in SZ cannot be simply described as either a problem of hyperfrontality or hypofrontality. Past studies have also reported discrepant findings on this issue, depending on the task difficulty and/or performance. For example, CO exhibited increasing DLPFC activation as performance decreased while SZ had the opposite pattern in a verbal WM task [25]. A meta-analysis also indicates a complex pattern of hyper and hypoactivation in schizophrenia [5]. In group comparison of the present study, SZ exhibited greater activation than CO in superior frontal areas (Fig. 2c) but this ‘hyperfrontality’ was due to deactivation relative to the baseline in CO rather than an increased activation in SZ. The results from false memory trials suggest that sometimes both CO and SZ maintain incorrectly encoded internal representation with corresponding cortical activation.
Healthy control participants appear to recruit different neural networks for maintaining items in verbal WM in true memory compared with false memory trials as indicated by deactivated prefrontal regions in true memory. Furthermore, the pattern and extent of activation is more bilateral and increased in true memory trials, whereas it is shifted leftward in false memory. Thus, CO seems to recruit a wider network during the maintenance of correctly encoded information. This was also true for SZ; patients also showed greater and less lateralized activation in true memory compared with false memory trials (Fig. 2b left and Fig. 3b). Unlike CO, however, SZ did not have deactivation relative to baseline in prefrontal regions.
Therefore, the most evident difference in activation patterns between groups related with our task is whether the superior prefrontal area (BA 8/9) was deactivated relative to baseline. However, ‘deactivation’ for correct memory in CO is not easy to explain and should be interpreted cautiously. On the basis of the results from CO in true memory trials, it is possible to assume that the activated frontal/parietal areas and deactivated prefrontal areas comprise or would be parts of a fully functioning network for verbal working memory. The activated areas in false memory would be also parts of the network (in fact, these areas overlap). Since deactivated areas were observed in correct trials only (note that there is also deactivation in correct guess trials), this deactivation is likely to be involved in maintaining correctly encoded internal representation. Therefore, this task-induced deactivation may reflect beneficial processes, for example, efficient reallocation of resources from default to task-relevant processes [26], [27], associated with correctly encoded information rather than reflecting detrimental processes [28]. Considering lack of such functionally relevant deactivation in SZ group and in false memory trials in CO, less task-induced deactivation in the prefrontal area during maintenance may have contributed to maintaining false representations. However, we do not argue that this deactivation is entirely responsible for maintenance of correctly encoded information because SZ did not show such deactivation even in true correct trials. At least, it is tempting to speculate that prefrontal deactivation would be beneficial for maintenance of correct information.
With respect to the activation pattern difference between true and false memory trials (i.e. bilateral vs. left-lateralized activation), it is worth noting that the participants had to phonologically decode visually presented stimuli during WM encoding in our task. It is hypothesized that during maintenance period, internal representations of the stimuli were supported. In our experiment design, we tried to minimize visual perceptual influences that could be used for encoding and retrieval. That is, if both the target and probe words were shown in identical cases or fonts, it may be possible to make a correct response by exclusively using the visual information (e.g. identical shape, font, or size). By making sure that the target and probe words were presented in different cases, we were trying to minimize the visual perceptual influence and the use of “visual features”, and to maximize the potential for phonological processing. However, our manipulation does not eliminate visual coding. Therefore, it would be more accurate to suppose that subjects had access to both visual and phonological representations that were maintained during true correct trials. During the retrieval stage, phonological-visual transformation must occur again because the probe word is visual. This effort may be reflected in a more bilateral activation pattern. Phonological decoding (grapheme-to-phoneme conversion) involves a network of the anterior left precentral gyrus and the left ventral occipitotemporal cortex [29]. One can maintain visual as well as the phonological representation of the nonsense words during the delay. Therefore, the bilateral activity observed in true correct trials might reflect this dual strategy. Activation in right parietal regions, which is involved in maintaining spatial and object information and possibly in WM manipulation [30]–[32], may also reflect active processing of visuospatial information during maintenance. Dual coding of stimuli and maintenance of both visual and phonological features could increase accuracy.
On false memory trials, the activation pattern was more left-lateralized. This may mean that what was maintained during false memory trials was probably phonological and perhaps the locus of the error lies in grapheme-phoneme conversion during encoding.
In the context of laterality, Lee et al. [13] found that CO had a right hemisphere advantage for processing visuospatial information while SZ exhibited more symmetrical activation pattern. Other studies also reported reduced or reversed hemispheric asymmetry in schizophrenia [33], [34]. In verbal domain, one might expect that CO would exhibit more left lateralized activation [35], [36] while SZ would have reduced asymmetry [22], [33]. Past studies have suggested that the lateralized activation in CO may reflect efficient and specialized processing and reduced asymmetry in SZ may indicate their inefficient and/or compensatory mechanisms [14], [22], [33].
However, other studies found bilateral activation for both verbal and spatial WM tasks [37]–[39]. A recent fMRI study [40] also suggested that a common bilateral frontoparietal network subserves both verbal and spatial domains but recruits additional left-lateralized frontal and temporal regions for further verbal processing. These studies suggest that the activation pattern of the frontoparietal network is shaped more by the task demands (manipulation, maintenance and/or both), and task difficulty than by laterality. Our data suggest that both CO and SZ recruit wider bilateral network of task-relevant brain areas perhaps reflecting the dual strategy to maintain true correct memory. As discussed above, prefrontal deactivation in CO in true memory trials might be associated with correct maintenance.
There are limitations and caveats. First, all patients were taking antipsychotic medication at the time of testing. Past results on the effect of antipsychotic medication on WM in schizophrenia are variable. For instance, atypical antipsychotic drugs appear to improve verbal and spatial WM performance in schizophrenia [41]–[43]. Other studies argue that improved performance on tasks after treatment is due to learning and practice rather than medication effect [44], [45]. Our SZ subjects did not perform significantly worse than CO overall, suggesting that medication effect may not be a critical confounding factor in interpreting our data. In addition, we examined correct and error trials separately, so the performance, by definition, was matched between the two groups. Second, our sample size is on the small side. However, the effect sizes were robust. We used a very conservative statistical criterion, i.e. very low false discovery rate of <0.005 to find difference between conditions or between groups in activation maps. Third, our primary purpose was to investigate brain activation related with verbal, phonological working memory. However, we had to extend discussion into visual domain because our task was not purely verbal by visually presenting verbal information. Comparing our results with future data collected by auditory presentation could reveal activation difference in processing visual-verbal and auditory-verbal working memory.
To summarize, we observed different patterns of brain activation in maintaining true memory and false memory in both CO and SZ: a wider frontoparietal network was recruited to maintain correctly encoded internal representation compared with maintenance of incorrectly encoded information. We found a subtle group difference in activation patterns in our study. CO showed prefrontal deactivation relative to resting activation in correct memory trials. Perhaps, a lack of such task-induced deactivation in schizophrenia may correspond to false memory.
Overall, these findings underscore the utility of parsing out different sources of WM errors to investigate accompanying brain activation and more broadly, the importance of elucidating the non-linear nature of brain-behavior relationship.
Materials and Methods
Participants
Twelve outpatients with chronic schizophrenia (SZ) were recruited from two private psychiatric facilities in Nashville, TN. The patients met the DSM-IV criteria for schizophrenia or schizoaffective disorder, based on structured clinical interviews (SCID) and chart reviews [46]. Clinical symptoms were evaluated using the Brief Psychiatric Rating Scale (BPRS) [47], the Scale for the Assessment of Negative Symptoms (SANS) [48], and the Scale for the Assessment of Positive Symptoms (SAPS) [49]. All patients were taking atypical antipsychotic drugs (clozapine, risperidone, or olanzapine) at the time of testing. Thirteen healthy control participants (CO) were recruited through advertisements in Nashville, TN. The two groups were matched in age, education level, handedness and IQ (See Table 4). All of the participants were native English speakers. No one had past or current substance abuse, head injury, neurological disease or medical illness affecting brain function. No CO had DSM-IV Axis I or II disorder, or a family history of psychotic disorders.
Table 4. Demographic information of the participants.
| SZ (n = 12; 5 women) | CO (n = 13; 5 women) | p | |
| Age | 40.2 (10.23)* | 40.4 (9.34) | 0.96 |
| IQ (WASI) | 92.0 (19.8) | 99.3 (17.9) | 0.39 |
| Years of Education | 14.1 (2.0) | 15.4 (3.12) | 0.23 |
| Illness Duration (years) | 14.1 (9.9) | - | - |
| BPRS | 13.75 (6.6) | - | - |
| SAPS | 10.0 (7.8) | - | - |
| SANS | 15.5 (10.1) | - | - |
| Handedness (Edinburgh) | +58.8 (64.49) | +77.75 (20.44) | 0.34 |
*Mean (standard deviation).
Ethics Statement
Written informed consent was obtained from all participants after they were given a complete description of the study. The Institutional Review Board of Vanderbilt University approved the protocol and consent procedure.
Phonological Verbal WM task
Functional images were obtained while participants performed a phonological delayed-response task (Fig. 1). At the beginning of each trial, a fixation cross was presented for 1 s. Then three nonsense words were presented in black on a gray background, each in a different location for 3 s. The stimuli were Dutch words between 4–6 letters and were phonologically similar to English words but were meaningless to non-Dutch speakers. Subjects were asked to silently read these stimuli. A delay period of 6 s followed. After the delay, a probe nonsense word was presented for 2.5 s and participants were asked to decide whether the probe was the same as one of the three target words by pressing one of the two assigned buttons. To minimize potential visual influence and visual strategy based on identical shape, font, or size on the screen, the probe nonsense word was presented in lower case if the targets were in upper case and vice versa.
After making the memory response, participants were given 2.5 s to indicate their confidence level of the memory response that they had just given, on a 3-point rating scale.
The inter-trial interval was 8.25 s and subjects completed 4 runs containing 27 trials per run. Each run had 5 pseudo trials (fixation only) and BOLD signals associated with these trials were regarded as baseline. The first and the last trials in each run were discarded prior to analysis for MR saturation. The trials with missing WM response and/or missing confidence rating were also discarded. Therefore, the total number of trials included in analyses varied across individuals.
Image acquisition
All brain images were collected on a 3-Tesla Phillips Intera Achieva system with a birdcage head coil at Vanderbilt University Medical Center, Nashville, TN. Twenty five T1-weighted anatomical images parallel to the AC-PC line were acquired with T2*-weighted functional images for BOLD-based images, using echoplanar (EPI: TR = 1500 ms, matrix = 128×128, slice thickness = 4.5 mm, slice gap = 0.4 mm, FOV = 240×240 mm) sequence. High-resolution T1-weighted anatomical volumes were also acquired with a T1 3D turbo field echo (T1TFE) sequence (TR = 8.877 ms, matrix = 256×256, slice thickness = 1 mm, gap = 0 mm, number of slices = 170).
fMRI data analysis
The imaging data were preprocessed and analyzed using Brain Voyager QX 1.10.2 (Brain Innovation, Maastricht, The Netherlands). Anatomical volumes were transformed into a common stereotaxic space [50]. Functional volumes for each subject were aligned to the anatomical volumes, thereby transforming the functional data into a common brain space across participants. Data pre-processing included image alignment, three-dimensional motion correction, linear de-trending, temporal frequency filtering with high pass filter, slice-time correction, and spatial smoothing with 4 mm Gaussian kernel (FWHM). Statistical analysis was based on the application of the multi-study general linear model (GLM) to the time-series of task-related functional volumes. A GLM with predictors of interest (i.e. correct vs. incorrect trials with/without confidence from behavioral data) was applied for the individual z-normalized volume time courses. To reduce possible mixture of signals from encoding and maintenance phases, the BOLD signals coupled with the latter 3TR (4.5 s) of the delay period were analyzed as WM maintenance activity. Significant difference among the conditions was assessed with contrast (t) maps at a false discovery rate (FDR) of q<0.005, using random effects statistical parametric maps (SPM).
Acknowledgments
We are grateful to Junghee Lee, Crystal Gibson, Bradley Folley, Katy Thakkar, Jutta Mayer and Adam Anderson for their input and support throughout the various stages of this project.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Supported by MH-073028 and the Discovery Grant to Sohee Park. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Park S, Lee J. Spatial working memory function in schizophrenia. In: Lenzenweger MF, Hooley JM, editors. Principles of experimental psychopathology. Washington, DC: APA; 2002. pp. 83–106. [Google Scholar]
- 2.Baddeley AD. New York: Oxford University Press; 1986. Working Memory. [Google Scholar]
- 3.Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory. In: Carroll B, editor. Psychopathology and the Brain. Raven Press: New York; 1991. pp. 1–22. [Google Scholar]
- 4.Park S, Püschel J, Sauter B, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia; A 4-month follow-up study. Biol Psychiatry. 1999;46:392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Park S. Working memory abnormalities in schizophrenia: A meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 6.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual cortex in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–348. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 9.Funahashi S, Bruce CJ, Goldman-Rakic PS. Visuospatial coding in primate prefrontal neurons revealed by oculomotor paradigms. J Neurophysiol. 1990;63:814–831. doi: 10.1152/jn.1990.63.4.814. [DOI] [PubMed] [Google Scholar]
- 10.Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesion & oculomotor delayed-response performance: Evidence for mnemonic “scotomas”. J Neuroscience. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 12.Wexler BE, Anderson M, Fulbright RK, Gore JC. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry. 2000;157:1694–1697. doi: 10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Folley BS, Gore J, Park S. Origins of Spatial Working Memory Deficits in Schizophrenia: An Event-Related fMRI and Near-Infrared Spectroscopy Study. PLoS One. 2008;3:e1760. doi: 10.1371/journal.pone.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 15.Barch DM, Csernansky JG. Abnormal parietal cortex activation during working memory in schizophrenia: verbal phonological coding disturbances versus domain-general executive dysfunction. Am J Psychiatry. 2007;164:1090–1098. doi: 10.1176/ajp.2007.164.7.1090. [DOI] [PubMed] [Google Scholar]
- 16.Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 17.Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 18.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophr Res. 2003;60:285–98. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg TE, Berman KF, Fleming K, Ostrem J, Van Horn JD, et al. Uncoupling cognitive workload and prefrontal cortical physiology: a PET rCBF study. Neuroimage. 1998;7:296–303. doi: 10.1006/nimg.1998.0338. [DOI] [PubMed] [Google Scholar]
- 20.Leung HC, Seelig D, Gore JC. The effect of memory load on cortical activity in the spatial working memory circuit. Cogn Affect Behave Neurosci. 2004;4:553–563. doi: 10.3758/cabn.4.4.553. [DOI] [PubMed] [Google Scholar]
- 21.Jansma JM, Ramsey NF, Kahn RS. Is prefrontal activation in the schizophrenic brain abnormal? It depends! Schizophr Res. 2002;53:111. [Google Scholar]
- 22.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, et al. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 23.Cairo TA, Liddle PF, Woodward TS, Ngan ETC. Assessing differential patterns of load dependence between working memory system components. Cog Brain Res. 2004;21:377–387. doi: 10.1016/j.cogbrainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on- line storage of spatial memoranda. J Cogn Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- 25.Karlsgodt KH, Sanz J, van Erp TGM, Bearden CE, Nuechterlein KH, et al. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res. 2009;108:143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 27.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otten LJ, Henson RNA, Rugg MD. Depth of processing effects on neural correlates of memory encoding – Relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- 29.Dietz N, Jones KM, Gareau L, Zeffiro TA, Eden GF. Phonological decoding involves left posterior fusiform gyrus. Human Brain Mapping. 2005;26:81–93. doi: 10.1002/hbm.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139:173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- 31.Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13:257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- 32.Olsen IR, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiol Learn Mem. 2009;91:155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter H, Wunderlich AP, Blankenhorn M, Schafer S, Tomczak R, et al. No hypofrontality, but absence of prefrontal lateralization comparing verbal and spatial working memory in schizophrenia. Schizophr Res. 2003;61:175–184. doi: 10.1016/s0920-9964(02)00225-6. [DOI] [PubMed] [Google Scholar]
- 34.Angrilli A, Spironelli C, Elbert T, Crow TJ, Marano G, et al. Schizophrenia as failure of left hemispheric dominance for the phonological component of language. PLoS One. 2009;4:e4507. doi: 10.1371/journal.pone.0004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- 36.Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, et al. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11:424–446. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- 37.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 38.D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, et al. Functional MRI studies of spatial and nonspatial working memory. Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 39.Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci USA. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray MK, Mackay CE, Harmer CJ, Crow TJ. Bilateral generic working memory circuit requires left-lateralized addition for verbal processing. Cereb Cortex. 2008;18:1421–1428. doi: 10.1093/cercor/bhm175. [DOI] [PubMed] [Google Scholar]
- 41.Green MF, Marshall BD, Jr, Wirshing WC, Ames D, Marder SR, et al. Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiatry. 1997;154:799–804. doi: 10.1176/ajp.154.6.799. [DOI] [PubMed] [Google Scholar]
- 42.Snyder PJ, Jackson CE, Piskulic D, Olver J, Norman T, et al. Spatial working memory and problem solving in schizophrenia: the effect of symptom stabilization with atypical antipsychotic medication. Psychiatry Res. 2008;30:316–32. doi: 10.1016/j.psychres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Surguladze SA, Chu EM, Evans A, Anikumar AP, Patel MX, et al. The effect of long-acting risperidone on working memory in schizophrenia: a functional magnetic resonance imaging study. J Clin Psychopharmacology. 2007;27:560–570. doi: 10.1097/jcp.0b013e31815a256c. [DOI] [PubMed] [Google Scholar]
- 44.Godlberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 45.Boulay LJ, Labelle A, Bourget D, Robertson S, Habib R, et al. Dissociating medication effects from learning and practice effects in a neurocognitive study of schizophrenia: Olanzapine versus haloperidol. Cogn Neuropsychiatry. 2007;12:322–338. doi: 10.1080/13546800601069534. [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatric Association. Washington, DC: American Psychiatric Press; 1991. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. [Google Scholar]
- 47.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 48.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 49.Andreasen NC. Iowa City, Iowa: The University of Iowa; 1984. Scale for the Assessment of Positive Symptoms (SAPS). [Google Scholar]
- 50.Talairach J, Tournox P. Co-planar Stereotaxic Atlas of the Human Brain, Thieme Medical, New York 1988.