Abstract
The possibility of providing the acetogenic microorganism Sporomusa ovata with electrons delivered directly to the cells with a graphite electrode for the reduction of carbon dioxide to organic compounds was investigated. Biofilms of S. ovata growing on graphite cathode surfaces consumed electrons with the reduction of carbon dioxide to acetate and small amounts of 2-oxobutyrate. Electrons appearing in these products accounted for over 85% of the electrons consumed. These results demonstrate that microbial production of multicarbon organic compounds from carbon dioxide and water with electricity as the energy source is feasible.
IMPORTANCE
Reducing carbon dioxide to multicarbon organic chemicals and fuels with electricity has been identified as an attractive strategy to convert solar energy that is harvested intermittently with photovoltaic technology and store it as covalent chemical bonds. The organic compounds produced can then be distributed via existing infrastructure. Nonbiological electrochemical reduction of carbon dioxide has proven problematic. The results presented here suggest that microbiological catalysts may be a robust alternative, and when coupled with photovoltaics, current-driven microbial carbon dioxide reduction represents a new form of photosynthesis that might convert solar energy to organic products more effectively than traditional biomass-based strategies.
INTRODUCTION
The intermittent nature of renewable sources of energy, most notably solar and wind, is leading to a search for strategies to capture the electrical energy produced from these sources in covalent chemical bonds, producing compounds that can readily be stored and consumed on demand, preferably within the existing infrastructure (1). One particularly attractive option is to reduce carbon dioxide to produce multicarbon organic compounds that are precursors for desirable organic chemicals or liquid transportation fuels (2). Basic requirements for a practical system to fix carbon dioxide in this manner include (i) the ability to use electrons derived from water as an abundant, inexpensive source of reductant (1, 2); and (ii) inexpensive, durable catalysts (3).
Reaction thermodynamics suggests that it should be readily feasible to electrochemically reduce carbon dioxide to a diversity of organic compounds, and this process has been studied for over a hundred years (4, 5). However, in practice, abiotic electrochemical reduction of carbon dioxide has not proven practical in large part due to (i) poor long-term stability of the cathodes, (ii) nonspecificity of products produced, (iii) sluggishness of carbon dioxide reduction, (iv) competition with hydrogen production, and (v) cathode expense (3). Incorporating enzyme catalysts on electrodes may promote more specific product formation from electrochemical reduction of carbon dioxide and lower the energy required for reduction (3), but experiments on enzymatic reduction have typically lasted only a matter of hours, reflecting the fact that enzymes adsorbed to electrodes do not have long-term stability.
Acetogenic bacteria can reduce carbon dioxide to acetate and other multicarbon extracellular products with hydrogen as the electron donor (6, 7). However, supplying acetogens with hydrogen that is produced electrochemically is unlikely to be practical because it would require expensive catalysts and/or substantial energy inputs (8). An alternative might be to directly feed acetogens electrons with electrodes. Geobacter and Anaeromyxobacter species have previously been shown to accept electrons from graphite electrodes for the reduction of fumarate to succinate (9), the reduction of nitrate to nitrite (9), U(VI) reduction (10), or reductive dechlorination (11, 12). It has also been suggested that it may be possible to directly supply methanogenic microorganisms electrons at electrode surfaces for carbon dioxide reduction to methane (13), but this has been difficult to verify because there can be significant electrochemical hydrogen production at the low electrode potentials required for active methanogenesis (14).
Culturing in H cells.
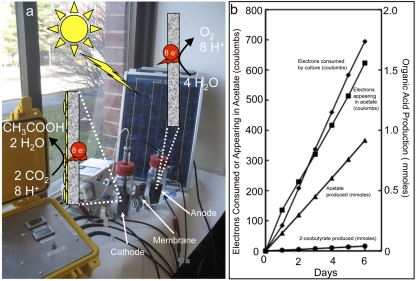
We evaluated the possibility of feeding an acetogen electrons from an electrode with Sporomusa ovata (15) (Deutsche Sammlung Mikroorganismen und Zellkulturen [DSMZ] culture 2662). Cells were grown in the cathode chambers of “H cells” (Fig. 1a), which have previously been used to evaluate other forms of electrode-driven anaerobic respiration (9–12). The cathode and anode were comprised of unpolished graphite sticks. The anode and cathode chambers, each containing 200 ml of medium, were separated with a Nafion cation-exchange membrane. A potentiostat maintained a potential difference between the anode and cathode. Electrons extracted from water at the anode were delivered to the cathode at −400 mV (versus standard hydrogen electrode), a potential well above the −600 mV necessary to produce even low levels of hydrogen with unpolished graphite (8). The lack of hydrogen production was verified by directly measuring hydrogen concentrations with a reduction gas analyzer as previously described (9). In most instances, the electrical current was obtained from a standard electrical outlet, but a solar-powered potentiostat (Fig. 1a), comprised of a solar panel and voltage control unit built with standard electrical components, could also support the system.
FIG 1.
(a) H-cell device for supplying cathode biofilms of S. ovata electrons derived from water. The solar-powered option is illustrated. 8 e−, 8 electrons. (b) Electron consumption and product formation by a representative S. ovata biofilm over time. The data shown were obtained with a system connected to a standard electric current. The mean standard errors of the organic acid and current measurements were 2% and 13%, respectively.
An inoculum of S. ovata was grown with hydrogen as the electron donor (H2-CO2 [80:20]) in the DSMZ-recommended growth medium (DSMZ 311) with betaine, Casitone, and resazurin omitted. The hydrogen-grown cells were introduced into the cathode chamber in the same medium but with the yeast extract and the cysteine and sulfide reductants omitted. This bicarbonate-based medium contained no organic compounds other than a vitamin mixture, and carbon dioxide was the sole electron acceptor. The culture was initially bubbled with a hydrogen-containing gas mixture (N2-CO2-H2 [80:13:7]) as an additional electron donor to accelerate the growth of a biofilm on the cathode surface. Acetate was measured with high-performance liquid chromatography (HPLC) (16). Once acetate reached 10 mM, 50% of the medium was replaced with fresh medium. This process was repeated three times. This periodic removal of planktonic cells promoted biofilm growth on the cathode. The gas phase was then switched to N2-CO2 (80:20). Once the consumption of current was observed (within 24 h), the system was switched to flowthrough mode in which fresh medium maintained under N2-CO2 was continuously introduced (0.1 ml/min; dilution rate of 0.03 h−1) as previously described (16, 17). Hydrogen partial pressures in the headspace remained less than 10 ppm throughout the study, ca. 2 orders of magnitude below the minimum threshold for acetate production from hydrogen by acetogens (18).
Systems with S. ovata steadily consumed current with the production of acetate and small amounts of 2-oxobutyrate (Fig. 1b). Uninoculated controls did not consume current or produce organic acids. If the current supply to the S. ovata biofilm was interrupted, acetate and 2-oxobutyrate production stopped.
Although it was not possible to measure carbon dioxide consumption due to the high concentrations of bicarbonate in the medium, it was possible to calculate an electron balance. Electrons appearing in acetate accounted for a high proportion of the electrons that the cultures consumed (Fig. 1b). In three replicate cultures, the electron recovery in acetate and 2-oxobutyrate was 86% ± 21% of the electrons transferred at the cathodes. These results demonstrated that S. ovata could accept electrons from graphite electrodes with the reduction of carbon dioxide and that most of the electrons transferred from the electrodes to the cells were diverted toward extracellular products, rather than biomass formation. The S. ovata cathode biofilms were robust and have been run for periods of more than 3 months without losing their capacity for current consumption and acetate production.
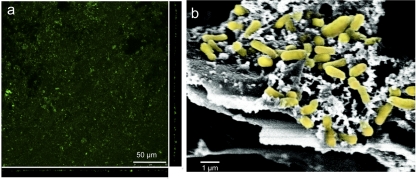
The long-term viability of S. ovata biofilms was further evident from confocal scanning laser microscopy of a biofilm that had been fixing carbon dioxide for 3 months. The cells in biofilms treated with LIVE/DEAD BacLight viability stain, as previously described (16, 17), stained green, suggesting that they were healthy and metabolically active (Fig. 2a). The biofilms were relatively thin, similar to the biofilms previously described for other microorganisms growing on cathodes (9, 11, 12). This was further confirmed with scanning electron micrographs of the cathode surface (Fig. 2b), prepared as previously described (19). The cells appeared to be intimately associated with the graphite surface, as would be expected for direct electrode-to-cell electron transfer (9, 11, 12). There was no visible turbidity in the cathode chamber, consistent with previous studies on direct electrode-driven respiration (9–12) and further suggesting that biofilm cells were primarily responsible for current consumption and carbon dioxide reduction.
FIG 2 .
Cathode biofilms of S. ovata. (a) Confocal scanning laser microscopic images (top down and side views) of cathode surface. Cells were stained with LIVE/DEAD BacLight viability stain. (b) Scanning electron microscopic image of cathode surface with cells highlighted in yellow.
Implications.
These results demonstrate that S. ovata is capable of using electrons derived from an electrode as the sole electron donor for the reduction of carbon dioxide to acetate. The high coulumbic efficiencies of acetate production (electrons appearing in acetate/electrons consumed as current) are consistent with the following reaction: 2CO2 + 2 H2O → CH3COOH + 2O2.
This conversion of carbon dioxide and water to an organic compound and oxygen is the same net reaction as oxygenic photosynthesis. We propose the term microbial electrosynthesis for the reduction of carbon dioxide to multicarbon compounds with electrons donated from an electrode as the electron donor. Microbial electrosynthesis differs significantly from photosynthesis in that carbon and electron flow is directed primarily to the formation of extracellular products, rather than biomass. Biomass typically requires extensive additional processing for chemical or fuel production. When coupled to a photovoltaic system, microbial electrosynthesis offers a new photosynthetic technology for the production of organic products with the added advantage that photovoltaic technology is orders of magnitude more effective in capturing solar energy than photosynthesis is (20).
Although acetate has economic value (6), a more important consideration is that acetate is formed from acetyl coenzyme A (acetyl-CoA) (6, 7), which is the central intermediate for the genetically engineered production of a wide range of chemical commodities as well as potential liquid transportation fuels (21, 22). The fact that small amounts of 2-oxobutyrate were produced, in addition to acetate, demonstrates that even without any engineering, some carbon and electron flow was diverted away from acetate production. The acetogen Clostridium ljungdahlii has recently been genetically engineered to produce the gasoline substitute butanol from acetyl-CoA (23). Attempts to genetically engineer S. ovata and other acetogens to produce products other than acetate via microbial electrosynthesis are under way.
ACKNOWLEDGMENTS
This research was supported by the Office of Science (BER), U.S Department of Energy, Cooperative Agreement DE-FC02-02ER63446.
We thank Daniel Drell, Department of Energy, for thought-provoking discussions that led to the development of this project.
Footnotes
Citation Nevin, K. P., T. L. Woodard, A. E. Franks, Z. M. Summers, and D. R. Lovley. 2010. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1(2):e00103-10. doi:10.1128/mBio.00103-10.
REFERENCES
- 1. Lewis N. S., Nocera D. G. 2006. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. U. S. A. 103:15729–15735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centi G., Perathoner S. 2009. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 148:191–205 [Google Scholar]
- 3. Cole E. B., Bocarsly A. B. 2010. Photochemical, electrochemical, and photochemical reduction of carbon dioxide, p. 291–316 In Aresta M., Carbon dioxide as chemical feedstock. Wiley-VCH Verlag GmbH & Co., Weinheim, Germany [Google Scholar]
- 4. Gattrell M., Gupta N., Co A. 2006. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 594:1–19 [Google Scholar]
- 5. Olomon C., Li H. 2008. Electrochemical processing of carbon dioxide. ChemSusChem 1:385–391 [DOI] [PubMed] [Google Scholar]
- 6. Drake H. L., Gössner A. S., Daniel S. L. 2008. Old acetogens, new light. Ann. N. Y. Acad. Sci. 1125:100–128 [DOI] [PubMed] [Google Scholar]
- 7. Muller V. 2003. Energy conservation in acetogenic bacteria. Appl. Environ. Microbiol. 69:6345–6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aulenta F., Reale P., Catervi A., Panero S., Majone M. 2008. Kinetics of trichlorethene dechlorination and methane formation by a mixed anaerobic culture in a bio-electrochemical system. Electrochim. Acta 53:5300–5305 [Google Scholar]
- 9. Gregory K. B., Bond D. R., Lovley D. R. 2004. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 6:596–604 [DOI] [PubMed] [Google Scholar]
- 10. Gregory K. B., Lovley D. R. 2005. Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ. Sci. Technol. 39:8943–8947 [DOI] [PubMed] [Google Scholar]
- 11. Strycharz S. M., Gannon S. M., Boles A. R., Nevin K. P., Franks A. E., Lovley D. R. 2010. Anaeromyxobacter dehalogenans interacts with a poised graphite electrode for reductive dechlorination of 2-chlorophenol. Environ. Microbiol. Rep. 2:289–294 [DOI] [PubMed] [Google Scholar]
- 12. Strycharz S. M., Woodward T. L., Johnson J. P., Nevin K. P., Sanford R. A., Loeffler F. E., Lovley D. R. 2008. Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by Geobacter lovleyi. Appl. Environ. Microbiol. 74:5943–5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng S., Xing D., Call D. F., Logan B. E. 2009. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 43:3953–3958 [DOI] [PubMed] [Google Scholar]
- 14. Villano M., Aulenta F., Ciucci C., Ferri T., Giuliano A., Majone M. 2010. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresource Technol. 101:3085–3090 [DOI] [PubMed] [Google Scholar]
- 15. Moller B., Obmer R., Howard B. H., Gottschalk G., Hippe H. 1984. Sporomusa, a new genus of gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch. Microbiol. 139:388–396 [Google Scholar]
- 16. Nevin K. P., Richter H., Covalla S. F., Johnson J. P., Woodard T. L., Jia H., Zhang M., Lovley D. R. 2008. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ. Microbiol. 10:2505–2514 [DOI] [PubMed] [Google Scholar]
- 17. Reguera G., Nevin K. P., Nicoll J. S., Covalla S. F., Woodard T. L., Lovley D. R. 2006. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 72:7345–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cord-Ruwisch R., Seitz H., Conrad R. 1988. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch. Microbiol. 149:350–357 [Google Scholar]
- 19. Araujo J. C., Téran F. C., Oliveira R. A., Nour E. A. A., Montenegro A. P., Campos J. R., Vazoller R. F. 2003. Comparison of hexmethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J. Electron Microsc. 52:429–433 [DOI] [PubMed] [Google Scholar]
- 20. JASON, Mitre Corporation 2006. Engineering microorganisms for energy production. Department of Energy Report JSR-05-300. JASON, Mitre Corporation, McLean, VA [Google Scholar]
- 21. Fortman J. L., Chhabra S., Mukhopadhyay A., Chou H., Lee T. S., Steen E., Keasling J. D. 2008. Biofuel alternatives to ethanol: pumping the microbial well. Trends Biotechnol. 26:375–381 [DOI] [PubMed] [Google Scholar]
- 22. Yan Y., Liao J. C. 2009. Engineering metabolic systems for the production of advanced fuels. J. Ind. Microbiol. Biotechnol. 36:471–479 [DOI] [PubMed] [Google Scholar]
- 23. Kopke M. 2009. Genetische Veranderung von Clostridium ljungdahlii zur Produktion von 1-butanol aus Synthesegas. Ph.D. Dissertation. Ulm University, Hamburg, Germany [Google Scholar]