Abstract
Background
Reduced metabolism, blood flow, and tissue volume have been detected in the dorsolateral prefrontal cortex (dlPFC) of neurologically intact alcoholic subjects and these deficits are accompanied by lower density of neurons and glial cells. Another prefrontal region, the orbitofrontal cortex (ORB), functionally and structurally differentiated from the dlPFC, and heavily involved in decision-making processes, also shows functional alterations in alcoholic subjects. However, it is unknown whether changes in the packing density of neurons or glial cells also occur in the ORB and whether that density may be related to the increased suicide probability of alcoholic subjects or to the duration of alcohol dependence.
Methods
The present study used a 3-dimensional cell-counting method in postmortem brain tissue to determine the packing density of neurons and glial cells in the ORB (area 47) of 15 subjects with alcohol dependence (8 suicides, 7 nonsuicides) and 8 normal controls and to determine whether cell density is correlated with suicide and duration of alcohol dependence.
Results
There was a significantly lower density of both neurons (by 27%) and glial cells (by 25%) in the ORB of alcoholic subjects compared with controls. Packing density of either neurons or glial cells was not significantly different in alcoholic suicides compared with alcoholic nonsuicides. Age was not correlated with neuronal or glial density in either group. However, the duration of alcohol dependence and the ratio of that duration to the length of life span were significantly and negatively correlated to the overall density of neurons.
Conclusion
The present results indicate that alcohol dependence is associated with a decrease in the packing density of neurons and glia in the ORB and that the reduction in neuronal but not glial density progresses with the duration of alcohol dependence.
Keywords: Addiction, Alcoholism, Prefrontal Cortex, Neuropathology, Morphometry
Recent research in alcoholic subjects without major neurological impairments has shown a reduction in the packing density of glial cells in the dorsolateral prefrontal cortex (dlPFC) (Miguel-Hidalgo et al., 2002) and the hippocampus (Korbo, 1999). Furthermore, the magnitude of this reduction in glial density contrasts with lesser or nonsignificant reduction in the numbers of neurons found in these and other cortical regions in alcoholic subjects without Wernicke's encephalopathy or Korsakoff's syndrome (Korbo, 1999; Kril et al., 1997; Kril and Harper, 1989). Several areas within the prefrontal cortex (PFC) demonstrate physiological and structural alterations in alcoholism and other psychiatric disorders, and these alterations correlate with disturbances of cognition, emotion, and motivation (Catafau et al., 1999; Dao-Castellana et al., 1998; Freund and Anderson, 1996; Steketee, 2003; Sullivan et al., 2000). The dlPFC is significantly affected by chronic alcohol-use disorders and there are functional and histopathological studies demonstrating a heavier pathology in the dlPFC than in other brain areas outside of the PFC (Kril and Harper, 1989; Kril et al., 1997). However, for the orbitofrontal cortex (ORB), a prefrontal region of great relevance to behaviors of addiction (Volkow and Fowler, 2000; Yan et al., 1998), there is little information on the cellular pathology that may be related to functional and behavioral alterations in alcoholic subjects. The study of putative histopathological anomalies in the ORB, however, is not trivial as the ORB exerts features of behavioral control distinct from those of the dlPFC and can even show patterns of activation opposite to the dlPFC when confronted with particular stimuli in normal subjects or in depressed patients compared with controls (Bechara et al., 2000; Drevets, 2001). The ORB is also involved in the emotional aspects of decision-making processes (Bechara et al., 2000), and given the significantly increased risk of suicide in alcoholic subjects (Cornelius et al., 1996; Kendall, 1983), it is pertinent to determine whether there are cellular alterations in the ORB that might be associated with increased risk for suicide. Moreover, the ORB is also particularly sensitive to the effects of aging as demonstrated by gross structural deficiencies in normal elderly subjects (Tisserand et al., 2002, 2004). Finally, recent studies in major depressive disorder (MDD) have also shown remarkable significant decreases in neuronal density in the ORB of elderly depressed subjects with major depression (Rajkowska et al., 2005).
The present study sought to determine whether there are alterations in the packing density of glial cells and neurons in Brodmann's area 47 of the ORB of neurologically intact alcoholic subjects compared with nonalcoholic control subjects. We also examined the possibility of differential cellular pathology in the alcoholic subjects dying by suicide compared with subjects dying by other causes.
Materials and Methods
Human Subjects (Table 1)
Table 1.
Characteristics of the Subjects Included in the Study
| Subject | Cause of death | Age/gender/race | Duration of dependencea | PMI | pH | TF |
|---|---|---|---|---|---|---|
| Control | ||||||
| 1 | N | 71/M/C | 24 | 6.82 | 24 | |
| 2 | N | 58/M/C | 22 | 6.78 | 35 | |
| 3 | H | 24/M/AAm | 15 | 6.84 | 37 | |
| 4 | N | 30/F/C | 9 | 6.75 | 32 | |
| 5 | N | 47/M/C | 17 | 6.89 | 7 | |
| 6 | A | 23/F/C | 11 | 6.85 | 23 | |
| 7 | H | 46/F/C | 27 | 6.32 | 13 | |
| 8 | N | 27/F/C | 15 | 7.01 | 22 | |
| Mean ± SD | Age 41 ± 18 | 18 ± 6 | 6.78 ± 0.20 | 24 ± 10 | ||
| Alcoholism | ||||||
| 9 | S | 50/M/C | 2 | 17 | 6.67 | 78 |
| 10 | S | 36/M/C | 21 | 15 | 6.72 | 12 |
| 11 | S | 37/M/C | 23 | 19 | 6.89 | 68 |
| 12 | S | 24/M/C | 4 | 12 | 6.89 | 64 |
| 13 | S | 45/M/C | 7 | 29 | 6.86 | 59 |
| 14 | S | 51/M/C | 33 | 22 | 7.07 | 52 |
| 15 | S | 44/M/C | 10 | 20 | 6.77 | 27 |
| 16 | S | 30/F/C | 9 | 17 | 6.77 | 31 |
| 17 | N | 61/M/AAm | 46 | 27 | 6.68 | 75 |
| 18 | N | 29/M/C | 14 | 27 | 6.75 | 26 |
| 19 | A | 30/M/C | 15 | 12 | 6.49 | 66 |
| 20 | N | 54/M/C | 39 | 24 | 6.42 | 61 |
| 21 | N | 41/F/C | 20 | 24 | 6.74 | 60 |
| 22 | A | 50/M/C | 20 | 14 | 6.66 | 45 |
| 23 | N | 40/F/C | 21 | 27.5 | 6.67 | 34 |
| Mean ± SD | Age 41 ± 11 | 19 ± 13 | 20 ± 6 | 6.74 ± 0.16 | 50 ± 20 | |
AAm, African American; A, accident; C, Caucasian; F, female; H, homicide; M, male; N, natural; PMI, postmortem interval (hours), defined as the time between death and the beginning of the formalin fixation; S, suicide, TF, time in formalin (months).
The duration (years) of alcohol dependence covers the time between the first display (and not necessarily diagnosis) of signs of dependence and the date of death.
Brain tissue was collected at autopsies performed at the Cuyahoga County Coroner's Office in Cleveland, Ohio. Institutional Review Board policies were followed in obtaining informed consent from the next of kin and performing retrospective psychiatric assessments for all subjects. Retrospective psychiatric assessments and establishment of diagnoses were based on information obtained from knowledgeable informants (significant others or first-degree family members) and medical records of control and alcoholic subjects. Information regarding psychiatric symptoms or the lack thereof was collected by means of the Structured Clinical Interview for DSM-IV Psychiatric Disorders (First et al., 1995). Diagnosis of alcohol dependence for individual subjects was made according to the Diagnostic and Statistic Manual of Mental Disorders (DSM-IV) (American Psychiatric Association, 1995). Kelly and Mann (1996) have shown that there is good agreement between informant-based retrospective psychiatric assessments of deceased subjects and chart diagnoses generated by clinicians treating the same subjects before death. Estimates of the duration of abuse were based on the interviews of next of kin and close relationships and on available medical records of the deceased. Only those subjects for whom a good estimate of duration of abuse can be made (using the above sources) are included in our studies. Additional information on the subjects was gathered from hospital medical records, prior medical or substance abuse problems, and postmortem toxicology reports. Potential subjects with evidence of head trauma or neurologic disease were excluded from the study. One of the alcoholic subjects was diagnosed with cirrhosis of the liver at the time of death. Tremors and blackouts were described for several subjects in the family interviews, but these episodes were all reported to be associated with the periods of alcohol intoxication. The medical records available and family reports did not report that any of the alcoholic subjects in the present study had Korsakoff's psychosis or Wernicke's encephalopathy. In the alcohol-dependent group (subjects 9–23, Table 1), 6 subjects were found to have had episodes of MDD (cases 9, 10, 11, 13, 15, 16), 1 was diagnosed with alcohol-related mood disorder (case 14), and 1 was diagnosed with dysthymia (case 12). Seven subjects with alcohol dependence died by suicide (Table 1). The mood disorders detected in the alcohol-dependent group were reported as starting following the emergence of alcohol dependence. Subjects with a history of MDD or depressed mood (depression that did not reach DMS-IV criteria for MDD or bipolar disorder) beginning before the onset alcohol dependence were excluded from the study. The normal control group was composed of 8 subjects who were included in our previous study on cell pathology in the ORB in MDD (Rajkowska et al., 1999). Four subjects in the control group and 12 subjects with alcohol dependence were tobacco smokers. The subjects included in this study were younger than 71 years of age at the time of death, had a postmortem interval less than 32 hours, and had fixation time in formalin (TF) of less than 66 months (Table 1). For each subject, a pH value (Table 1) was obtained using nonfixed brain tissue stored frozen at −80 °C at the time of autopsy. Postmortem ethanol toxicology was performed in all subjects. In controls, there was no detectable blood ethanol. Among alcoholic subjects, 6 did not have detectable levels of blood ethanol and the level of ethanol in the remaining 9 subjects ranged between 0.02 and 0.27 g/dL, with an average of 0.135 ± 0.0276 (mean ± standard error of the mean) in these 9 subjects.
Tissue Sampling
Brain tissue from the ORB (Brodmann's area 47) was examined in 15 subjects who were retrospectively diagnosed with alcohol dependence and in 8 psychiatrically normal control subjects. These 2 groups were closely matched by age, gender, race, postmortem delay, and tissue pH (see Table 1) and there was no significant differences in any of these variables between the groups. There was, however, a significant difference in the storage TF (defined as the period between the immersion in formalin and the starting of the celloidin embedding process) between those groups (Table 1). In spite of this difference, the values of glial and neuronal density were not correlated with TF in any of our cohorts (in controls, for glial density r2 = 0.004, p = 0.889; for neuronal density r2 = 0.017, p = 0.755; in alcoholic subjects, for glial density r2 = 0.025, p = 0.571; for neuronal density r2 = 0.037, p = 0.490). The detection of the borders of the rostral part of Brodmann's area 47 located in front of the transverse sulcus was based on cytoarchitectonic criteria reported previously (Rajkowska et al., 1999). Blocks of tissue (3×3 cm) dissected from area 47 were embedded in 12% celloidin, cut into 40-μm-thick sections, and stained for Nissl substance with cresyl violet.
3-Dimensional Cell Counting
Neuronal and glial cell packing density was measured in a microscope with a ×100 magnification objective (numerical aperture 1.3). The stained cell nucleus was the structure designated for counting in both neurons and glial cells. The counting method involved a computer-assisted image analysis with 3-dimensional (3-D) cell counting capabilities based on the optical dissector probe (Gundersen, 1986; Williams, 1989; Williams and Rakic, 1988). The morphometric parameters were measured using 3 sections per brain. The 3 sections were evenly spaced at 400-μm intervals in the anteroposterior extent of area 47, with the first section chosen at random within the anterior third of area 47. In each section, 1 probe was randomly located within rostral area 47. Each probe consisted of an uninterrupted series of 3-D counting boxes (90×60×25 μm), or sampling sites, spanning the entire depth of the cortex [for further details on the method, see Rajkowska et al., 1998; Selemon et al., 1995]. With this method, each section produced about 20 sampling sites. Consequently, from each brain, we counted cells in approximately 60 sampling sites. As there are 6 cortical layers, each layer of the brain was sampled 10 times per brain, although using our method some layers (e.g., layer III) may have been in all represented with a higher frequency than the other layers. As in each sampling site we counted all cells and there was an average of 8 neurons and about 14 glial cells per counting box, the number of neurons counted per brain was about 480 and the number of glial cells counted was about 840. Cell densities are expressed as number of cells×103/mm3. Alternating brain sections were coded and counted by 2 researchers who were unaware of the diagnoses of the subjects while counting.
Glial cells were distinguished from small neurons in cresyl violet–stained sections by the lack of a Nissl-stained cytoplasm, the presence of several dark chromatin granules (with the exception of the nucleolus, chromatin in neuronal nuclei has a more uniform and smooth appearance), and a thick condensation of stained material in the periphery of the nucleus that gives the appearance of a heavily stained membrane enclosing the nuclear content (Fig. 1).
Fig. 1.
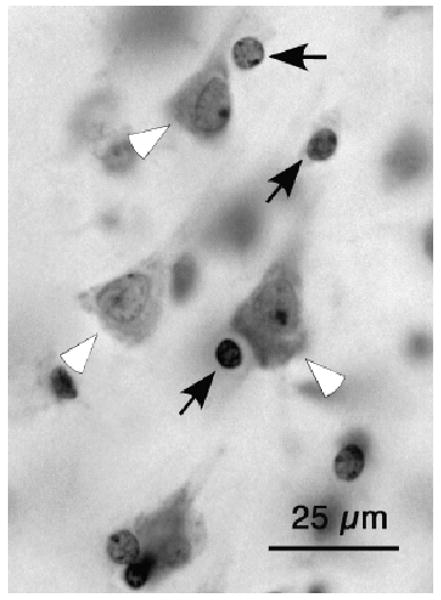
Grayscale micrograph of neuronal somata (white arrowheads) and glial cell nuclei (black arrows) stained with cresyl violet (a Nissl-type staining) in layer III of human cortical area 47.
Statistics
Individual values for the 3 main dependent variables (neuronal density, glial cell density, and cortical thickness) included in the statistical analysis were obtained by averaging the mean values in the 3 probes per subject. These individual values were obtained first for all layers combined and compared between controls and alcoholic subjects. Comparisons between the groups for each dependent variable were performed with analysis of covariance (ANCOVA) with TF, age at the time of death, postmortem delay, and brain pH as covariates. Although TF significantly differed between controls and alcohol-dependent subjects (X ± SD; 23.95 ± 10.31 months in controls, 50.34 ± 20.10 months; see Table 1), there was no correlation between glial or neuronal density and TF in either group studied. The groups were also compared using repeated measures ANCOVA, considering the values of cell density in each of the 6 different cortical layers as repeated measures. When significant comparisons were obtained with the ANCOVA, univariate selected contrast analyses were performed between pairs of groups for all layers combined or for individual cortical layers. Multiple correlation analysis was used to examine any potential influence of age, postmortem delay, TF, and pH of the brain on the dependent variables studied. Mean values in Table 2 and the figures are presented without adjusting for the covariates.
Table 2.
Mean Values of Glial and Neuronal Packing Density
| Control | Alcoholic | F(1, 17) | Significance, p < | |
|---|---|---|---|---|
| Glia by layers | ||||
| I | 99.0 ± 8.2 | 80.1 ± 3.1 | 13.59 | 0.002 |
| II | 93.9 ± 11.7 | 75.4 ± 3.6 | 2.29 | 0.149 |
| III | 93.5 ± 6.1 | 74.5 ± .5 | 8.23 | 0.011 |
| IIIa | 88.1 ± 4.2 | 69.1 ± 4.1 | 4.77 | 0.043 |
| IIIb | 91.2 ± 6.5 | 71.0 ± 2.5 | 7.22 | 0.016 |
| IIIc | 100.1 ± 7.3 | 87.6 ± 3.4 | 3.33 | 0.086 |
| IV | 109.2 ± 5.9 | 88.1 ± 2.9 | 6.06 | 0.025 |
| V | 107.2 ± 8.4 | 83.9 ± 3.4 | 4.92 | 0.040 |
| Va | 103.6 ± 8.8 | 81.6 ± 3.1 | 5.01 | 0.039 |
| Vb | 110.7 ± 8.6 | 86.1 ± 4.8 | 2.79 | 0.113 |
| VI | 138.7 ± 7.8 | 96.0 ± 3.2 | 16.84 | 0.001 |
| All layers | 109.1 ± 6.6 | 82.0 ± 2.3 | 14.85 | 0.001 |
| Glia by layers | ||||
| I | 26.8 ± 1.8 | 11.4 ± 1.0 | 37.93 | 0.0001 |
| II | 125.1 ± 4.7 | 101.5 ± 2.9 | 8.12 | 0.011 |
| III | 62.8 ± 2.0 | 55.9 ± 1.1 | 3.67 | 0.072 |
| IIIa | 80.2 ± 3.3 | 77.2 ± 6.1 | 0.12 | 0.736 |
| IIIb | 52.9 ± 1.8 | 48.0 ± 1.1 | 0.83 | 0.376 |
| IIIc | 69.9 ± 2.8 | 54.9 ± 2.1 | 7.52 | 0.014 |
| IV | 123.3 ± 4.6 | 102.4 ± 3.6 | 3.85 | 0.066 |
| V | 60.9 ± 2.4 | 59.5 ± 2.6 | 0.25 | 0.623 |
| Va | 73.2 ± 3.8 | 67.4 ± 2.8 | 0.29 | 0.595 |
| Vb | 50.8 ± 2.7 | 45.7 ± 3.7 | 0.00 | 0.989 |
| VI | 45.2 ± 1.7 | 46.9 ± 1.3 | 0.31 | 0.585 |
| All layers | 64.4 ± 1.0 | 54.9 ± 2.6 | 27.23 | 0.0001 |
Values of density are expressed as mean ± standard error of the mean. F(1, 17) corresponds to the values of the F statistic of the univariate contrasts between control subjects and alcoholic subjects. Significance, p <, significance values for each univariate contrast.
Results
Packing Density of Glial Cell Nuclei
When considering all cortical layers combined, the ANCOVA (using TF, age at the time of death, postmortem interval, and pH as covariates) revealed a significantly lower (25%) overall packing density of glial cells [F(2, 17) = 14.85, p = 0.001] in the group of alcohol-dependent subjects (82.0 ± 2.3 cells×103/mm3) compared with the group of control subjects (109.1 ± 6.6 cells×103/mm3; Fig. 2A). Repeated-measures ANCOVA with the packing density in each of layers I, II, IIIa, IIIb, IIIc, IV, Va, Vb, and VI as repeated measures also revealed a significant difference between both groups (F = 9.728, p = 0.006). Comparison of both groups for individual layers revealed that the reduction of glial packing density in alcohol-dependent subjects was particularly marked in layers I [F(1, 17) = 13.59, p < 0.002], IIIb [F(1, 17) = 7.22 p = 0.016; Fig. 2B], and VI [F(1, 17) = 16.84, p < 0.001; Fig. 2D; Table 2, GLIA]. There was a trend of a significant difference in layers IIIa, IIIc, and Va (Table 2, GLIA) and there was no significant difference between groups in layer Vb (Fig. 2C). A generalized reduction of glial cell density was consistent with the absence of a significant interaction between density in individual layers and diagnostic group [F(8, 136) = 1.24, p = 0.281].
Fig. 2.
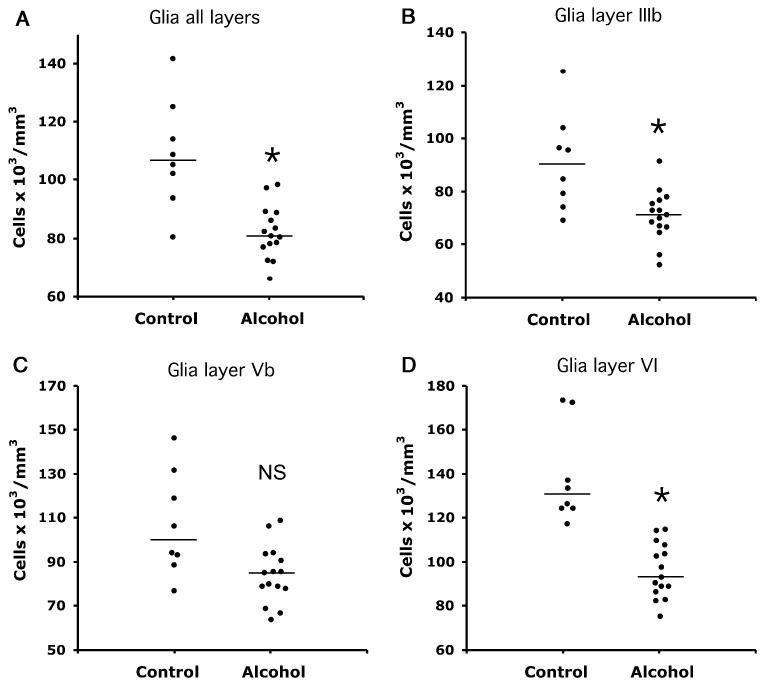
Plots of glial packing density in all layers combined (A), and in individual layers IIIb (B), V (C), and VI (D) in cortical area 47 of control and alcohol-dependent subjects. Horizontal lines denote median values. *Significant differences: (A) p < 0.001, (B) p = 0.016, (D) p < 0.001 (C) NS, nonsignificant difference.
Relation of Glial Cell Density to Death By Suicide
The alcohol-dependent group was sorted into 2 subgroups according to their mode of death (suicide versus nonsuicide), and a new ANCOVA was used to compare the overall (all layers combined) density of glial cells between the resulting 3 groups (controls, alcoholic subjects dying by suicide, and alcoholic subjects dying by other causes). There was a significant group difference in the overall glial density [F(2, 16) = 7.5, p = 0.005]. Univariate contrast analyses revealed comparable significant differenes between the controls and alcoholic suicide subjects [F(1, 16) = 14.8, p = 0.001] and between the controls and alcoholic nonsuicide subjects [F(1, 16) = 7.1, p = 0.017]. However, there was no significant difference between alcoholic suicides and alcoholic nonsuicides [F(1, 16) = 0.542, p = 0.472]. There was a significant difference in the density of glial cells among the 3 groups using repeated-measures ANCOVA, with density in individual cortical layers as repeated measures [F(2, 16) = 5.2, p = 0.003]. However, there was no significant difference between alcoholic suicide subjects and alcoholic nonsuicide subjects in any of the layers.
Packing Density of Neuronal Cell Bodies
In all cortical layers combined, there was a dramatically lower packing density of neurons (by 27.1%) [F(1, 17) = 27.23, p < 0.0005] in the group of alcohol-dependent subjects (46.9 ± 1.4 neurons×103/mm3) compared with the group of control subjects (64.4 ± 1.0 neurons × 103/mm3; Fig. 3A). Repeated-measures ANCOVA with the packing density in each of layers I, II, IIIa, IIIb, IIIc, IV, Va, Vb, and VI as repeated measures also showed a significant difference in neuronal density between both groups [F(1, 17) = 12.76, p < 0.002]. Comparison of both groups for individual layers revealed that the reduction of the packing density of neurons in alcohol-dependent subjects was significantly lower only in layers I [F(1, 17) = 37.93, p < 0.0001], II [F(1, 17) = 8.12, p = 0.011], and IIIc [F(1, 17) = 7.52, p = 0.014; Figs. 3B and 3C], but not in the other layers (e.g., Fig. 3D) (Table 2, neurons). This restriction of lower neuronal density to specific cortical layers in alcoholic subjects was reflected in a significant interaction of packing density in individual layers by group [F(8, 136) = 2.60, p = 0.011].
Fig. 3.
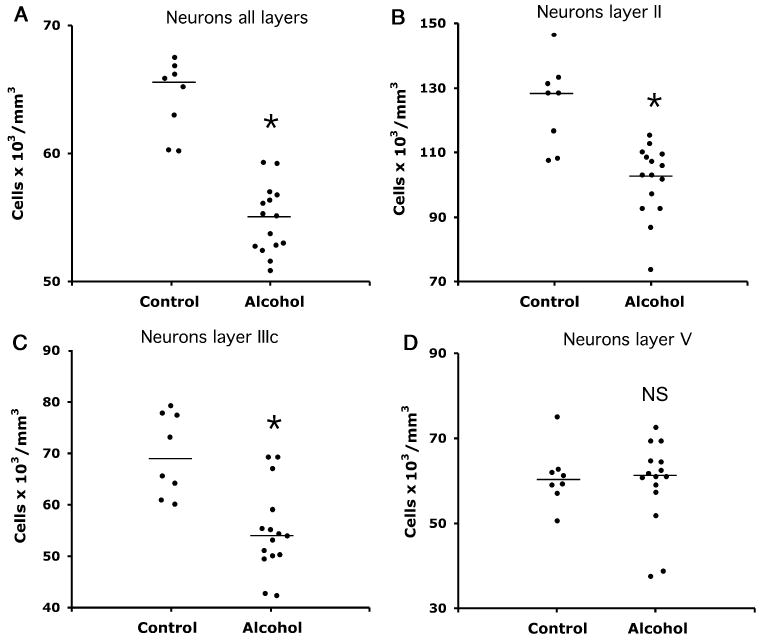
Plots of neuronal packing density in all layers combined (A) and in individual layers II (B), IIIc (C), and V (D) in cortical area 47 of control and alcohol-dependent subjects. Horizontal lines denote median values. *Significant differences: (A) p < 0.0005, (B) p = 0.011, (C) p < 0.014. (D) NS indicates nonsignificant difference.
Relation of Neuronal Density to Death by Suicide
Analysis of covariance comparing 3 groups (controls, alcoholic suicide subjects, and alcoholic nonsuicide subjects) revealed a significant group difference in overall neuronal density [F(2, 16) = 14.074, p < 0.0005], and univariate contrast analysis showed significant differences between the controls and each of the alcohol-dependent subgroups [suicides, F(1, 16) = 21.846, p < 0.0001; nonsuicides, F(1, 16) = 23.137, p < 0.0001]. However, there was no significant difference in overall neuronal density between alcoholic suicide subjects and alcoholic nonsuicide subjects [F(1, 16) = 0.967, p = 0.340]. Repeated-measures ANCOVA for the 3 groups, with density in individual layers as repeated measures, showed a significant difference in neuronal density as well [F(2, 16) = 5.241, p = 0.017]. Univariate contrast analysis revealed a significantly lower neuronal density in layers I, II, and IIIc in the alcoholic nonsuicide subjects compared with controls. There were no significant differences in the laminar density of neurons between alcoholic suicide subjects and alcoholic nonsuicide subjects or between alcoholic suicide subjects and controls.
Correlation of Glial and Neuronal Density With Age and Duration of Alcohol Dependence
Neither glial nor neuronal densities were significantly correlated with the age at the time of death in either the alcoholic group (neurons, r = −0.155, p = 0.581; glia, r = 0.213, p = 0.446) or the control group (neurons, r = 0.642, p = 0.086; glia, r = 0.509, p < 0.198). However, there was a significant negative correlation between the estimated duration of alcohol dependence in alcoholic subjects and the overall packing density of neurons (r = −0.596, p = 0.019; Fig. 4A). When the duration of dependence was expressed relative to the life span, there was again a negative, highly significant correlation between the density of neurons and that ratio (r = −0.695, p = 0.004; Fig. 4B). However, no significant correlation between glial density and duration of alcohol dependence was detected (r = −0.037, p = 0.896; Fig. 4C).
Fig. 4.
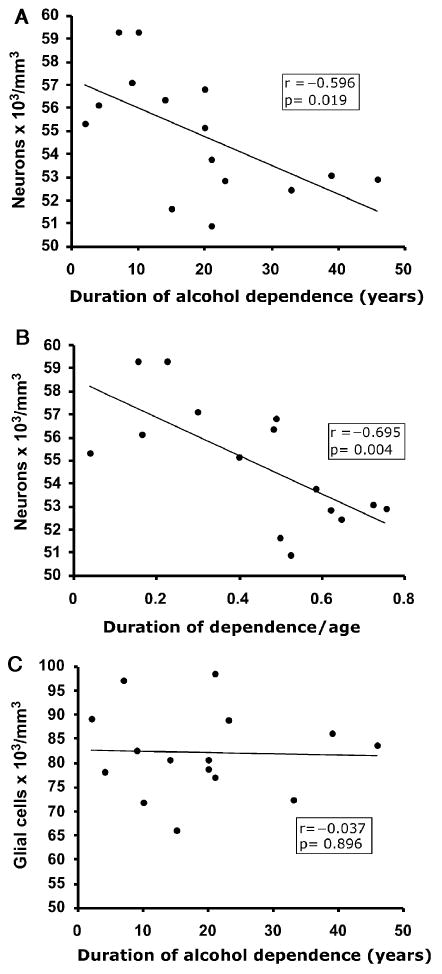
Scatter plots of the average neuronal (A and B) and glial (C) densities versus the estimated duration of alcohol dependence. (A) Duration of alcohol dependence versus neuronal density; (B) Ratio of the duration of dependence to the age at the time of death versus neuronal density; (C) Duration of alcohol dependence versus glial cell density. Note the significant negative correlation of neuronal density with the duration of abuse (A) or with the duration expressed in proportion to the life span (B). No correlation was observed between glial cell density and the age at the time of death (C).
Ratio of Glial Cells to Neurons
One of the possibilities for a general mechanism involving glial cell pathology in the pathophysiology of alcoholism is reduced neuronal support caused by a reduced number of glial cells per neuron. Accordingly, an additional analysis was performed by calculating the ratio of the overall glial density to the overall neuronal density (glia/neurons ratio) for each subject. ANCOVA using only age as a covariate showed a significantly lower ratio in the alcoholic group (1.50 ± 0.05) compared with the control group (1.70 ± 0.09) [ANCOVA, age, F(1, 17) = 5.01, p = 0.037]. Another ANCOVA including age, postmortem delay, TF, and pH as covariates showed that a tendency for a lower glia/neurons ratio was still present in the alcoholic group, although it was not statistically significant [F(1, 17) = 3.79, p = 0.068; Fig. 5A]. When considering cortical layers individually, the glia/neurons ratio was significantly lower in alcoholic subjects compared with control subjects in layers IIIa (0.90 ± 0.053 alcoholic subjects, 1.11 ± 0.068 controls; p = 0.026) and VI (2.08 ± 0.099 alcoholic subjects, 3.09 ± 0.169 controls; p = 0.002; Figs. 5B and 5C). However, there was no significant difference between alcoholic suicide subjects and alcoholic nonsuicide subjects.
Fig. 5.
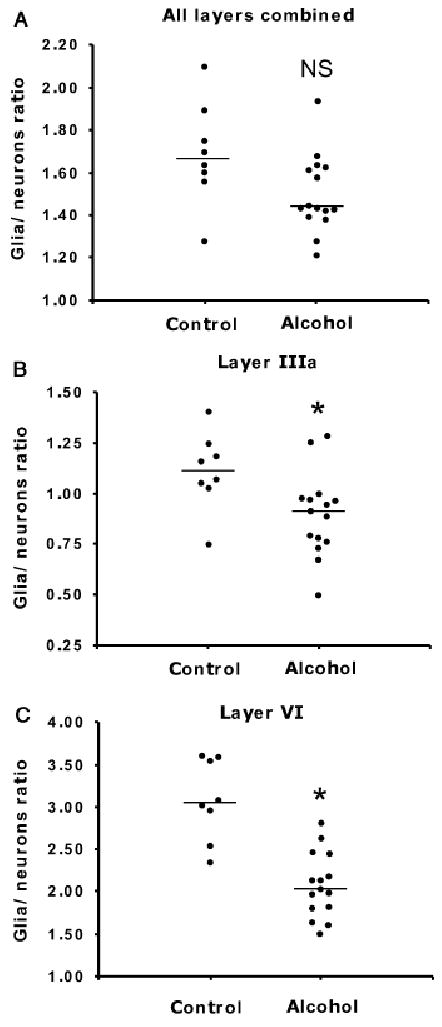
Plots illustrating average individual values of the ratio of glial cells to neurons in controls and alcoholic subjects in all cortical layers combined (A), layer IIIa (B), and layer VI (C). In (A), NS indicates nonsignificant; p = 0.068. *Significant differences after ANCOVA using age, postmortem interval, time in formalin, and tissue pH as covariates: in (B) p = 0.026, in (C) p = 0.002.
Ethanol Toxicology and Neuronal and Glial Density
There was no ethanol detected in control subjects. Among alcoholic subjects, 6 subjects had no detectable levels of ethanol. There was no significant difference in the density of glial cells or neurons between alcoholic subjects without detectable ethanol blood levels and those with detectable blood ethanol: overall glial cell density 81.1 ± 3.2 cells×103/mm3 (with ethanol), 83.5 ± 3.6 (without ethanol), p = 0.619; neuronal density 52.6 ± 6.9 cells×10/mm (with ethanol), 47.0 ± 8.5 (without ethanol), p = 0.612. Blood ethanol level was not correlated with glial (r2 = 0.0188, p = 0.626) or neuronal (r2 = 0.0362, p = 0.497) density in alcohol-dependent subjects. Moreover, in none of the cortical layers, glial and neuronal densities were significantly correlated to blood ethanol levels in the alcohol-dependent group.
Discussion
The present study revealed a significantly lower packing density of both glial and neuronal cells in area 47 of the ORB in alcohol-dependent subjects compared with nonalcoholic control subjects. A comparison of glial density in individual cortical layers indicates that the glial deficit is spread across cortical layers, as there was no significant statistical interaction between the densities of glia in particular layers and the diagnostic group. The density of neuronal cell bodies was also significantly lower in the alcohol-dependent subjects compared with controls. However, analysis of individual layers and a significant layer by diagnosis interaction suggests that the neuronal deficit was rather limited to layers I, II, and IIIc. A more widespread laminar deficit of glial cells than neurons in area 47 of alcoholic subjects is also consistent with the tendency for a lower glia-to-neuron ratio in several of the alcoholic subjects included in the present study.
As 8 of 15 alcohol-dependent subjects died by suicide, the difference between alcohol-dependent subjects and controls may have been related to the cause of death. Studies of postmortem brain tissue have provided some evidence for neurochemical or cellular changes in subjects dying by suicide, even if suicide probability is significantly elevated under many different disorders and circumstances (Arora and Meltzer, 1989a, 1989b; Lecomte and Fornes, 1998; Mann et al., 1999; Moscicki, 1995; Stockmeier et al., 1998; Suominen et al., 1997). However, in the present study, there was no difference in glial or neuronal densities between alcoholic subjects dying by suicide or not by suicide. The absence of a suicide-related difference in cell densities does not support a specific role of changes in neuronal or glial densities in the ORB as a factor related to suicide. This lack of a difference in cell density, however, does not rule out that changes in cell numbers are related to the increased probability of suicide in alcoholic subjects compared with the general population (Sher, 2006). Alcoholism also increases mortality due to causes other than suicide (Laatikainen et al., 2003), and in a given sample of subjects, many subjects who might have died later by suicide actually may have died by other causes unrelated to suicide. Thus, the increased mortality by causes other than suicide might lead to underestimate the influence of prefrontal pathology on suicidal behavior in alcoholic subjects.
That the layers with the most prominent glial deficits do not match those layers where deficits of neuronal somata were found may be explained by the known dendritic cytoarchitecture of the cortex, where somata located in a layer send dendrites that reach across several layers (this will also be consistent with more widespread glial deficits). For instance, this cross-layers dendritic distribution could be the reason why a significantly lower density of neurons in layers II and IIIc is accompanied by a reduction of glial cells in overlaying layers I and IIIb, where many dendrites from neurons in layers II and IIIc, respectively, are located. However, further studies are necessary to determine what is the mechanism, if any, that links putative dendritic deficits in particular layers to a decrease in the glial cells of those layers.
The lower density of glial cells generalized across layers of the ORB adds evidence for localized glial deficits in the cortex of alcoholic subjects, as has been reported previously in the dlPFC and the hippocampus (Korbo, 1999; Miguel-Hidalgo et al., 2002). In addition, the present results indicate that a glial deficit in the PFC is not restricted to the dlPFC (area 9) but it also extends to the ventral ORB (area 47). Nevertheless, the relationship of this glial deficit with age is different in dlPFC and ORB. In dlPFC, glial cell density in alcoholic subjects was significantly and positively correlated with age (Miguel-Hidalgo et al., 2002) while in the ORB no such correlation was found in the present study. The difference in the relationship of overall glial cell density with age between dlPFC and ORB does not rule out that 1 of the 3 different glial cell types (astrocytes, oligodendrocytes, or microglia) are equally correlated to age in both brain regions. The need still remains to detail the changes in each of the glial cell types to fully explain the different relationship of glial cell density with age in the ORB compared with dlPFC.
The generalized glial deficit observed across areas might argue for a slow degeneration or a reduction in glial cell proliferation related to chronic alcohol abuse. Regressive responses of astrocytes to chronic alcohol exposure would also be consistent with the known antiproliferative and toxic properties of ethanol on astrocytes and oligodendrocytes in vivo and in vitro (Davies and Cox, 1991; Davies and Ross, 1991; Davies and Vernadakis, 1984; Isenberg et al., 1992; Khokhrina et al., 1991; Snyder et al., 1992). Alternatively, although not necessarily in exclusion, reductions in glial cells might be an indirect result of pathological neuronal function in the PFC of alcoholic subjects. Chronic ethanol acting on neuronal GABA or glutamate receptors or other effector neuronal proteins will change neuronal activity and neurotransmitter release from those neurons. Changes in extracellular levels of neurotransmitters may affect the numbers of glial cells because the proliferation of astrocytes can be regulated by the extracellular levels of neurotransmitters released by neurons (Ciccarelli et al., 1997, 2000; Feinstein and Rozelman, 1997; Guizzetti et al., 1996; Rathbone et al., 1999). In addition, there is evidence for deficits in some neurotransmitter systems caused by chronic ethanol consumption in humans (Arendt, 1994; Melis et al., 1996) and ethanol itself inhibits glial cell proliferation caused by activation of neurotransmitter receptors (Guizzetti and Costa, 1996).
Another mechanism that might contribute to the apparently reduced number of glia, at least in some subjects at risk, is a lower packing density of glial cells predating the intake of alcohol. A preexisting deficit would be consistent with the fact that in several of the subjects with alcoholism in the present study the glia/neurons ratio was lower than in the majority of controls and the highest values of glial density and glia/neurons ratio were found only among subjects older than 40 years of age. Further support for the possibility of a preexisting deficit of glial cells, at least in some alcoholic subjects, is provided by recent work with alcohol-preferring (P) rats, a model for alcohol preference and increased risk for alcohol dependence. In this rat model, it was found that the packing density of astrocytes immunoreactive for GFAP (an astrocytic cytoskeletal marker) or glutamine synthetase (the astrocytic enzyme in charge of recycling released glutamate) is significantly lower in P rats (whether ethanol drinking or ethanol naïve) than in alcohol nonpreferring (NP) rats or nonselected Wistar rats (Miguel-Hidalgo, 2005, 2006). Clearly, the different mechanisms proposed here to explain the lower glial numbers in alcohol-dependent subjects are not mutually exclusive, and their different combinations might be at work in different subgroups of alcoholic subjects. Nevertheless, the predominance of one of the possible mechanisms of glial attrition might have different implications for the onset and progression of the alcoholism. A preexisting glial deficit might facilitate the onset of behaviors conducive to alcohol dependence, while alcohol-related glial damage caused by abusing alcohol might contribute to the maintenance of alcohol dependence.
Interestingly, we found no correlation between the lower numbers of glial cells and the duration of alcohol dependence. However, the 3 main glial cell types (astrocytes, oligodendrocytes, and microglia), all of which can be toxically affected by alcohol (Snyder, 1996), may each respond differently to chronic alcohol exposure and alter their proportions without changing their combined packing density. In fact, later in life, the combined number of glial cells in the dlPFC of alcohol-dependent subjects appears to increase to levels comparable to those in the normal brain (Miguel-Hidalgo et al., 2002).
According to the results presented here, the packing density of glial cells in alcoholic subjects appears to be lower starting relatively early in the manifestation of alcohol dependence, while lower neuronal numbers appear only later, and are strongly dependent on the duration of dependence. Thus, there is the possibility that relatively early lower glial numbers or impaired glial function in the ORB contribute to the progressive depletion of neurons in the PFC of chronic alcoholic subjects.
The lower neuronal packing density detected in the ORB in area 47 of alcoholic subjects suggests that neuronal loss associated with chronic alcoholism is not restricted to the superior frontal gyrus, where the dlPFC is located (Kril et al., 1997), but can also be found in the orbitofrontal region of the PFC, located on the inferior frontal gyrus. During aging, and in neurodegenerative and psychiatric disorders, the ORB also shows proclivity for reduced cortical thickness and reduced number, packing density, or soma size of neurons, particularly in layer III (Holthoff et al., 2005; Rahman et al., 1999a, 1999b; Rajkowska et al., 1999, 2005; Tisserand et al., 2002, 2000). Alcoholism-related neuronal deficits in this cortical region will affect those features of behavior that are dependent on the normal function of the ORB, provided that the packing density of neurons influences the output of ORB neurons to other brain regions.
In neuroimaging studies, it is well documented that the duration of alcoholism is inversely related to the volume of some cerebral regions (Crews, 1999; Harper and Matsumoto, 2005; Laakso et al., 2002). Volumes of the dlPFC and the ORB are inversely correlated to the duration of alcoholism. Other neuroimaging studies that used magnetic resonance imaging have also found a positive correlation between cortical T1 relaxation times and lifetime consumption of ethanol (Chick et al., 1989). In addition, research on the influence of alcohol dependence on the numbers of hypothalamic neurons has revealed that the numbers of vasopressin neurons in the paraventricular nucleus inversely correlate with the duration alcohol dependence and the dose of alcohol ingested (Harding et al., 1996; Harper, 1998). The duration-dependent decrease in neuronal density found in the present investigation in the ORB adds support to the hypothesis that neuronal loss is an important contributor to duration-dependent decreases in the volume of prefrontal areas in alcoholic subjects (Laakso et al., 2002). In addition, the absence of a correlation of detected autopsy ethanol levels and the density of neurons and glial cells is also consistent with a major effect of prolonged alcohol dependence, rather than an acute effect of elevated ethanol, on the changes in glial and neuronal density detected in this study in the ORB.
The results in the present report do not necessarily rule out an influence of aging on the cellular consequences of alcoholism in the ORB, as there were only 2 subjects older than 60 (1 in the alcoholic group and 1 in the control group), and many aging-related changes are more conspicuous after that age. Nevertheless, when the duration of alcohol dependence was expressed in proportion to the extent of the life span, there was still a strong negative correlation between this ratio and neuronal packing density. The strong correlation between the portion of the life spent with alcohol dependence and the decline in neuronal density is consistent with a very important role of duration of dependence in the neuronal neuropathology of the ORB in alcoholism. The correlation also points to a complex interaction between the ability of neurons to resist alcohol-related pathology, the duration of alcohol dependence, and possibly age. Further studies focusing on each of these factors and including higher numbers of subjects are necessary.
Acknowledgments
The authors gratefully acknowledge the assistance of Bryan Roth. MD, PhD, George Jurjus, MD, and Ginny Dilley in the establishment of retrospective psychiatric diagnoses. We thank Jingrong Wei for assistance with tissue preparation and cell counting. The excellent assistance of the Cuyahoga County Coroner's Office, Cleveland, OH, is greatly appreciated, as is the cooperation and support of the next of kin of the deceased.
This research was supported by grants from the National Institute of Mental Health (MH45488, MH61387, MH61578, MH60451), the National Center for Research Resources (RR017701), and the Alcohol Beverage Medical Research Foundation.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 1995. [Google Scholar]
- Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm. 1994;44(suppl):173–187. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- Arora RC, Meltzer HY. 3H-imipramine binding in the frontal cortex of suicides. Psychiatry Res. 1989a;30:125–135. doi: 10.1016/0165-1781(89)90154-6. [DOI] [PubMed] [Google Scholar]
- Arora RC, Meltzer HY. Serotonergic measures in the brains of suicide victims: 5-HT2 binding sites in the frontal cortex of suicide victims and control subjects. Am J Psychiatry. 1989b;146:730–736. doi: 10.1176/ajp.146.6.730. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Catafau AM, Etcheberrigaray A, Perez de los Cobos J, Estorch M, Guardia J, Flotats A, Berna L, Mari C, Casas M, Carrio I. Regional cerebral blood flow changes in chronic alcoholic patients induced by naltrexone challenge during detoxification. J Nucl Med. 1999;40:19–24. [PubMed] [Google Scholar]
- Chick JD, Smith MA, Engleman HM, Kean DM, Mander AJ, Douglas RH, Best JJ. Magnetic resonance imaging of the brain in alcoholics: cerebral atrophy, lifetime alcohol consumption, and cognitive deficits. Alcohol Clin Exp Res. 1989;13:512–518. doi: 10.1111/j.1530-0277.1989.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, D'Alimonte I, Giuliani P, Florio T, Caciagli F, Middlemiss PJ, Rathbone MP. Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the co-presence of microglia. Glia. 2000;29:202–211. [PubMed] [Google Scholar]
- Ciccarelli R, Sureda FX, Casabona G, Di Iorio P, Caruso A, Spinella F, Condorelli DF, Nicoletti F, Caciagli F. Opposite influence of the metabotropic glutamate receptor subtypes mGlu3 and -5 on astrocyte proliferation in culture. Glia. 1997;21:390–398. doi: 10.1002/(sici)1098-1136(199712)21:4<390::aid-glia6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Day NL, Thase ME, Mann JJ. Patterns of suicidality and alcohol use in alcoholics with major depression. Alcohol Clin Exp Res. 1996;20:1451–1455. doi: 10.1111/j.1530-0277.1996.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Crews FT. Alcohol and neurodegeneration. CNS Drug Rev. 1999;5:379–394. [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Féline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Davies DL, Cox WE. Delayed growth and maturation of astrocytic cultures following exposure to ethanol: electron microscopic observations. Brain Res. 1991;547:53–61. doi: 10.1016/0006-8993(91)90573-e. [DOI] [PubMed] [Google Scholar]
- Davies DL, Ross TM. Long-term ethanol-exposure markedly changes the cellular composition of cerebral glial cultures. Brain Res Dev Brain Res. 1991;62:151–158. doi: 10.1016/0165-3806(91)90162-c. [DOI] [PubMed] [Google Scholar]
- Davies DL, Vernadakis A. Effects of ethanol on cultured glial cells: proliferation and glutamine synthetase activity. Brain Res. 1984;318:27–35. doi: 10.1016/0165-3806(84)90059-2. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Rozelman E. Norepinephrine suppresses l-arginine uptake in rat glial cells. Neurosci Lett. 1997;223:37–40. doi: 10.1016/s0304-3940(97)13402-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis 1 Disorders–Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute, Biometrics Research; New York: 1995. [Google Scholar]
- Freund G, Anderson KJ. Glutamate receptors in the frontal cortex of alcoholics. Alcohol Clin Exp Res. 1996;20:1165–1172. doi: 10.1111/j.1530-0277.1996.tb01106.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Inhibition of muscarinic receptor-stimulated glial cell proliferation by ethanol. J Neurochem. 1996;67:2236–2245. doi: 10.1046/j.1471-4159.1996.67062236.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Ng JL, Harper CG, Kril JJ. Loss of vasopressin-immunoreactive neurons in alcoholics is dose-related and time-dependent. Neuroscience. 1996;72:699–708. doi: 10.1016/0306-4522(95)00577-3. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Holthoff VA, Beuthien-Baumann B, Kalbe E, Ludecke S, Lenz O, Zundorf G, Spirling S, Schierz K, Winiecki P, Sorbi S, Herholz K. Regional cerebral metabolism in early Alzheimer's disease with clinically significant apathy or depression. Biol Psychiatry. 2005;57:412–421. doi: 10.1016/j.biopsych.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Isenberg K, Zhou X, Moore BW. Ethanol inhibits C6 cell growth: fetal alcohol syndrome model. Alcohol Clin Exp Res. 1992;16:695–699. doi: 10.1111/j.1530-0277.1992.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Kendall RE. Alcohol and suicide. Substance and alcohol actions/misuse. Subst Alcohol Actions Misuse. 1983;4:121–127. [PubMed] [Google Scholar]
- Khokhrina NT, Kazakova PB, Rakhmanova VI. Morphometric analysis of the changes in the cerebral cortex of rats after long-term administration of alcohol. Zh Nevropatol I Psikhiatr Im S S Korsakova. 1991;91:66–67. [PubMed] [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999;23:164–168. [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathol (Berlin) 1989;79:200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Res. 2002;114:95–102. doi: 10.1016/s0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Laatikainen T, Manninen L, Poikolainen K, Vartiainen E. Increased mortality related to heavy alcohol intake pattern. J Epidemiol Commun. 2003;57:379–384. doi: 10.1136/jech.57.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecomte D, Fornes P. Suicide among youth and young adults, 15 through 24 years of age. A report of 392 cases from Paris, 1989–1996. J Forensic Sci. 1998;43:964–968. [PubMed] [Google Scholar]
- Mann JJ, Oquendo M, Underwood MD, Arango V. The neurobiology of suicide risk: a review for the clinician. J Clin Psychiatry. 1999;60(suppl 2):7–11. [PubMed] [Google Scholar]
- Melis F, Stancampiano R, Imperato A, Carta G, Fadda F. Chronic ethanol consumption in rats: correlation between memory performance and hippocampal acetylcholine release in vivo. Neuroscience. 1996;74:155–159. doi: 10.1016/0306-4522(96)00109-1. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Lower packing density of glial fibrillary acidic protein-immunoreactive astrocytes in the prelimbic cortex of alcohol-naive and alcohol-drinking alcohol-preferring rats as compared with alcohol-nonpreferring and Wistar rats. Alcohol Clin Exp Res. 2005;29:766–772. doi: 10.1097/01.alc.0000164378.92680.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol. 2006;41:379–385. doi: 10.1093/alcalc/agl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicki EK. Epidemiology of suicidal behavior. Suicide Life Threat Behav. 1995;25:22–35. [PubMed] [Google Scholar]
- Rahman S, Robbins TW, Sahakian BJ. Comparative cognitive neuropsychological studies of frontal lobe function: implications for therapeutic strategies in frontal variant frontotemporal dementia. Dement Geriatr Cogn Disord. 1999a;1(suppl):15–28. doi: 10.1159/000051207. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999b;122:1469–1493. doi: 10.1093/brain/122.8.1469. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan RR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Sher L. Alcoholism and suicidal behavior: a clinical overview. Acta Psychiatr Scand. 2006;113:13–22. doi: 10.1111/j.1600-0447.2005.00643.x. [DOI] [PubMed] [Google Scholar]
- Snyder A. Responses of Glia to alcohol. In: Aschner N, Kimelberg H, editors. The Role of Glia in Neurotoxicity. CRC Press; Boca Raton: 1996. pp. 111–135. [Google Scholar]
- Snyder AK, Singh SP, Ehmann S. Effects of ethanol on DNA, RNA, and protein synthesis in rat astrocyte cultures. Alcohol Clin Exp Res. 1992;16:295–300. doi: 10.1111/j.1530-0277.1992.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression—postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Suominen K, Isometsa E, Henriksson M, Ostamo A, Lonnqvist J. Hopelessness, impulsiveness and intent among suicide attempters with major depression, alcohol dependence, or both. Acta Psychiat Scand. 1997;96:142–149. doi: 10.1111/j.1600-0447.1997.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14:966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Williams RW. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1989;281:335. doi: 10.1002/cne.902780305. Erratum. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Yan QS, Reith ME, Yan SG, Jobe PC. Effect of systemic ethanol on basal and stimulated glutamate releases in the nucleus accumbens of freely moving Sprague-Dawley rats: a microdialysis study. Neurosci Lett. 1998;258:29–32. doi: 10.1016/s0304-3940(98)00840-4. [DOI] [PubMed] [Google Scholar]


