Abstract
The purpose of this study was to determine and compare the bioadhesive profiles of hydroxypropylcellulose (HPC) polymer matrices as a function of Δ9-tetrahydrocannabinol (THC) content. In addition, the effect of processing temperature on the stability of THC and its extent of degradation to cannabinol (CBN) was investigated. A hot-melt cast molding method was used to prepare HPC polymer matrix systems incorporated with THC at 0, 4, 8, and 16 percent. Bioadhesive measurements including peak adhesive force, area under the curve, and elongation at adhesive failure were recorded utilizing the TA.XT2i Texture Analyzer™. Data obtained from these tests at various contact time intervals suggested that the incorporation of THC led to an increase in the bioadhesive strength of the HPC polymer matrices. To determine the stability of THC and the resulting CBN content in the matrices, three different processing temperatures were utilized (120, 160, and 200°C). Post-production High Performance Liquid Chromotography (HPLC) analysis revealed that the processed systems contained at least 94% of THC and the relative percent formation of CBN was 0.5% at 120°C and 0.4% at 160°C compared to 1.6% at 200°C. These findings indicate that the cannabinoid may be a plausible candidate for incorporation into systems utilizing hot-melt extrusion techniques for the development of an effective mucoadhesive transmucosal matrix system for delivery of THC.
Keywords: Tetrahydrocannabinol, Hot-melt, Stability, Bioadhesion, Cannabinol, Polymer matrix
INTRODUCTION
Δ9-Tetrahydrocannabinol (THC) is the primary active ingredient of Cannabis sativa (marijuana) and is responsible for the majority of the pharmacological effects of the plant. People have utilized the plant since ancient times for medicinal purposes as well as for its intoxicating properties. To date, the most promising clinical applications approved by the Food and Drug Administration (FDA) are for the control of nausea and vomiting associated with chemotherapy and for appetite stimulation of AIDS patients suffering from anorexia as a result of wasting syndrome (Joy et al., 1999; Martin, 2002). Δ9-Tetrahydrocannabinol (THC), however, demonstrates other biological activities, which lend themselves to possible additional therapeutic applications. These include glaucoma, migraine headaches, spasticity, and anxiety (Voth & Schwartz, 1997). Also, more recently, THC is becoming recognized as an analgesic (Martin, 2002). Due to these promising biological activities of THC, marijuana has been advocated for its medicinal value.
One of the main issues brought into public debate by the medicinal marijuana proponents is the fact that the currently available soft gelatin formulation is very expensive and lacks consistency in its pharmacokinetic profiles. The latter point could be explained based on the fact that oral THC has erratic absorption from the gastrointestinal tract, is subject to extensive first-pass metabolism with the production of high levels of 11-hydroxy-tetrahydrocannabinol, and exhibits undesirable side effects (Ohlsson et al., 1980; Perlin et al., 1985). Indeed, the only formulations resulting in consistent and predictable bioavailability is an intramuscular injectable (Perlin et al., 1985) and an intravenous dosage form, both of which are not available commercially. Injectables carry the inherent problem of being invasive and requiring professional assistance, and therefore, in many cases, preclude self-medication. In addition, these routes of administration are subject to abuse. Other THC formulations may be forthcoming in light of the current interest in the therapeutic activities of cannabinoids and the recommendations made in the Institute of Medicine (IOM) report entitled “Marijuana and Medicine: Assessing the Science Base” (Joy et al., 1999).
Drug delivery systems to be administered by non-parenteral routes have gained renewed attention over the last decade. This stems from the significant limitations of traditional routes of drug administration. The non-parenteral routes under investigation include buccal (Li et al., 1997; Benes et al., 1997), sublingual (Cannon et al., 1996), rectal (ElSohly et al., 1991; Watanabe et al., 1996), nasal (Harris et al., 1988), and vaginal (Acarturk & Robinson, 1996). While each of these non-parenteral drug delivery routes has its associated advantages and disadvantages, the intra-oral transmucosal buccal route of drug administration has some unique benefits, including avoiding the first-pass effect, easy accessibility, and enhanced patient compliance. The nature of the oral mucosa itself includes a high cellular turnover rate that contributes to rapid cellular recovery (robustness) following local stress (Harris & Robinson, 1992). Oral mucosal delivery necessitates the use of mucoadhesive polymers in these dosage forms since they should ideally adhere to the mucosa and withstand salivation, muscular movement, and swallowing for a predetermined period of time. Examples of mucoadhesive polymers include sodium carboxymethyl cellulose, Carbopol® 934, hydroxypropylcellulose (HPC), hydroxypropyl methylcellulose, and acacia (Mathiowitz et al., 1999). The mucoadhesives may increase residence time at the absorption site, improve contact between the delivery system and the absorption site, and provide localization to specified oral mucosa regions to enhance bioavailability.
The effect of drug content on the force of adhesion of bioadhesive systems has received limited study. When hydrophobic drugs are incorporated into hydrophilic matrices, as in the present case, drug loading may significantly alter the mucoadhesive strength by changing the surface properties of the bioadhesive, thus retarding or enhancing the formation of intimate contact between the mucosal and the adhesive surfaces (Shojaei et al., 1998). For example, mucoadhesive force of hydroxypropyl methylcellulose-Carbopol® buccoadhesive tablets was found to decrease with an increase in drug content of morphine sulphate (Anlar et al., 1994). In contrast, Ponchel et al. (1987) reported no significant reduction in the bioadhesive bond strength due to the drug content of metronidazole in poly(acrylic) acid-hydroxypropyl methylcellulose tablets.
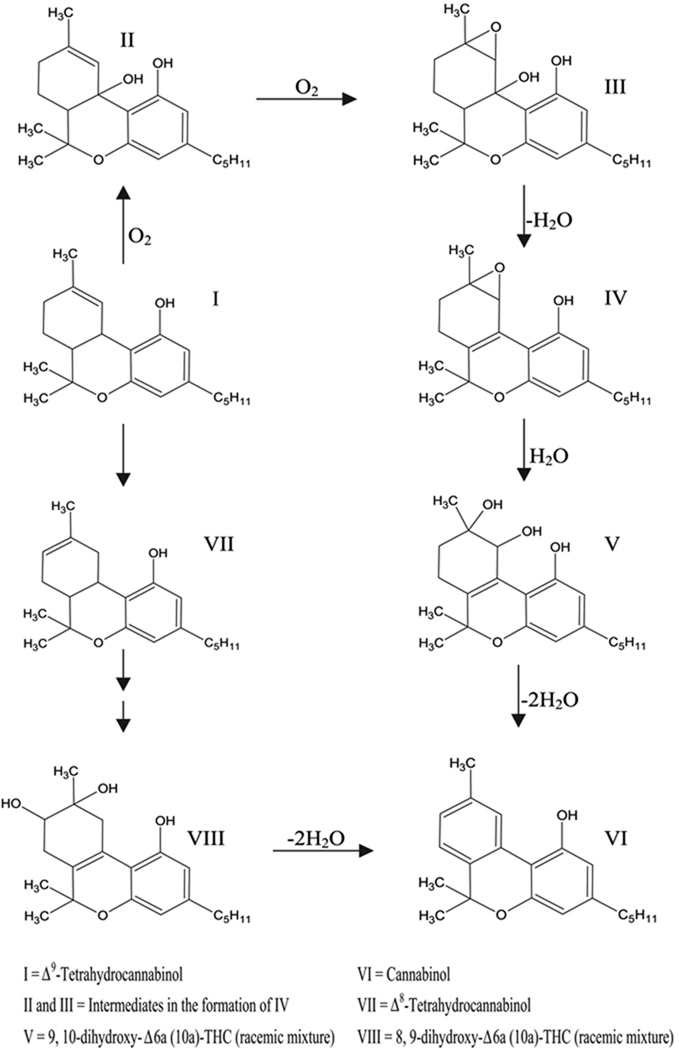
Due to THC’s high lipophillicity and sticky resin nature at room temperature, the authors hypothesized that the drug may enhance the bioadhesive properties of a polymeric delivery system. In addition, it has been reported that THC is susceptible to heat and oxidation, and the primary decomposition product observed is cannabinol (CBN), which is an oxidized degradant of THC (Ross & ElSohly, 1997–1998) (Fig. 1). Therefore, this study investigates a thermal processing method for the incorporation of THC into thin films to assess the stability and bioadhesive properties of the active-incorporated polymeric matrices.
FIGURE 1.
Proposed Pathway for Decomposition of THC (I) to Its Primary Degradant, Cannabinol (VI). (Adapted from Turner & ElSohly, 1979).
EXPERIMENTAL
Materials
Hydroxypropylcellulose (HPC) (Klucel® EF) was kindly gifted by Aqualon Div., Hercules Incorporated (Wilmington, DE,). Polycarbophil (Noveon® AA-1) was obtained from Noveon, Inc. (Cleveland, OH). Polyethylene glycol 400 NF and butylated hydroxytoluene (BHT) were purchased from Spectrum Quality Products (New Brunswick, NJ) and poly (ethylene oxide) was purchased from Aldrich Chemical Company Inc. (Milwaukee, WI). Δ9-Tetrahydrocannabinol (THC) and its degradant cannabinol were provided by the Coy Waller Laboratory Complex, University, MS.
Methods
Preparation of the THC-incorporated Polymeric Films
For evaluation of bioadhesive properties, a hot-melt cast molding method was used to prepare HPC polymer matrices incorporated with THC at 0, 4, 8, and 16%. Hydroxypropylcellulose (HPC), which possesses bioadhesive properties, was used as the matrix-forming polymer. Low molecular weight polyethylene oxide and polyethylene glycol-400 were used to facilitate film processing. BHT was included in the formulation to reduce oxidative degradation of the polymer and the drug. Noveon®, a bioadhesive polymer, was also incorporated into the formulation.
The polymeric systems were prepared by melting and homogenizing the polymers with other excipients followed by controlled cooling. The maximum temperatures used for the preparation of the matrices ranged from 150–160°C and the total time for preparation of each matrix varied from 75–90 min. The prepared matrices were sealed in foil-lined ziploc bags and stored at −18°C. These films were cut into predetermined strips for the bioadhesion studies and were also analyzed for post-production content of THC and cannabinol.
To investigate the thermal stability of THC, a similar hot-melt cast molding method was utilized to incorporate THC into the HPC matrix systems to investigate the effect of processing temperature on the stability of THC and its extent of degradation to CBN within the matrix systems. For this purpose, THC was incorporated into the matrices at a 4% concentration. The final temperature in the matrix preparation technique was varied to compare the post-production stability of THC and the resulting CBN content in the matrices. The three different processing temperatures used were 120, 160, and 200°C.
Measurement of Bioadhesive Properties
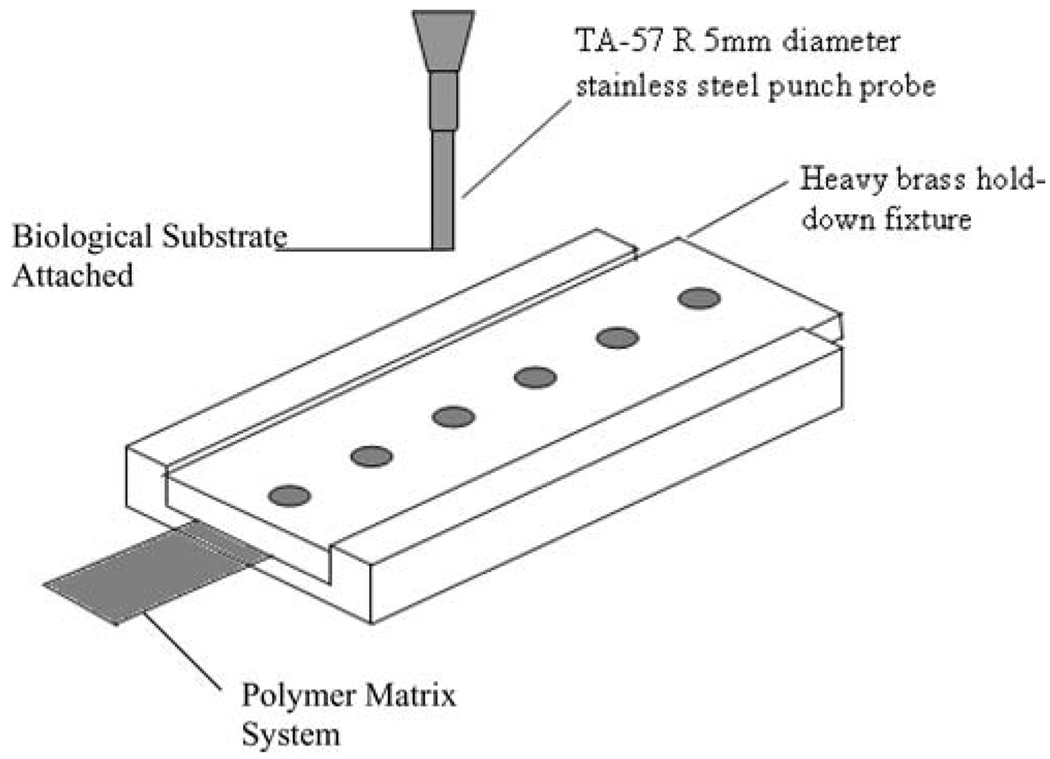
Adhesive properties are important factors for the polymer matrices intended for intra-oral administration in that the polymer system has to adhere to the tissue for a considerable period of time for a dosage form to be effective. Bioadhesive measurements including peak adhesive force (PAF), area under the curve (AUC), and elongation at adhesive failure (EAF) were recorded utilizing a TA.XT2i Texture Analyzer™ (Texture Technologies Corp., Scarsdale, NY/Stable Micro Systems, Godalming, Surrey, UK). Rabbit intestinal mucosa was used as the model biological membrane for conducting the bioadhesion tests. The fixture used for the test consisted of an indexable tackiness hold-down fixture and a TA-57 R 5mm diameter stainless steel punch probe with a flat end (Fig. 2). The tissue was mounted onto the probe using a cyanoacrylate adhesive and wetted with simulated artificial saliva (2.38 g Na2HPO4, 0.19 g KH2PO4, and 8.00 g NaCl per liter of nanopure water adjusted with phosphoric acid to pH 6.75) every 30 sec. The film was positioned onto the base plate that was secured by a brass fixture. This whole system was placed at the base of the texture analyzer. Prior to conducting the tests, the polymeric patches were wetted with nanopure water for 1 min. Optimum wetting is a necessary condition for achieving effective adhesion as it provides intimate contact between adhesive and adherent.
FIGURE 2.
Indexable Tackiness Hold-down Fixture Used in Bioadhesive Experiments.
The instrumental parameters were adjusted such that the pre-test speed, the test speed, and the post-test speed of the probe was 1, 0.1, and 0.5 mm/s, respectively. These parameters were chosen based on previous studies (Repka & McGinity, 2000, 2001). A force of 3.5 N was applied for the contact time intervals of 10, 20, 60, and 120 s. Data were collected at 200 points per second. Data acquisition and calculations were performed using the Texture Expert™ software provided with the system. The measurements reported are each the average of six test replicates. Furthermore, since the strength of the observed mucoadhesive bond has been shown to depend on temperature, this parameter was set at the physiological temperature of 37 ± 0.5°C (Tobyn et al., 1994).
Bioadhesive parameters such as work of adhesion and peak adhesive force were used to evaluate the bioadhesive strength of the polymeric matrices. Elongation at adhesive failure and post-peak area under the curve were also calculated to compare the stringiness and cohesiveness of the films, respectively.
Sample Preparation
A portion of the polymeric drug-containing film was dissolved in methanol by sonicating for 20–25 min followed by centrifugation for 5 min. The supernatant was filtered, transferred into vials, and injected into the HPLC column for THC and CBN content analysis.
Post-production Drug Analysis
The post-production THC and CBN content in the polymeric matrices were determined by a Waters HPLC-UV system (Waters 600 solvent delivery system and a dual wavelength Waters Model 2487 UV detector) with Millennium™ software. The HPLC conditions for THC consisted of a reversed phase C18 column Partisil 5µ ODS (3), 250 × 4.60 mm utilizing an injection volume of 20 µL and a detection wavelength of 228 nm. The mobile phase consisted of 80:20:0.01 methanol: water:acetic acid (pH ~5) set at a flow rate of 1.0 mL/min. The run time was maintained at 25 min. The intraday and interday variability for the HPLC method was less than 3.0%. The limit of detection for THC and CBN was 0.3 and 0.1 µg/mL, while the limit of quantitation was 0.9 and 0.3 µg/mL, respectively. Calibration curves for THC and CBN were plotted and used to calculate the amount of THC remaining and the percent degraded to CBN in the post-production polymer matrices.
Thermal Analysis
Differential scanning calorimetry (DSC) studies on pure drug, physical mixtures (with and without drug), and hot-melt films were performed using a Perkin-Elmer Pyris-1 DSC instrument. The samples were heated from 10°C to 300°C at a linear heating rate of 10°C per min. The flow rate of the nitrogen gas was maintained at 30 mL/min.
Thermogravimetric Analysis (TGA) was performed with the pure drug, physical mixtures (with and without drug), and the drug loaded polymeric films fabricated at different temperatures, to determine the stability of the formulation as a function of weight loss. A Perkin-Elmer Pyris-1 TGA instrument was utilized to obtain the thermograms. Samples of 7–8 mg were heated from 30°C to 500°C at the rate of 10°C/min.
Data Analysis
Statistical analysis was carried out using Microsoft Excel®. A t-test was utilized to analyze the results and a statistically significant difference was considered when p < 0.05.
RESULTS AND DISCUSSION
Chromatography and THC Degradants
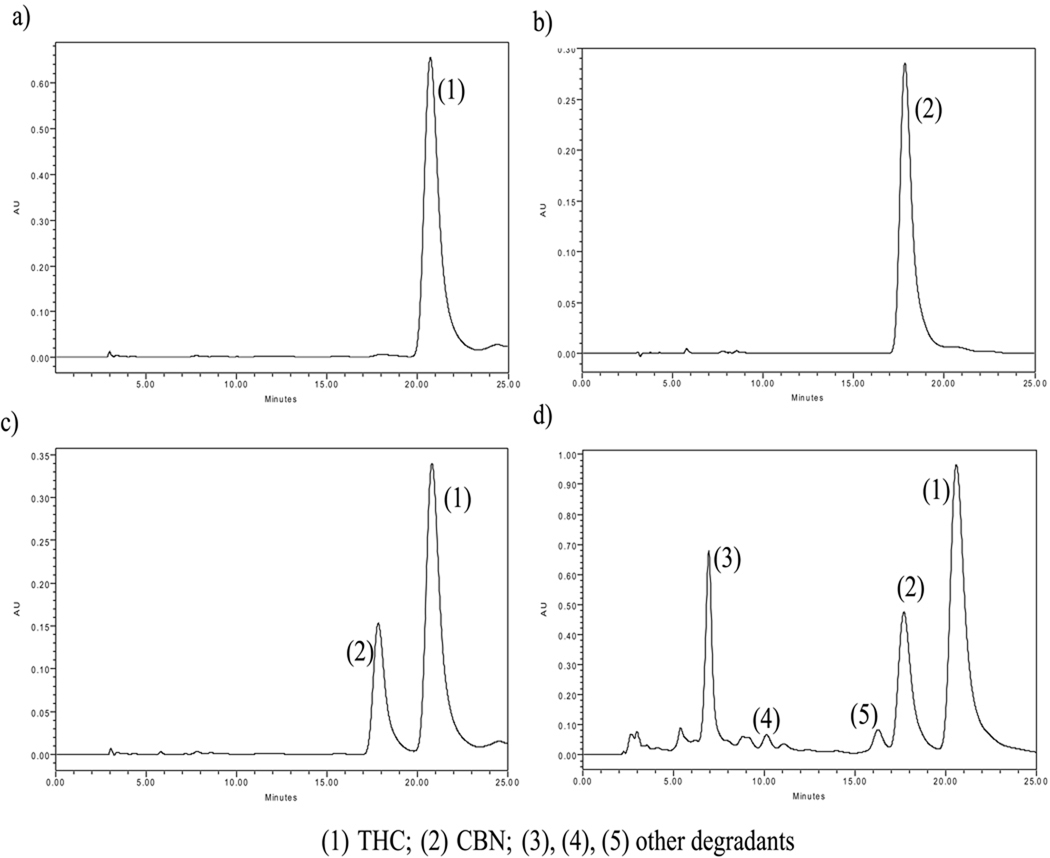
Δ9-Tetrahydrocannabinol (THC) and other related cannabinoids, separated using the HPLC method, are shown in Fig. 3. Chromatographs obtained using THC and CBN are illustrated in Fig. 3a and 3b, respectively. Upon spiking the THC sample with CBN, it becomes apparent that CBN elutes before THC (Fig. 3c) similar to what was reported by Flora et al., (1980). Figure 3d illustrates the presence of THC and its degradant CBN (formed during preparation/storage) in the polymeric films in addition to other several relatively polar degradants (3, 4, 5) (Flora et al., 1980). Analysis and quantitation of these compounds is not the subject of the present study. The extent of CBN formation during preparation of the films is described in the next section.
FIGURE 3.
HPLC Chromatograms of (a) Pure THC, (b) CBN, (c) THC Spiked with CBN, and (d) Hot-melt Cast Polymeric Film Showing CBN, THC, and Other Degradant Products.
Effect of Processing Temperature on the THC Content in Polymeric Matrices
Hydroxypropylcellulose (HPC) was used as the primary matrix-forming polymer since it has excellent thermoplastic properties and may be processed by many fabrication methods such as hot-melt molding and extrusion. Hydroxypropylcellulose is a nonionic water-soluble cellulose ether and has a melting temperature in the range of 100–150°C based on its molecular weight (Aqualon Company, 2001). The films processed for investigation in this study required a temperature of at least 120°C for the polymers to be homogenized and fabricated into a film. Small amounts of plasticizers may be used with HPC to ensure a smooth, uniform melt and homogeneity. Plasticizers function by reducing the glass transition temperature of polymers (Repka et al., 1999). In this study, polyethylene glycol-400 was used as the plasticizer for HPC to allow the reduction of the extrusion/molding temperatures, which potentially improves the stability of the active compound.
The influence of temperature on the amount of THC remaining and percent THC degraded to cannabinol during the processing of hot-melt cast systems is illustrated in Table 1. It has been reported that THC is highly unstable at room temperature and its primary degradant is CBN (Harvey, 1990). It has also been reported that UV light and heat accelerate THC degradation (Ross & ElSohly, 1997–1998). Coffman and Gentner (1974) studied the effect of temperature on the stability of THC and found that little decomposition occurred at 65°C, but considerable losses occurred from 85–100°C. Turner et al. (1973) reported that at 37°C and 50°C significant THC degradation occurred. These studies were focused on the long-term stability/degradation of THC. However, processing conditions of THC into polymeric matrices are relevant to novel delivery systems of the cannabinoid. The results of our studies, as shown in Table 1, indicate that the extent of degradation of THC was 2.8, 5.1, and 5.7% at 120, 160, and 200°C, respectively, when heated for approximately 75–90 min. Therefore, the percent THC remaining in the post-production polymeric films was at least 94% theoretical. These findings are encouraging for the possibility of processing THC via hot-melt extrusion.
TABLE 1.
THC Content Remaining and Percent CBN Formed by Degradation of THC in the Polymer Matrix Films after Processing as a Function of Temperature
| Temperature (°C) | % THC remaining | % THC degraded | Relative % formation of CBN |
Relative % CBN formation from % THC degraded |
|---|---|---|---|---|
| 120 | 97.20 | 2.80 | 0.49 | 9.01 |
| 160 | 94.91 | 5.09 | 0.39 | 7.79 |
| 200 | 94.34 | 5.66 | 1.55 | 29.08 |
In addition, Table 1 illustrates that at 120, 160, and 200°C, the percent CBN increase in the polymeric systems was 0.5, 0.4, and 1.6%, respectively. An interesting parameter as reported in this table is the relative % CBN formation from % THC degraded. Our findings indicate that at 120 and 160°C, only 9.0% and 7.8%, respectively, of the total THC degraded appeared as CBN. However at 200°C, the THC converted to CBN was 29.1%. This could be attributed to the fact that decomposition of THC occurs at temperatures in the range of 200–250°C as depicted in Fig. 5. Thus, although the degradation of THC is relatively low at 200°C, the extent of conversion of THC to CBN is high. The remaining percentage is most likely composed of other cannabinoids/degradants. These may be cannabidiol, Δ8-THC, and other polymeric forms of THC and/or CBN, which cannot be detected by usual chromatographic methods (Fairbairn et al., 1976). Similar observations were reported by Lewis & Turner (1978), in that the amount of CBN produced could not account for the decrease in THC concentration over a period of time when the latter is kept under the conditions suitable for decomposition. Turner & ElSohly (1979) addressed this outcome and proposed a possible pathway for the decomposition of THC to CBN as illustrated in Fig. 1. Their work cited the formation of epoxy and hydroxylated intermediates 9, 10-dihydroxy-Δ6a (10a)-THC (racemic mixture) and 8, 9-dihydroxy-Δ6a (10a)-THC (racemic mixture). These researchers also indicated that these compounds were susceptible to heat and acid and that the final product was CBN.
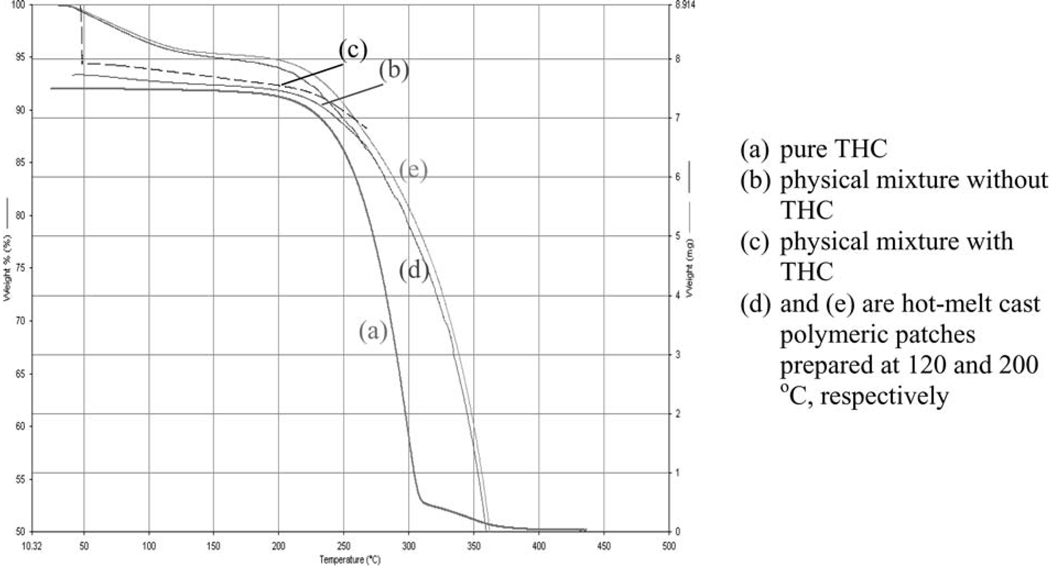
FIGURE 5.
TGA Thermograms of Pure THC, Physical Mixtures and Polymeric Films.
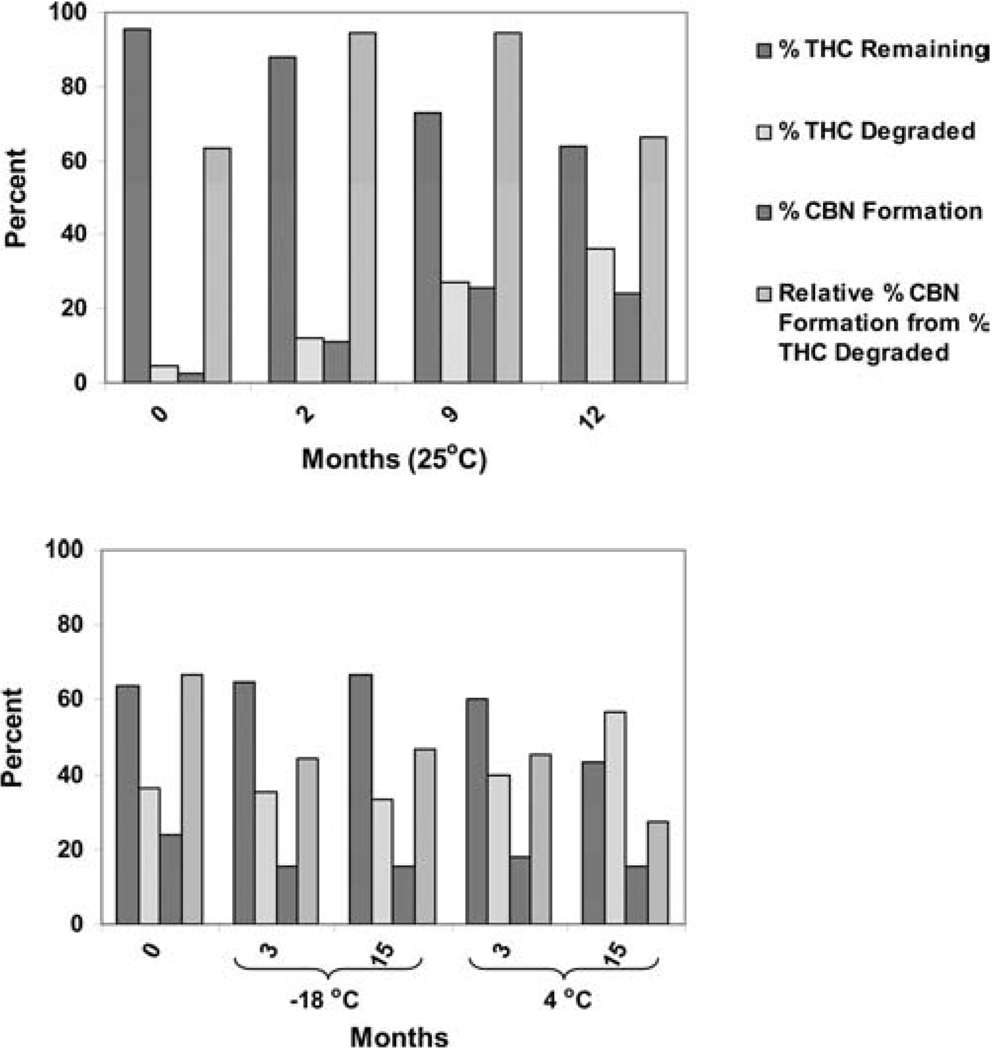
Long-term Stability of THC in Polymeric Matrices
Few studies have investigated the stability of THC in a formulated system. For example, Flora et al. (1980) prepared solutions containing THC in ethanol-Emulphor EL620-sodium chloride injection USP, and ethanol-Cremophor EL-sodium chloride injection USP. The solution formulations were sealed under room air in clean glass ampoules. After 10 days, the THC remaining in these vehicles at 25°C was determined to be 81.1 and 84.2%, respectively. Therefore, an objective of the present research was to study the long-term stability of THC in a polymeric system. For this purpose, an HPC polymeric film containing THC was fabricated using a hot-melt cast technique, and stored at 25°C for 12 months. After 12 months, the matrix was divided into two parts: one-half was stored at −18°C and the other at 4°C. At predetermined time intervals, the film was analyzed for THC content and its extent of degradation to CBN. The results are illustrated in Fig. 4. THC content remaining in the system after 12 months at 25°C was 63.8%. However, when the same film was transferred to −18°C, THC degradation was insignificant for up to 15 months. The THC content in the film at 4°C continued to degrade but at a much slower rate. Also, % CBN formation from % THC degraded was higher when the film was kept at 25°C. This suggests that more CBN was formed from THC at 25°C than at the lower storage temperatures. It should be noted that packaging of the stored matrix systems was less than optimal. Stabilizing THC in the hot-melt extrudates is the subject of ongoing studies.
FIGURE 4.
Stability of THC Incorporated into a Polymeric Film System.
Thermal Analysis
Differential scanning calorimetry (DSC) was performed on the polymeric systems processed at 120, 160, and 200°C to investigate any change in the physical state of THC and/or interaction between the various components of the matrix system that may have occurred at higher temperatures. Differential Scanning Calorimetry thermograms, however, appeared to be the same for all of the systems, which suggested that there was no phase separation or that the interaction between components in the films was similar. The thermograms also suggested that THC might be present as a solid dispersion in the polymeric systems prepared at various temperatures. Further studies are underway to confirm these results.
Figure 5 depicts the thermogravimetric profiles of drug, physical mixtures, and the films, which demonstrate the change in mass of the samples as a function of temperature. TGA scan of the pure drug showed decomposition of the drug after 200°C while the physical mixture containing the drug showed initial loss of moisture followed by decomposition of the constituents after 200°C. Similar results were obtained when a TGA scan was performed on the THC film systems. This suggests that the drug, physical mixture, and the film matrix system are likely to be thermally stable under the conditions of high temperatures employed during hot-melt cast molding processes for incorporation of THC into polymeric systems.
The hot-melt cast molding method utilizes thermal energy as the primary source of energy that softens the polymers in addition to a small amount of mechanical energy obtained from stirring. The polymeric systems utilized in the present study were processed under the conditions of heat, air, and light (suitable for THC degradation), and the processing time was 75–90 min. During hot melt extrusion, polymers soften and become flexible primarily due to the heat generated by the shearing effect of the rotating screw in addition to the heat from thermal devices that aid in the softening of the polymers (Newton, 2002; Repka et al., 2002). This suggests that a polymer would require less heat to be fabricated into a film system utilizing extrusion processing rather than a hot-melt cast molding method. Thus, under the potential conditions of hot-melt extrusion (90–140°C depending on the polymer and processing time of approximately 2 min) and an optimized formulation, the stability of THC may be greatly enhanced during processing.
Evaluation of Bioadhesive Properties of THC
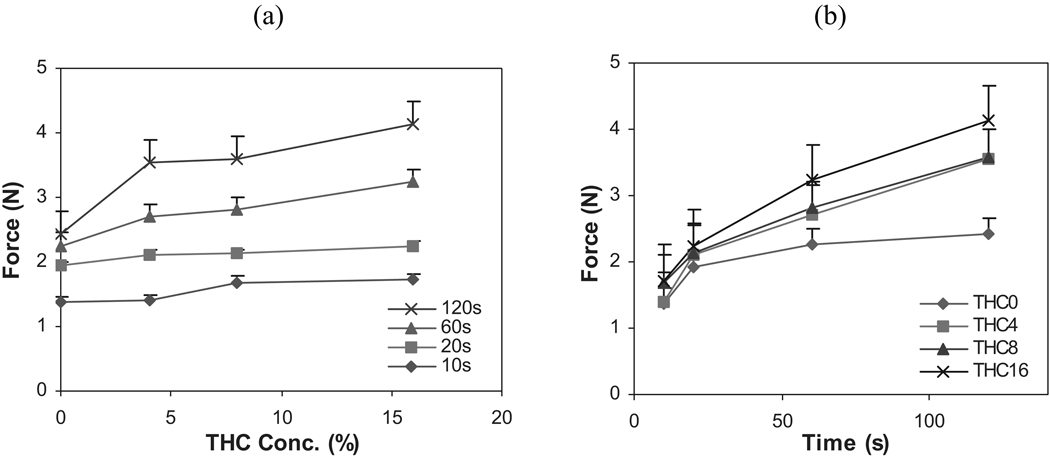
The influence of THC on two primary bioadhesive parameters, peak adhesive force and work of adhesion, of the films at different contact time intervals is illustrated in Figs. 6 and 7, respectively. The maximum force required to detach the THC film from the mucosa, known as the peak adhesive force, was recorded as a function of elongation. Figure 6a indicates that there is a statistical increase in peak adhesive force with a corresponding increase in THC concentration at contact time intervals of 60 and 120 sec as compared to the control (0% THC). In addition, Fig. 6b demonstrates that there was an increase in peak adhesive force with an increase in contact time duration.
FIGURE 6.
Influence of (a) THC Concentration, and (b) Contact Time Interval on the Peak Adhesive Force of HPC Polymeric Films (n = 6).
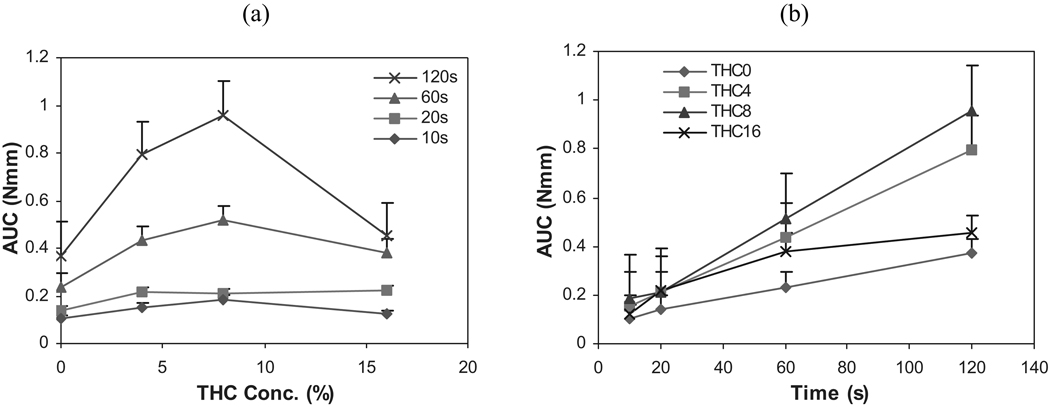
FIGURE 7.
Influence of (a) THC Concentration, and (b) Contact Time Interval on the Work of Adhesion of HPC Polymeric Films (n = 6).
The area under the curve (AUC) of the force-deflection profile, which denotes the work of adhesion, is the total amount of energy required to sever the adhesive bond. As depicted in Fig. 7a, at contact times of 60 and 120 sec, there was a corresponding increase in work of adhesion with an increase in THC concentration up to the 8% level. Further addition of THC in the matrix system (16%) decreased the work of adhesion. Figure 7b demonstrates that the work of adhesion of the films increased as a function of contact time interval.
The above findings revealed that bioadhesion was not influenced by THC concentration at lower contact time intervals of 10 and 20 sec. As such, contact time is critical for the bioadhesion process. Sufficient time is needed for the polymer chains to uncoil so that entanglement and bond formation with mucin molecules occur when the force is applied (Wong et al., 1999). Several theories (Longer & Robinson, 1986; Chickering & Mathiowitz, 1999) have been advocated to explain the mechanism of bond formation between the polymers and mucus glycoproteins, suggesting interpenetration and physical entanglement of polymeric chains with the mucin complex at the molecular level. In addition, secondary chemical bonds formed by electrostatic and van der Waals attraction, as well as hydrogen and hydrophobic bonds, are potential factors.
Although, it is not easy to explain various bioadhesive phenomena obtained in this study by any one theory, the following mechanism has been proposed. The increase in peak adhesive force and work of adhesion with increasing THC concentration may be based on the sticky, viscous tar-like nature of THC and the structural presence of a relatively flexible carbon chain (Seymour & Carraher, 1984) in addition to interpenetration of macromolecular chains at the polymer-polymer interface between the adhesive and the mucin complex. Since work of detachment is a function of the degree of linking between the two networks, mobility of the polymer chains and polymer concentration (Lee et al., 2000) are two significant factors that must be considered. The relatively small molecular size and flexibility of the THC molecule may increase the polymer chain mobility in bioadhesive matrices, resulting in increased interpenetration and entanglement of the polymeric chains with the mucin molecules. At the 16% THC level, however, the polymer fraction of the system decreased beyond a threshold to have a negative impact on this bioadhesive parameter. The polymeric film chains were more flexible, but the matrix polymer content decreased such that the interaction between the film and mucin chains was negatively affected, resulting in a lower work of adhesion.
In contrast, peak adhesive force, which is recorded at the point when the probe starts withdrawing from the film, is indicative of the binding strength of the film to the mucosa. The results obtained for the peak adhesive force may be explained based on the glutinous nature of THC (Flora et al., 1980).
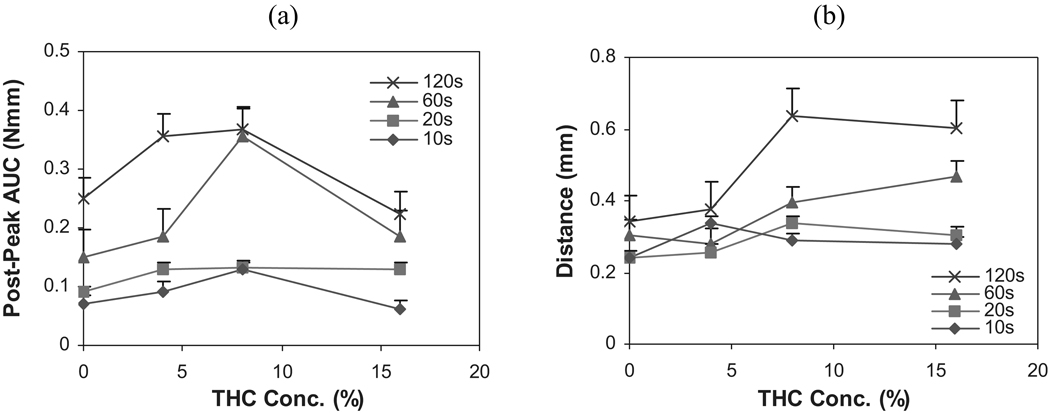
In addition to possessing adhesive properties, an intra-oral polymer matrix system should also possess adequate cohesivity and elasticity, so that it can withstand musculatory and salivary activity. As illustrated in Fig. 8a, cohesivity increased with increasing THC content up to the 8% level at high contact intervals, similar to work of adhesion. Indeed, the ratio post-peak-area/pre-peak-area was the least for systems incorporated with 8% THC, which indicates strong relative cohesivity (Johnson, 1999).
FIGURE 8.
Influence of THC Concentration on (a) Cohesivity, and (b) Elongation at Adhesive Failure of HPC Polymeric Films (n = 6).
Elongation at adhesive failure, equivalent to elongation at break in the tensile testing of films, was also investigated. The EAF is the distance the upper probe traveled to the point of film and mucosa separation, and is indicative of the stringiness of bioadhesives. The lingering separation is often well after the peak adhesive force, and therefore, EAF also reflects the cohesive nature of the system. The stringiness of the film matrices is depicted in Fig. 8b that suggests that the maximum EAF for the polymeric matrix is at 8% THC concentration.
CONCLUSIONS
Δ9-Tetrahydrocannabinol (THC) was found to be relatively stable under the hot-melt casting conditions. Processing temperatures up to 200°C were utilized without imparting considerable degradation of the drug. The hot-melt casting method used in this study was, therefore, determined to be appropriate for production of polymeric matrix films on the laboratory scale. In the matrix formulation, THC degraded fairly rapidly at room temperature; however, the degradation slowed significantly at 4°C and was arrested at −18°C. Optimization of the formulation could further enhance the stability of THC in the polymeric films. In addition, the incorporation of THC in HPC polymeric matrices led to an increase in bioadhesive strength and work of adhesion of the systems, demonstrating a very unique, significant feature of THC, in that THC itself possess mucoadhesive properties. These studies have demonstrated the potential use of thermally processed intra-oral matrix systems for systemic controlled delivery of THC.
ACKNOWLEDGEMENTS
This research was partially funded by NIH grants #1R41 GM067304-01 and #P20RR17701. The authors also thank the Coy Waller Laboratory Complex for providing THC samples.
Contributor Information
Michael A. Repka, Department of Pharmaceutics, School of Pharmacy, The University of Mississippi, University, MS 38677-1848 and Research Institute of Pharmaceutical Sciences, The University of Mississippi, University, MS 38677-1848
Manish Munjal, Department of Pharmaceutics, School of Pharmacy, The University of Mississippi, University, MS 38677-1848.
Mahmoud A. ElSohly, Department of Pharmaceutics, School of Pharmacy, The University of Mississippi, University, MS 38677-1848 and Research Institute of Pharmaceutical Sciences, The University of Mississippi, University, MS 38677-1848
Samir A. Ross, Research Institute of Pharmaceutical Sciences, The University of Mississippi, University, MS 38677-1848
REFERENCES
- Acarturk F, Robinson JR. Vaginal permeability and enzymatic activity studies in normal and ovariectomized rabbits. Pharmaceutical Research. 1996;13(5):779–783. doi: 10.1023/a:1016016120392. [DOI] [PubMed] [Google Scholar]
- Anlar S, Capan Y, Guven O, Gogus A, Dlakara T, Hincal AA. Formulation and in vitro-in vivo evaluation of buccoadhesive morphine sulphate tablets. Pharmaceutical Research. 1994;11(2):231–236. doi: 10.1023/a:1018951323522. [DOI] [PubMed] [Google Scholar]
- Benes L, Claustrat B, Horriere F, Geoffriau M, Konsil J, Parrott KA, DeGrande G, McQuinn RL, Ayres JW. Transmucosal, oral controlled-release, and transdermal drug administration in human subjects: a crossover study with melatonin. Journal of Pharmaceutical Sciences. 1997;86(10):1115–1119. doi: 10.1021/js970011z. [DOI] [PubMed] [Google Scholar]
- Cannon JB, Adjei LA, Fu Lu MY, Garren K. Alternate drug delivery routes for A-71623, a potent cholecystokinin-A receptor agonist tetrapeptide. Journal of Drug Targetting. 1996;4(2):69–78. doi: 10.3109/10611869609046264. [DOI] [PubMed] [Google Scholar]
- Chickering DE, III, Mathiowitz E. Definitions, mechanisms, and theories of bioadhesion. Drugs and the Pharmaceutical Sciences. 1999;98:1–10. (Bioadhesive Drug Delivery Systems) [Google Scholar]
- Coffman CB, Gentner WA. Cannabis sativa L.: Effect of drying time and temperature on cannabinoid profile of stored leaf tissue. Bulletin on Narcotics. 1974;26(1):67–70. [Google Scholar]
- ElSohly MA, Stanford DF, Harland EC, Hikal AH, Walker LA, Little TL, Jr, Rider JN, Jones AB. Rectal bioavailability of delta-9-tetrahydrocannabinol from the hemisuccinate ester in monkeys. Journal of Pharmaceutical Sciences. 1991;80(10):942–945. doi: 10.1002/jps.2600801008. [DOI] [PubMed] [Google Scholar]
- Fairbairn JW, Liebmann JA, Rowan MG. The stability of cannabis and its preparation on storage. Journal of Pharmacy and Pharmacology. 1976;28(1):1–7. doi: 10.1111/j.2042-7158.1976.tb04014.x. [DOI] [PubMed] [Google Scholar]
- Flora KP, Cradock JC, Davignon JP. Determination of delta-9-tetrahydrocannabinol in pharmaceutical vehicles by high-performance liquid chromatography. Journal of Chromatography. 1980;206(1):117–123. doi: 10.1016/s0021-9673(00)82611-4. [DOI] [PubMed] [Google Scholar]
- Harris AS, Ohlin M, Lethagen S, Nilsson IM. Effects of concentration and volume on nasal bioavailability and biological response to desmopressin. Journal of Pharmaceutical Sciences. 1988;77(4):337–339. doi: 10.1002/jps.2600770412. [DOI] [PubMed] [Google Scholar]
- Harris D, Robinson JR. Drug delivery via the mucous membranes of the oral cavity. Journal of Pharmaceutical Sciences. 1992;81(1):1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. Stability of cannabinoids in dried samples of cannabis dating from around 1896–1905. Journal of Ethnopharmacology. 1990;28(1):117–128. doi: 10.1016/0378-8741(90)90068-5. [DOI] [PubMed] [Google Scholar]
- Johnson M. How to Measure the Adhesiveness of Bioadhesives. Adhesives & Sealants Fall Convention. 1999 October;:24–26. [Google Scholar]
- Joy JE, Watson SJ, Benson JA. Marijuana and Medicine: Assessing the Science Base. Washington DC: National Academy press; 1999. [PubMed] [Google Scholar]
- Klucel Hydroxypropylcellulose. Physical and Chemical Properties. Aqualon Company, Booklet. 2001;250–2F:5. [Google Scholar]
- Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: The next generation. Journal of Pharmaceutical Sciences. 2000;89(7):850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lewis GS, Turner CE. Constituents of Cannabis sativa L.: XIII. Stability of dosage form prepared by impregnating synthetic (−)-delta-9-trans-tetrahydrocannabinol on placebo cannabis plant material. Journal of Pharmaceutical Sciences. 1978;67(6):876–878. doi: 10.1002/jps.2600670645. [DOI] [PubMed] [Google Scholar]
- Li C, Bhatt PP, Johnston TP. Transmucosal delivery of oxytocin to rabbits using a mucoadhesive buccal patch. Pharmaceutical Development and Technology. 1997;2(3):265–274. doi: 10.3109/10837459709031446. [DOI] [PubMed] [Google Scholar]
- Longer MA, Robinson JR. Fundamental aspects of bioadhesion. Pharmacy International. 1986;7(5):114–117. [Google Scholar]
- Martin BR. The use of cannabinoids in patients with chronic illness. U.S. Pharmacist. 2002;1:61–72. [Google Scholar]
- Mathiowitz E, Chickering DE, Lehr CM. In: Bioadhesive Drug Delivery Systems. Swarbrick J, editor. vol. 98. New York: Marcel Dekker, Inc.; 1999. [Google Scholar]
- Newton JM. Extrusion and extruders. In: Swarbrick J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology. New York: Marcel Dekker, Inc.; 2002. May, pp. 1220–1236. [Google Scholar]
- Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking with erratic bioavailability which is dependant on food intake. Clinical Pharmacology and Therapeutics. 1980;28(3):409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- Perlin E, Smith CG, Nichols AI, Almirez R, Flora KP, Cradock JC, Peck CC. Disposition and bioavailability of various formulations of tetrahydrocannabinol in the Rhesus monkey. Journal of Pharmaceutical Sciences. 1985;74(2):171–174. doi: 10.1002/jps.2600740213. [DOI] [PubMed] [Google Scholar]
- Ponchel G, Touchard F, Wouessidjewe D, Duchene D, Peppas NA. Bioadhesive analysis of controlled-release systems III. Bioadhesive and release behavior of metronidazole containing poly (acrylic) acid-hydroxypropyl methylcellulose systems. International Journal of Pharmaceutics. 1987;38(1–3):65–70. [Google Scholar]
- Repka MA, Gerding TG, Repka SL, McGinity JW. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot-melt extrusion. Drug Development and Industrial Pharmacy. 1999;25(5):625–633. doi: 10.1081/ddc-100102218. [DOI] [PubMed] [Google Scholar]
- Repka MA, McGinity JW. Physical-mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Biomaterials. 2000;21(14):1509–1517. doi: 10.1016/s0142-9612(00)00046-6. [DOI] [PubMed] [Google Scholar]
- Repka MA, McGinity JW. Bioadhesive properties of hydroxypropylcellulose topical films produced by hot-melt extrusion. Journal of Controlled Release. 2001;70(3):341–351. doi: 10.1016/s0168-3659(00)00365-5. [DOI] [PubMed] [Google Scholar]
- Repka MA, McGinity JW, Zhang F, Koleng JJ. Hot-melt extrusion technology. In: Swarbrick J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology. New York: Marcel Dekker, Inc.; 2002. pp. 203–266. [Google Scholar]
- Ross SA, ElSohly MA. CBN and delta-9-tetrahydrocannabinol ratio as an indicator of the age of stored marijuana samples. Bulletin on Narcotics. 1997–1998;49/50(1&2):139–147. [Google Scholar]
- Seymour RB, Carraher CE. Thermal properties of polymers. In: Seymour RB, Carraher CE, editors. Structure-Property Relationships in Polymers. New York: Plenum Publishing Corporation; 1984. pp. 83–93. [Google Scholar]
- Shojaei AH, Zhou S, Li X. Transbuccal delivery of acyclovir (II): Feasibility, system design, and in vitro permeation studies. Journal of Pharmacy and Pharmaceutical Sciences. 1998;1(2):66–73. [PubMed] [Google Scholar]
- Tobyn M, Johnson JR, Gibson S. Use of a TA.XT2 Texture Analyzer in Mucoadhesive Research. International Labmate. 1994;XVII(VI) [Google Scholar]
- Turner CE, ElSohly MA. Constituents of Cannabis sativa L.: XVI. A possible decomposition pathway of delta-9-tetrahydrocannabinol to cannabinol. Journal of Heterocyclic Chemistry. 1979;16(8):1667–1668. [Google Scholar]
- Turner CE, Hadley KW, Fetterman PS, Doorenbos NJ, Quimby MW, Waller C. Constituents of Cannabis sativa L. IV: Stability of Cannabinoids in Stored Plant Material. Journal of Pharmaceutical Sciences. 1973;62(10):1601–1605. doi: 10.1002/jps.2600621005. [DOI] [PubMed] [Google Scholar]
- Voth EA, Schwartz RH. Medicinal applications of delta-9-tetrahydrocannabinol and marijuana. Annals of Internal Medicine. 1997;126(10):791–798. doi: 10.7326/0003-4819-126-10-199705150-00008. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kiriyama M, Ito R, Kikuchi R, Mizufune Y, Nomura H, Miyazaki M, Matsumoto M. Pharmacodynamics and pharmacokinetics of recombinant human granulocyte colony-stimulating factor (rhG-CSF) after administration of a rectal dosage vehicle. Biological and Pharmaceutical Bulletin. 1996;19(8):1059–1063. doi: 10.1248/bpb.19.1059. [DOI] [PubMed] [Google Scholar]
- Wong CF, Yuen KH, Peh KK. An in-vitro method for buccal adhesion studies: importance of instrument variables. International Journal of Pharmaceutics. 1999;180(1):47–57. doi: 10.1016/s0378-5173(98)00402-5. [DOI] [PubMed] [Google Scholar]