Abstract
In order to ex vivo produce clinically useful quantity of platelets, we may need to firstly enhance early self-renewal of hematopoietic stem cells (HSCs) and/or megakaryocyte (Mk) progenitors. The homeodomain transcription factor HoxB4 has been shown to be an important regulator of stem cell renewal and hematopoiesis; however, its effect on megakaryopoiesis is unclear. In this study, we investigated the effect of HoxB4 overexpression or RNA silencing on megakaryocytic development in the human TF1 progenitor cell line; we then used recombinant tPTD-HoxB4 fusion protein to study the effect of exogenous HoxB4 on megakaryocytic development of human CD34 positively-selected cord blood cells. We found that ectopic HoxB4 in TF1 cells increased the antigen expression of CD61and CD41a, increased the gene expression of thrombopoietin receptor (TpoR), Scl-1, Cyclin D1, Fog-1 and Fli-1 while it decreased c-Myb expression. HoxB4 RNA silencing in TF1 cells decreased the expression of CD61 and CD41a and decreased Fli-1 expression while it increased the expression of c-Myb. Recombinant tPTD-HoxB4 fusion protein increased the percentages and absolute numbers of CD41a and CD61 positive cells during megakaryocytic differentiation of CD34 positively-selected cord blood cells and increased the numbers of colony forming unit-megakaryocyte (CFU-Mk). Adding tPTD-HoxB4 fusion protein increased the gene expression of TpoR, Cyclin D1, Fog-1 and Fli-1 while it inhibited c-Myb expression. Our data indicate that increased HoxB4 enhanced early megakaryocytic development in human TF1 cells and CD34 positively-selected cord blood cells primarily by upregulating Tpo R and Fli-1 expression and downregulating c-Myb expression. Increasing HoxB4 expression or adding recombinant HoxB4 protein might be a way to expand Mks for the production of platelets for use in transfusion medicine.
Keywords: HoxB4, megakaryocytic development, Tpo Receptor, CFU-Mk
Introduction
Overexpression of HoxB4 enhances the self-renewal of murine and human hematopoietic stem cells (HSCs) [1, 2], and expansion of HSCs in vitro can be achieved by HoxB4 overexpression or by directly using recombinant HoxB4 fusion protein [3, 4]. HoxB4 can also be used to promote hematopoietic differentiation from human embryonic stem cells or from human induced pluripotent stem cells [5, 6]. Increased numbers of committed hematopoietic progenitors, including granulocyte-macrophage [7] and lymphoid [8] progenitors, have been demonstrated as a result of ectopic HoxB4. However, the effect of increased HoxB4 on megakaryocyte (Mk) development is still unknown. Studies on HoxB4’s functions during megakaryocytopoiesis may enhance ex vivo production of platelets for clinical use.
Mk development is a complex process of continuous production of platelets from CD34+ multipotent hematopoietic progenitors. The differentiation process is driven primarily by thrombopoietin (TPO) [9]. Many genes have been found to play roles in this process, including Tpo receptor (Tpo R) [10], Scl-1[11], GATA-1[12], NF-E2 [13], Cyclin D1[14], Fli-1[15], Fog-1 [16] and c-Myb [17]. Among those genes, Scl-1, GATA-1, NF-E2, Cyclin D1, Fli-1 and Fog-1 are positive regulators of megakaryocytic development while c-Myb is a negative modulator. The cell surface antigens CD41 (integrin αIIb) and CD61 (integrin β3) are expressed in cells of the megakaryocytic lineage, and these two antigens form the receptor complex glycoprotein (GP) IIb/IIIa. The expression of CD41 and CD61 is a characteristic of the megakaryocytic lineage as it progresses from progenitor cells to megakaryocytes and then platelets [18].
TF1 is a human erythro-megakaryocytic progenitor cell line which is completely dependent on interleukin 3 (IL-3) or granulocyte-macrophage colony-stimulating factor (GM-CSF) for long term growth. This cell line can be used as a model to study the mechanism of proliferation and differentiation of hematopoietic progenitor cells (HPCs), as TF1 cells share several phenotypic characteristics of HPCs, including growth factor-dependence and expression of CD34 [19]. This cell line is also useful to study the erythro-megakaryocytic differentiation because TF1 cells can differentiate into erythroid or megakaryocytic lineage cells upon appropriate stimulation [20]. Cord blood is a unique source of HSCs, and in vitro differentiation from cord blood cells provides a great tool for studies of cell development and differentiation.
In the study reported here, we introduced overexpressed or RNA silenced HoxB4 into human TF1 cells by lentivirus and found that increased Hoxb4 enhanced the expression of CD41a and CD61, upregulated the gene expression of TpoR, Scl-1, Cyclin D1, Fog-1and Fli-1 and inhibited c-Myb expression. We also investigated the effect of recombinant tPTD-HoxB4 fusion protein on Mk development of CD34 positively-selected cord blood cells and found that tPTD-HoxB4 fusion protein increased the numbers of CD61 and CD41a positive cells and also increased the numbers of CFU-Mk. Recombinant tPTD-HoxB4 fusion protein mainly increased the gene expression of TpoR, Cyclin D1, Fog-1and Fli-1 and inhibited c-Myb expression. These data indicated that increased HoxB4 enhanced human early megakaryocytic development.
Materials and methods
Cell culture
TF1 cells were purchased from ATCC (Manassas, VA) and were cultured in RPMI1640 with 2 ng/ml GM-CSF (StemCell Technologies, Vancouver, BC, Canada), 10% fetal bovine serum (FBS), and 1% Penicillin-Streptomycin. For Mk induction, TF1 cells were cultured in the presence of 20 ng/ml PDB and 20 ng/ml TPO for 4 days.
CD34 positively selected cord blood cells were isolated from umbilical cord blood units from normal full–term deliveries after institutional review board approval and informed consent. Light–density cells were isolated from citrated cord blood using discontinuous density centrifugation over Ficoll–Paque Plus (GE Healthcare BioSciences, Uppsala, Sweden). CD34-positive selection was conducted using a MACS Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Anaheim, CA, USA). To induce megakaryocytic differentiation, the CD34 positive cells were cultured in IMDM medium with 10% BIT 9500 Serum Substitute (Stemcell Technologies), 50ng/ml SCF, 50ng/ml TPO, 50ng/ml Flt, 10ng/ml IL6, 10ng/ml IL3 and 10ng/ml IL11 (All cytokines from R&D Systems Inc., Minneapolis, MN).
Lentiviral vectors
A Xba I/Cla I fragment containing human HoxB4 cDNA-IRES-GFP was cloned from the tricistronic vector [21] (a gift from Dr. Dhanalakshmi Chinnasamy) using the following primers: forward primer 5′-tttctagaatggctatgagttcttttttg-3′ and reverse primer 5′-ttatcgatttacttgtacagctcgtccat-3′, and this fragment was subcloned into the lentiviral vector iDuet101 [22] (a gift from LZ Cheng).
Lentivirus production, concentration and titration
Lentivirus was produced in HEK 293T cells (from ATCC) using the method as described by Karolewski et al. with modifications [23]. The day before transfection, 5 × 106 HEK 293T cells were plated in poly-L-lysine-coated 10 cm dishes. One hour prior to transfection, the media was replaced with Opti-MEM® I reduced serum medium (Invitrogen, Carlsbad, CA). The envelope plasmid pMD2.G (Addgen, Cambridge, MA), packaging plasmid psPAX (Addgene) and lentiviral vectors were used at a ratio of 1.2:3:4 for all transfections. Lentiviral supernatant was concentrated by ultrafiltration using Centicon ® Plus-70 (Millipore, Billerica, MA) according the manual. Titers of lentivirus were tested on HEK293T cells. 5×104 HEK293T cells were plated in 12-well plates. The next day, we counted the cells on one well and then transduced the cells with 4, 5-fold serial dilutions of lentivirus in 1ml DMEM medium with 8ug/ml polybrene. Medium was changed one day after transduction, and GFP expression was analyzed by flow cytometry 4 days after transduction.
HoxB4 shRNA lentivirus was purchased from Santa Cruz Biotechnology (Santa Cruz, California).
Recombinant tPTD-HoxB4 fusion protein
The tPTD-HoxB4 fusion protein was kindly provided by Dr. Shi-Jiang Lu from Advanced Cell Technology (Santa Monica, CA) [24]. A concentration of 50 nM of tPTD-HoxB4 fusion protein was used in this study, and the protein was added into serum-free medium every two days.
CFU-Mk assays
CFU-Mk assays were performed using MegaCult™-C Staining Kit - CFU-Mk (StemCell Technologies) according to the manufacturer’s instructions. In short, 5,000 cells were seeded per chamber culture slide in serum-free medium containing thrombopoietin (50 ng/ml), IL-3 (10 ng/ml), IL-6 (10 ng/ml), and collagen (1.1 mg/ml). Cultures were incubated for 10–12 days, followed by dehydration and immunocytochemical staining of the slides. Megakaryocyte colonies were detected using the anti-CD41 antibody and alkaline phosphatase detection system and counterstained in Evan’s Blue. MK colonies consisting of at least five nucleated cells expressing CD41 were scored.
Flow cytometry
All antibodies including CD61, CD41a, CD34, CD42a and CD42b were purchased from BD Pharmingen (San Diego, CA). Flow cytometry was performed on a BD FACScalibur System flow cytometer (BD Biosciences, San Jose, CA). More than 10,000 cells were analyzed for each sample. The results were analyzed using the EXPO32 ADC Analysis software.
Western blot
1 × 106 cells were pelleted and lysed with 1x SDS sample buffer (62.5 mM Tris-HCL, 2% SDS, 10% glycerol, 50 mM DTT and 0.01% bromophenol blue), and boiled for 3 minutes at 95°C. 30 μl of total cell lysate were fractionated on SDS-PAGE gel and transferred to nitrocellulose membranes. The membranes were blocked with 5% dry nonfat milk and probed with a 1:1000 dilution of HoxB4 antibody (Developmental Studies Hybridoma Bank, University of Iowa). Antibody binding was detected with a 1:5000 dilution of anti-mouse IgG (Santa Cruz Biotechology) and luminescence was detected with ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ). Anti-beta-actin antibody was purchased from Cell Signaling (Cell Signaling, Boston, MA).
Reverse transcription and real time PCR
Total RNA was extracted by the RNeasy® Mini Kit (Qiagen, Valencia, CA) and cDNA was synthesized using SuperScript ™ III First-Strand (Invitrogen) according to the manufacturer’s protocol. Real time PCR was carried out using Applied Biosystems 7900HT Fast Real-Time PCR System with ABI PRISM. The reactions were run at 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles, 95°C for 15 seconds, 60°C for 1 minute. Primer sequences were listed in Supplementary Table S1. The gene expression levels were calibrated to the housekeeping gene GAPDH using the comparative threshold cycle (CT) method.
Results
Construction of lentiviral vectors and HoxB4 overexpression in human TF1 cells
GFP in the control lentiviral vector (iDuetA) was replaced by a fragment containing HoxB4-IRES-GFP as shown in Supplementary Fig. S1 A. Human TF1 cells were transduced by spinoculation at MOI 10, and transduced cells were selected by 200 ng/ml hygromycin for 4 days. The GFP expression in transduced TF cells was detected by flow cytometry (Supplementary Fig. S2 B). We noticed that GFP expression in TF1-HoxB4 cells decreased during culture while GFP expression in control TF1-GFP cells was almost constant (Supplementary Fig. S2 C). As verified by western blot, the expression of HoxB4 is proportional to GFP expression (Supplementary Fig. S2 D). As detected by reverse transcription PCR, there is low basal expression of HoxB4 in TF1 control cells and the expression was greatly increased by overexpression (Supplementary Fig. S2 E).
HoxB4 overexpression in human TF1 cells induced Mk development by upregulating gene expression of TpoR, Cyclin D1, Fog-1 and Fli-1 while inhibiting c-Myb expression
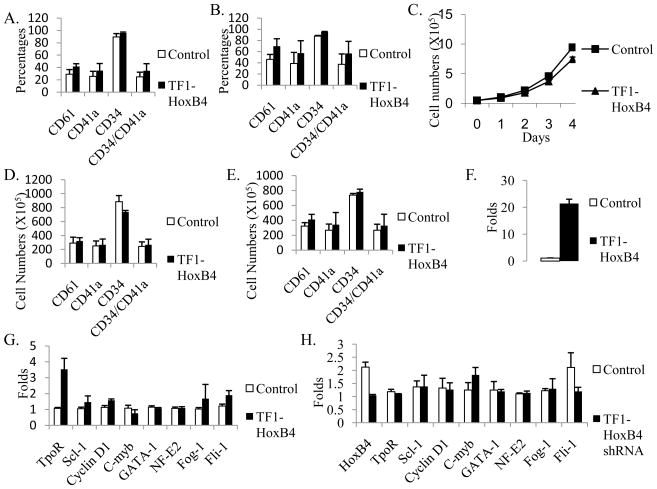
We analyzed the expression of several cell membrane antigens including CD34, CD41a and CD61 in transduced TF1 cells by flow cytometry. The percentages of CD61+, CD41a+ or CD34+/CD41a+ cells were increased 13%, 9% or 10% respectively in TF1-HoxB4 cells compared to control cells (Fig. 1A); HoxB4 overexpression also increased the percentages of CD61+, CD41a+ and CD34+/CD41a+ cells in TF1 stimulated by PDB and TPO (Fig. 1B). The cell numbers were counted every day during culture, and we found that HoxB4 overexpression inhibited the cell growth (Fig. 1C); thus increased HoxB4 slightly increased the absolute numbers of CD61+ or CD41a+ cells in untreated TF1(Fig. 1D) and in TF1 treated by PDB and TPO (Fig. 1E). When HoxB4 was silenced by lentiviral shRNA in untreated TF1 cells, the percentages of CD41a+ or CD61+ cells were decreased by 4% or 3%, respectively (Data not shown).
Fig. 1.
HoxB4 overexpression in TF1 cells increased the expression of CD61 and CD41a by primarily upregulating TpoR expression and downregulating c-Myb expression. (A) Percentages of CD61 or CD41a positive cells in untreated TF1 cells. (B) Percentages of CD61 or CD41a positive cells in TF1 cells treated by PDB and TPO. (C) Cell growth of untreated TF1 cells was inhibited by HoxB4 overexpression. (D) Absolute numbers of CD61 or CD41a positive cells in untreated TF1 cells. (E) Absolute numbers of CD61 or CD41a positive cells in TF1 cells treated by PDB and TPO. (F) HoxB4 expression in TF1 cells. (G) Gene expression in TF1 control and TF1-HoxB4 cells. (H) Gene expression in TF1 control and TF1-HoxB4 shRNA cells. Data is the averages of three independent experiments.
We then studied the effect of ectopic HoxB4 on gene expression in untreated TF1 cells using real time PCR. Compared to the expression in TF1 control cells, HoxB4 expression was increased about 20 fold with gene insertion and decreased to half of basal expression with shRNA silencing (Fig. 1F, 1H). The expression of TopR, Scl-1, Cyclin D1, Fog-1, and Fli-1 in TF1-HoxB4 was increased about 3.5 fold, 1.4 fold, 1.6 fold, 1.7 fold, and 1.9 fold respectively, and c-Myb expression was decreased while the expression of GATA-1 and NF-E2 had no changes compared to the expression in control cells (Fig. 1G). GATA-2 expression was also not changed by ectopic HoxB4 as detected by reverse transcription PCR. HoxB4 silencing in TF1 cells mainly increased c-Myb expression and decreased Fli-1 expression (Fig. 1H).
Morphological analysis indicated that increased HoxB4 slightly increased Mk polyploidy. There is a low rate of spontaneous Mk differentiation in TF1 cells [19], so few cells with multiple nuclei were observed in TF1 control cells. However, about 1.5 times more cells with multiple nuclei were observed in TF1-HoxB4 cells (Supplementary Fig. S2).
Recombinant tPTD-HoxB4 fusion protein increased the percentages and absolute numbers of CD61 or CD41a positive cells and increased the numbers of CFU-Mk during Mk development from CD34 positively-selected cord blood cells
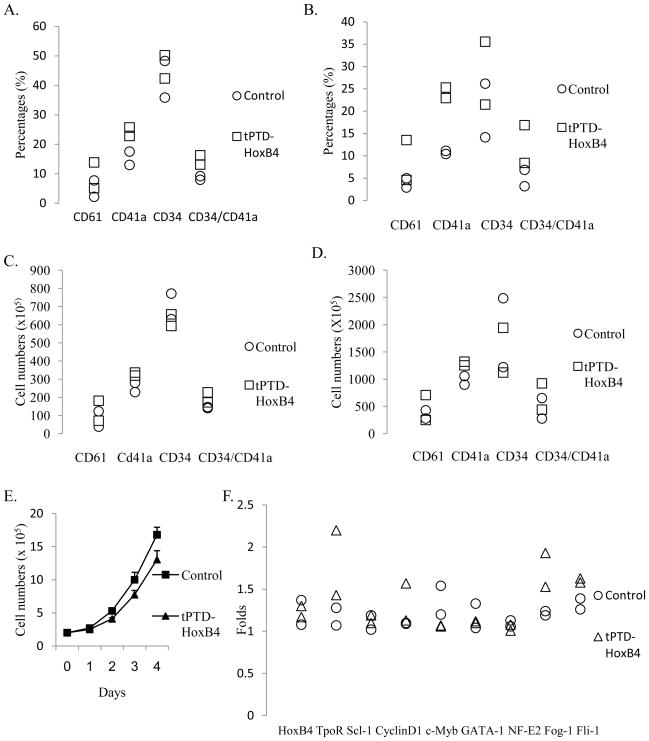
50nM recombinant tPTD-HoxB4 fusion protein or BSA was added every two days to the serum-free culture medium for CD34 positively-selected cord blood cells. The expression of CD34, CD41a, CD61, CD42a and CD42b was analyzed by flow cytometry on Day 5 and Day 8. During the differentiation of CD34 positively-selected cord blood cells, percentages of CD61+, CD41a+ and CD34+/CD41a+cells were increased by 4%, 9% and 6% respectively on Day 5 and increased by 5%, 13% and 8% on Day 8 by recombinant tPTD-HoxB4 fusion protein (Fig. 2A and 2B). Exogenous tPTD-HoxB4 protein still increased the absolute numbers of CD61+, CD41a+ and CD34+/CD41a+ cells (Fig. 2C and 2D) though it also inhibited cell growth (Fig. 2E). Ectopic HoxB4 slightly increased the percentages of CD42a+ or CD42b+ cells on Day 5 but had no effect on these two markers on Day 8. Real time PCR indicated that exogenous HoxB4 also increased the expression of TopR, Cyclin D1, Fog-1 and Fli-1 while it inhibited c-Myb expression as we found from human TF1 cells (Fig. 2F).
Fig. 2.
Recombinant tPTD-HoxB4 fusion protein increased the expression of CD61 and CD41a during Mk development from CD34 positively-selected cord blood cells by primarily increasing TpoR expression and decreasing c-Myb expression. (A) Percentages of CD61 or CD41a positive cells on Day 5. (B) Percentages of CD61 or CD41a positive cells on Day 8. (C) Absolute numbers of CD61 or CD41a positive cells on Day 5. (D) Absolute numbers of CD61 or CD41a positive cells on Day 8. (E) Cell growth was inhibited by HoxB4 fusion protein. (F) Gene expression on Day 8. Data is from two independent experiments.
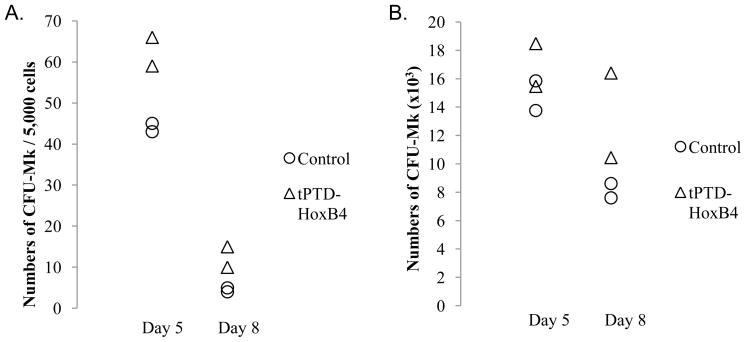
We performed CFU-Mk assays on Day 5 and Day 8 during megakaryocytic differentiation of CD34 positively-selected cord blood cells. Recombinant tPTD-HoxB4 fusion protein increased the colony numbers of CFU-Mk on both Day 5 and Day 8 (Fig. 3A and 3B).
Fig. 3.
CFU-Mk assays. (A) Numbers of CFU-Mk per 5,000 cells on Day 5 and Day 8. (B) Absolute numbers of CFU-Mk on Day 5 and Day 8. Data is from two independent experiments.
Discussion
HoxB4 has been shown to play important roles in HSCs and in some hematopoietic progenitor cells such as in granulocyte-macrophage progenitors and lymphoid progenitors [1, 8]. It is a promising tool to expand HSCs or progenitor cells in vitro, and to promote hematopoietic development of human embryonic stem cells or from human induced pluripotent stem cells [4, 5–6]. In this study, we found that ectopic HoxB4 enhanced Mk development in both TF1 cells and cord blood cells as evidenced by increased percentages and absolute numbers of two important Mk-specific markers, CD41a and CD61, and increased numbers of CFU-Mk derived from CD34 positively-selected cord blood cells.
The underlying molecular mechanisms of HOXB4’s actions in hematopoiesis have not been clearly identified yet. Sauvageau and coworkers reported that HOXB4 overexpression in Rat-1 fibroblasts increased the expression of JunB, Fra-1 and Cyclin D1 [25]. They also found that the DNA-binding ability of HoxB4 was very important for its HSC-expanding function [26]. In our study, several target genes of ectopic HoxB4 were identified and Tpo R was the most affected one. Many studies have highlighted the central role of TpoR (c-Mpl) during Mk development since TopR mediates the effects of TPO on megakaryopoiesis [10, 27]. TPO is a primary regulator of hematopoiesis and megakaryocytic development. One previous study indicated that the effect of TPO on hematopoietic cells is mediated by elevated expression of HoxB4 [28]. Our study found that ectopic HoxB4 increased the expression of TpoR, suggesting that the increased expression of TpoR was one of the molecular bases of the effect of increased HoxB4 expression on Mk development.
The changes of cell surface antigen expression and gene expression and the increased numbers of CFU-Mk caused by ectopic HoxB4 suggest that increased HoxB4 may primarily play roles in early megakaryocytic development. CD41 and CD61 are expressed in both early and later Mk development [18], and our study indicated increased HoxB4 greatly increased the expression of these two cell surface markers; CD42a and CD42b are two late Mk-specific markers and ectopic HoxB4 only slightly increased their expression. In this study, we found that ectopic HoxB4 did not change the expression of genes such as NF-E2 which play important roles in late Mk development [29] but changed the expression of genes such as TpoR, Scl-1, Cyclin D1, Fli-1, Fog-1 and c-Myb which may have roles in early or in both early and late megakaryocytic differentiation. The result that ectopic HoxB4 increased Fog-1 expression with no effects on GATA-1 expression may also indicate the role of HoxB4 in early Mk development because Fog-1 functions in a GATA-dependent pathway as a cofactor in late megakaryocytic differentiation but in a pathway unrelated to GATA-1 during early Mk development [16, 30]. One previous study also found that HoxB4 overexpression had no detectable effect on GATA-1 expression [31].
One previous study showed that HoxB4 overexpression inhibited cell growth in a dose-dependent manner and the inhibition was aggravated under reduced serum concentration, and they also found that this anti-proliferative effect was partially because of an increased sensitivity of cells with HoxB4 overexpression to induction of apoptosis [32]. In our study, we also found the inhibition of cell growth by increased HoxB4. A concentration of 50nM of HoxB4 fusion protein was used in our study and some other studies [4, 33]; however, a low concentration of 10nM or 15nM HoxB4 fusion protein also successfully expanded stem/progenitor cells [4, 34]. Since HoxB4 inhibits cell growth in a dose-dependent manner, a low concentration of HoxB4 fusion protein may be used to expand cells ex vivo more efficiently in serum-free conditions.
Conclusion
We report that increased HoxB4 in human TF1 progenitor cell line increased the expression of two important megakaryocyte specific markers CD41a and CD61, upregulated the gene expression of Tpo receptor, Scl-1, Cyclin D1, Fog-1and Fli-1 and downregulated the c-Myb expression. Silencing HoxB4 by lentiviral shRNA in TF1 cells decreased the expression of CD41a or CD61, increased c-Myb expression and decreased Fli-1 expression. We also found that recombinant tPTD-HoxB4 fusion protein increased the colony numbers of CFU-Mk derived from CD34 positively-selected cord blood cells, increased the percentages and absolute numbers of CD61 and CD41a positive cells, increased the gene expression of Tpo receptor, Cyclin D1, Fog-1and Fli-1 and decreased the c-Myb expression. These results indicated that ectopic HoxB4 enhanced early megakaryocytic development in human cells and suggested that increasing HoxB4 expression or adding recombinant HoxB4 fusion protein can be used to expand early megakaryocytic progenitors and will act to increase megakaryocytic differentiation.
Supplementary Material
Acknowledgments
This work was supported by the Center for Stem Cell and Regenerative Medicine (Tech 06–063) through the Biomedical Research and Commercialization Program, a component of the Third Frontier Program of Ohio (LCL) and NIH R21HL072088 (L.C.L.). Flow cytometry was performed at the University Cell Analysis and Sorting Core, a joint venture between The Comprehensive Cancer Center and The Davis Heart and Lung Research Institute at The Ohio State University. The authors thank Dr. Linzhao Cheng for supplying the iDuetA vector, Dr. Dhanalakshmi Chinnasamy for supplying the tricistronic vector, Dr. Shi-Jiang Lu for supplying the HoxB4 expression vector and HoxB4 recombinant protein and Cleveland Cord Blood Center for providing cord blood units used in this study.
Abbreviations
- HSCs
hematopoietic stem cells
- HPCs
hematopoietic progenitor cells
- CFU-Mk
colony-forming units megakaryocyte
- IL-3
interleukin 3
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- TpoR
thrombopoietin receptor
- Fog-1
Friend of GATA-1
- Fli-1
Friend leukemia integration 1
- Mk
megakaryocyte
- TPO
thrombopoietin
- Scl-1
stem cell leukemia-1
- PDB
phorbol dibutyrate
- FBS
fetal bovine serum
- RT-PCR
reverse transcription polymerase chain reaction
- TF1-WT
wild type TF1 cells
- TF1-GFP
TF1 cells transduced by control lentivirus containing GFP
- TF1-HoxB4
TF1 cells transduced by lentivirus containing HoxB4-IRES-GFP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antonchuk SGJ, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- 2.Kyba PRM, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 3.Amsellem PFS, Bardinet D, Izac B, Charneau P, Romeo PH, Dubart-Kupperschmitt A, Fichelson S. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat Med. 2003;9:1423–7. doi: 10.1038/nm953. [DOI] [PubMed] [Google Scholar]
- 4.Krosl APJ, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9:1428–32. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- 5.Bowles VLKM, Smith JR, Alexander MR, Pedersen RA. HOXB4 overexpression promotes hematopoietic development by human embryonic stem cells. Stem Cells. 2006;24:1359–69. doi: 10.1634/stemcells.2005-0210. [DOI] [PubMed] [Google Scholar]
- 6.Lengerke GMC, Niebuhr NI, Riedt T, Kanz L, Park IH, Daley GQ. Hematopoietic development from human induced pluripotent stem cells. Ann N Y Acad Sci. 2009;1176:219–27. doi: 10.1111/j.1749-6632.2009.04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyake BAN, Magnusson M, Miyake K, Scadden DT, Karlsson S. HOXB4-induced self-renewal of hematopoietic stem cells is significantly enhanced by p21 deficiency. Stem Cells. 2006;24:653–61. doi: 10.1634/stemcells.2005-0328. [DOI] [PubMed] [Google Scholar]
- 8.Haddad PFR, Vigon I, Visentin G, Auvray C, Fichelson S, Amsellem S. The HOXB4 Homeoprotein Differentially Promotes ex vivo Expansion of Early Human Lymphoid Progenitors. Stem Cells. 2008;26:312–22. doi: 10.1634/stemcells.2007-0721. [DOI] [PubMed] [Google Scholar]
- 9.Kaushansky DJK. The molecular and cellular biology of thrombopoietin: the primary regulator of platelet production. Oncogene. 2002;21:3359–67. doi: 10.1038/sj.onc.1205323. [DOI] [PubMed] [Google Scholar]
- 10.Edvardsson DJL, Olofsson T. Isolation and characterization of human myeloid progenitor populations--TpoR as discriminator between common myeloid and megakaryocyte/erythroid progenitors. Exp Hematol. 2006;34:599–609. doi: 10.1016/j.exphem.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Hall CDMA, Metcalf D, Elefanty AG, Sourris K, Robb L, Gothert JR, Jane SM, Begley CG. The critical regulator of embryonic hematopoiesis, SCL, is vital in the adult for megakaryopoiesis, erythropoiesis, and lineage choice in CFU-S12. Proc Natl Acad Sci U S A. 2003;100:992–7. doi: 10.1073/pnas.0237324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivdasani FYRA, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–73. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fock YFEL, Pan S, Chong BH. NF-E2-mediated enhancement of megakaryocytic differentiation and platelet production in vitro and in vivo. Exp Hematol. 2008;36:78–92. doi: 10.1016/j.exphem.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Sun ZJS, Toselli P, Thompson A, Jackson CW, Ravid K. Overexpression of cyclin D1 moderately increases ploidy in megakaryocytes. Haematologica. 2001;86:17–23. [PubMed] [Google Scholar]
- 15.Pang XHL, Szalai G, Wang X, Wang Y, Watson DK, Leonard WJ, Blobel GA, Poncz M. Maturation stage-specific regulation of megakaryopoiesis by pointed-domain Ets proteins. Blood. 2006;108:2198–206. doi: 10.1182/blood-2006-04-019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muntean CJAG. Differential requirements for the activation domain and FOG-interaction surface of GATA-1 in megakaryocyte gene expression and development. Blood. 2005;106:1223–31. doi: 10.1182/blood-2005-02-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalf CMD, Hyland C, Mifsud S, Dirago L, Nicola NA, Hilton DJ, Alexander WS. Anomalous megakaryocytopoiesis in mice with mutations in the c-Myb gene. Blood. 2005;105:3480–7. doi: 10.1182/blood-2004-12-4806. [DOI] [PubMed] [Google Scholar]
- 18.Levene LJRB, Broxmeyer HE, Lu L, Rabellino EM. Human megakaryocytes. V. Changes in the phenotypic profile of differentiating megakaryocytes. J Exp Med. 1985;161:457–74. doi: 10.1084/jem.161.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppola NLS, Feccia T, Bonci D, Calabrò L, Morsilli O, Gabbianelli M, De Maria R, Testa U, Peschle C. Enforced expression of KDR receptor promotes proliferation, survival and megakaryocytic differentiation of TF1 progenitor cell line. Cell Death Differ. 2006;13:61–74. doi: 10.1038/sj.cdd.4401698. [DOI] [PubMed] [Google Scholar]
- 20.Testa GFU, Hassan HJ, Rogaia D, Masciulli R, Gelmetti V, Guerriero R, Macioce G, Liberatore C, Barberi T, Mariani G, Pelicci PG, Peschle C. Terminal megakaryocytic differentiation of TF-1 cells is induced by phorbol esters and thrombopoietin and is blocked by expression of PML/RARalpha fusion protein. Leukemia. 1998;12:563–70. doi: 10.1038/sj.leu.2400967. [DOI] [PubMed] [Google Scholar]
- 21.Chinnasamy MMD, Shaffer J, Neuenfeldt J, Shaaban AF, Margison GP, Fairbairn LJ, Chinnasamy N. Multicistronic lentiviral vectors containing the FMDV 2A cleavage factor demonstrate robust expression of encoded genes at limiting MOI. Virol J. 2006;3 doi: 10.1186/1743-422X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou YZBY, Chen G, Gao ZP, Zhang YA, Cheng L. Inducible and reversible transgene expression in human stem cells after efficient and stable gene transfer. Stem Cells. 2007;25:779–89. doi: 10.1634/stemcells.2006-0128. [DOI] [PubMed] [Google Scholar]
- 23.Karolewski WDBA, Parente MK, Wolfe JH. Comparison of transfection conditions for a lentivirus vector produced in large volumes. Hum Gene Ther. 2003;14:1287–96. doi: 10.1089/104303403322319372. [DOI] [PubMed] [Google Scholar]
- 24.Lu FQSJ, Ivanova Y, Luo C, Li T, Li F, Honig GR, Lanza R. Recombinant HoxB4 fusion proteins enhance hematopoietic differentiation of human embryonic stem cells. Stem Cells Dev. 2007;16:547–59. doi: 10.1089/scd.2007.0002. [DOI] [PubMed] [Google Scholar]
- 25.Krosl SGJ. AP-1 complex is effector of Hox-induced cellular proliferation and transformation. Oncogene. 2000;19:5134–41. doi: 10.1038/sj.onc.1203897. [DOI] [PubMed] [Google Scholar]
- 26.Beslu KJN, Laurin M, Mayotte N, Humphries KR, Sauvageau G. Molecular interactions involved in HOXB4-induced activation of HSC self-renewal. Blood. 2004;104:2307–14. doi: 10.1182/blood-2004-04-1653. [DOI] [PubMed] [Google Scholar]
- 27.Ihara IEK, Eguchi M, Takada H, Suminoe A, Good RA, Hara T. Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc Natl Acad Sci U S A. 1999;96:3132–6. doi: 10.1073/pnas.96.6.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirito FNK, Kaushansky K. Thrombopoietin stimulates Hoxb4 expression: an explanation for the favorable effects of TPO on hematopoietic stem cells. Blood. 2003;102:3172–8. doi: 10.1182/blood-2003-03-0944. [DOI] [PubMed] [Google Scholar]
- 29.Lecine BVP, Shivdasani R. Characterization of the hematopoietic transcription factor NF-E2 in primary murine megakaryocytes. J Biol Chem. 1998;273:7572–8. doi: 10.1074/jbc.273.13.7572. [DOI] [PubMed] [Google Scholar]
- 30.Shivdasani R. Molecular and transcriptional regulation of megakaryocyte differentiation. Stem Cells. 2001;19:397–407. doi: 10.1634/stemcells.19-5-397. [DOI] [PubMed] [Google Scholar]
- 31.Helgason SGCD, Lawrence HJ, Largman C, Humphries RK. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood. 1996;87:2740–9. [PubMed] [Google Scholar]
- 32.Will SDE, Wang Z, Ghiaur G, Rimek A, Schiedlmeier B, Williams DA, Baum C, Ostertag W, Klump H. HOXB4 inhibits cell growth in a dose-dependent manner and sensitizes cells towards extrinsic cues. Cell Cycle. 2006;5:14–22. doi: 10.4161/cc.5.1.2304. [DOI] [PubMed] [Google Scholar]
- 33.Tang CJY, Young NS. Expansion of haematopoietic stem cells from normal donors and bone marrow failure patients by recombinant hoxb4. Br J Haematol. 2009;144:603–12. doi: 10.1111/j.1365-2141.2008.07509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang CPCH, Lu TC, Kung WM, Chiou TJ, Yang MH, Kao JY, Wu KJ. Purified recombinant TAT-homeobox B4 expands CD34(+) umbilical cord blood and peripheral blood progenitor cells ex vivo. Tissue Eng Part C Methods. 2010;16:487–96. doi: 10.1089/ten.TEC.2009.0163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.