Abstract
Understanding how the pancreas develops is vital to finding new treatments for a range of pancreatic diseases, including diabetes and pancreatic cancer. Xenopus is a relatively new model organism for the elucidation of pancreas development, and has already made contributions to the field. Recent studies have shown benefits of using Xenopus for understanding both early patterning and lineage specification aspects of pancreas organogenesis. This review focuses specifically on Xenopus pancreas development, and covers events from the end of gastrulation, when regional specification of the endoderm is occurring, right through metamorphosis, when the mature pancreas is fully formed. We have attempted to cover pancreas development in Xenopus comprehensively enough to assist newcomers to the field and also to enable those studying pancreas development in other model organisms to better place the results from Xenopus research into the context of the field in general and their studies specifically.
Keywords: Xenopus, pancreas, RA, Wnt, TGFβ, Pdx1, Ptf1a, metamorphosis, thyroid hormone
INTRODUCTION
The pancreas is an important organ in the digestive system. It is located next to the stomach and is composed of two types of cells, endocrine and exocrine, which secrete hormones, such as insulin and glucagon, and digestive enzymes, such as trypsin, respectively (Fig. 1). In Xenopus, the endocrine cells are arranged in clusters in the mature pancreas, and the exocrine cells in a floral pattern (Fig. 1D). The main disease associated with a malfunctioning pancreas is diabetes, a disease whereby sufferers are unable to store sugar from their bloodstream due to defects in either insulin production or recognition. Other diseases affecting the pancreas include exocrine pancreatic insufficiency, where the digestive enzymes are not made and the patient cannot properly digest food; pancreatitis, the inflammation of the pancreas, which can be caused by many conditions including cystic fibrosis and alcoholism; and pancreatic cancer, one of the most lethal cancers with a 5-year survival rate below 5% (Hezel et al., 2006; Nair et al., 2007; Maitra and Hruban, 2008).
Fig. 1.

Tadpole and froglet pancreas histology. A: Transverse section of a tadpole (NF55), showing the positioning of the pancreas relative to the rest of the gut. n, notochord; s, stomach; p, pancreas; l, liver; i, intestine. B: Higher magnification of the lower left-hand corner of A. C: Electron micrograph image (×4,500) of froglet pancreas endocrine cells. At least three different endocrine cells are visible, with two acinar cells in the top left corner. D: EM image (×2,500) of exocrine cells from the same froglet pancreas, illustrating the floral/floret pattern of acinar cells, surrounding a duct.
By understanding the way the pancreas forms and what genes are involved in its development, it is envisioned that new drug targets for the treatment of these diseases will be discovered or that techniques to produce insulin-expressing cells de novo will be developed. Most of the research in pancreas development thus far has been performed in more traditional model organisms, such as mouse and chick (Kume, 2005; Jorgensen et al., 2007), but recently more studies have been performed in other model organisms such as Xenopus and zebrafish. The benefits of using these organisms are numerous: their pancreases develop faster, the number of animals is not limiting as large numbers of embryos can be injected and processed in a single day, early development is more accessible allowing easy isolation of endodermal or pancreatic tissue, overexpression studies are accomplished by simple microinjection of mRNA (targeting to specific regions of the endoderm is done by injection into single blastomeres at the 8-, 16-, or 32-cell stage), transgenic tadpoles can be produced quickly, gene knockdown studies can be performed quickly using antisense morpholinos, and earlier patterning and specification events can be addressed more easily. Indeed, recent results demonstrate that early pancreas development in Xenopus closely resembles that of mice and humans, and is applicable to mammalian cells as we previously demonstrated (Horb et al., 2003; Cao et al., 2004; Li et al., 2005; Afelik et al., 2006; Blitz et al., 2006; Jarikji et al., 2007). In fact, it is becoming clear that the same genes used in mammalian pancreas development are involved in Xenopus pancreas development (Horb et al., 2003; Afelik et al., 2006; Jarikji et al., 2007).
With the emergence of Xenopus as a model system for pancreas development, now is the appropriate time for a comprehensive review of Xenopus pancreas development to be written. Although there have been several recent reviews that have touched on some aspects of Xenopus pancreas development (Blitz et al., 2006; Pieler and Chen, 2006; Spagnoli, 2007; Zorn and Wells, 2007; Pearl and Horb, 2008), none has comprehensively covered pancreas development in Xenopus from gastrulation through metamorphosis. This review aims to give detailed insight into pancreas development in Xenopus laevis from the end of gastrulation, when the endoderm becomes regionalized, through the emergence of pancreatic buds, to the cellular rearrangements that take place at metamorphosis resulting in a mature pancreas. We have tried to make this review differ from the numerous other pancreas reviews that have already been published, and have focused on the current state of pancreas development in Xenopus. Since pancreas development in Xenopus is a new topic, some aspects have not been covered in depth, and for more detail we refer the reader to some of the recent excellent reviews that have been written on pancreas development (Jensen, 2004; Cano et al., 2007; Murtaugh, 2007; Gittes, 2009).
MORPHOLOGICAL DEVELOPMENT
As with other vertebrates, the pancreas in Xenopus first arises as separate dorsal and ventral pancreatic buds, which subsequently fuse to give rise to the mature pancreas (Fig. 2). The dorsal pancreatic anlage is first visible at NF35/36 on the dorsal surface of the endoderm at the level of the pronephros (Table 1) (Nieuwkoop and Faber, 1967; Kelly and Melton, 2000). In this review, we routinely refer to the stages described by Nieuwkoop and Faber as NF followed by the stage number (Nieuwkoop and Faber, 1967). At NF35/36, the shape of the dorsal pancreas resembles a crescent straddling the dorsal endodermal midline. In contrast, the ventral pancreas develops from two symmetrical (left and right) anlagen that first become apparent at NF37/38, at the junction of the liver bud and developing duodenum. The two ventral buds fuse together around NF40 (Table 1) and look like two half circles joining together; their fusion, however, is not complete as a space between these two ventral buds remains for a short period (Kelly and Melton, 2000). It is here that the hepatopancreatic duct emerges. By stage 40, the dorsal and ventral pancreatic anlagen have fused; the fusion occurs at the junction of the right side of the dorsal pancreatic bud with the dorsal aspect of the fused ventral buds. From NF42 to 48, the intestine coils dramatically, and as a result the pancreas is displaced to the right side of the embryo (Fig. 2D). After fusion, the pancreas can now be isolated, along with the liver, separate from the intestine; the morphology of an isolated pancreas at NF40 is thin and long, it is also slightly curved in a horseshoe-type shape (Fig. 3A). By NF44, the pancreas appears more compact; it is shorter and wider than when it had just fused (Fig. 3B). This reduction in the length of the pancreas continues as the tadpole ages to NF46 when the pancreas has transformed from a rectangular shape to a square (Fig. 3C). The main difference between Xenopus and mammalian gut development is that the mammalian gut forms a tube early in development, from which the pancreatic buds emerge (reviewed in Jorgensen et al., 2007), whereas in Xenopus the gut does not form a tube until after the pancreatic buds have emerged and fused, around NF40 (Chalmers and Slack, 1998). The early morphological differences may be due to the different ways the embryos feed at these stages. Xenopus embryos develop ex vivo and require an external nutrient source (which is the yolk in the endoderm at this stage of development), while mice develop in vivo, gaining nutrients from their mother’s blood supply.
Fig. 2.

Developing pancreatic buds. A: NF32 tadpole showing Ptf1a RNA expression in the dorsal and ventral pancreatic buds prior to overt morphogenesis. B: Ptf1a expression in a NF40 tadpole, after buds have fused. C,D: Transgenic tadpoles expressing GFP under the control of the elastase promoter at (C) NF40 and (D) NF42. A–C: Anterior to the left, dorsal up (lateral view). D: Anterior to the left, ventral view. vp, ventral pancreas; dp, dorsal pancreas.
TABLE 1. Comparison of Major Morphological Events in Pancreas Development Between Xenopus, Mouse, Chick, and Zebrafish*.
| Xenopus laevis | Mus musculus | Gallus gallus | Danio rerio | |
|---|---|---|---|---|
| Regional specification of endoderm | NF11–20 | E7.5–8 | HH5–8 | |
| Pre-pancreatic region specified | NF27–30 expression of Pdx1a–c |
E8.5–8.75 (8–10 ss)g | HH10 (10 ss)g | 14 hpf, expression of Pdx1j,k |
| Dorsal pancreatic bud emerges | NF35/36d | E9.5–10.5 (20–25 ss)h | HH16 (26 ss)g,i | 24 hpfl |
| Ventral pancreatic buds emerge | NF37/38d | E10.25 (30 ss)h | HH22–23g,i | 40 hpfl |
| Ventral pancreatic buds fuse | NF40d,c | n/a (one bud regresses) | n/a (only one ventral bud) |
|
| Dorsal and ventral pancreatic buds fuse | NF40d,e | E12.5 | 52 hpfl | |
| β-cell clusters/islets formed | NF66f | E18.5h | 5.5 dpfl |
NF, Nieuwkwoop and Faber stages of Xenopus laevis development; ss, somite stage; E, embryonic day; HH, Hamburger and Hamilton stages of chick development; hpf, hours post-fertilization; dpf, days post-fertilization.
Fig. 3.
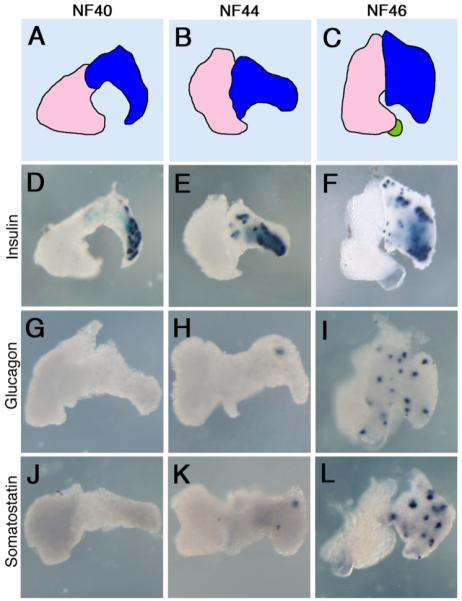
Endocrine marker expression in isolated liver and pancreas tissue. A–C: Diagrammatic representations of the livers and pancreases: pink, liver; bright blue, ventral pancreas; dark blue, dorsal pancreas; green, gall bladder. D–L: Whole mount in situ hybridization for insulin, glucagon, and somatostatin at NF40, NF44, and NF46.
Endocrine Cells
The endocrine pancreas is composed of 5 different cell types—α, β, δ, ε, and PP—that secrete glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide hormones into the bloodstream. In Xenopus, four of these cell types (α, β, δ, and PP) have been identified in the pancreas, and we will describe their development and differentiation in the next few paragraphs.
The first endocrine marker to be expressed in the pancreas at NF32 is insulin. RNA expression is first detected in the dorsal endoderm (where the future dorsal pancreatic anlage emerges) in a punctate manner at NF32. As the pancreas develops, expression initially remains localized to the dorsal pancreas, even after fusion of the dorsal and ventral pancreatic buds at NF40 (Fig. 3D,E). However, by NF46/47 insulin is expressed throughout the entire pancreas, including the ventral pancreas (Fig. 3F) (Kelly and Melton, 2000; Horb and Slack, 2002). Similarly, insulin protein expression is first detected only in the dorsal pancreas at NF39. At these early stages, insulin-expressing cells are found grouped as single cells or clusters of 2–4 cells.
Glucagon and somatostatin, unlike insulin, are not expressed in the pancreas until after fusion of the dorsal and ventral buds, but show similar expression profiles (Maake et al., 1998; Kelly and Melton, 2000; Horb and Slack, 2002). Expression is first detected at NF44/45 marking individual cells, initially in the dorsal portion of the pancreas (Fig. 3H,K). By NF46/47, expression of both markers is now detected throughout the entire pancreas (Fig. 3I,L). However, prior to expression in the pancreas, both somatostatin and glucagon are initially expressed at NF41 within the stomach and duodenum in a punctate pattern.
The two other endocrine cell types present within the pancreas are PP cells, producing pancreatic polypeptide (PP) and ε cells, producing ghrelin. In Xenopus, the cDNAs for these two genes remain to be cloned, although genomic sequence for the PP gene is found in the X. tropicalis genome. Antibodies to mammalian PP do detect scattered expression in mature Xenopus pancreas beginning at NF46 (Maake et al., 1998); the majority (79%) of these PP-expressing cells also co-express glucagon. Ghrelin-producing cells are yet to be discovered in Xenopus.
Exocrine Cells
The mammalian exocrine pancreas is a lobulated branched tissue, consisting of acinar and ductal cells that secrete and transport digestive enzymes into the duodenum. The expression of acinar differentiation markers follows a temporal and spatial distribution at the early stages of pancreas development, opposite to that seen with insulin. Expression of most exocrine markers, including amylase, trypsinogen and elastase, is first detected at NF41 exclusively in the ventral pancreas (Fig. 4A). Shortly thereafter, their expression diffuses into the dorsal pancreas, such that by NF45 expression is detected throughout the entire pancreas (Fig. 4B) (Horb and Slack, 2002). A fourth exocrine marker, carboxypeptidase A, was detected by immunostaining throughout the entire pancreas at NF44 (Kelly and Melton, 2000). In contrast to these markers, expression of a fifth exocrine marker, pancreatic protein disulfide isomerase (XPDIp), is first observed at NF39 in both dorsal and ventral pancreatic buds (Afelik et al., 2004). The reason for these differences is unclear, although it may be that XPDIp is simply expressed at an earlier stage in acinar cell differentiation, and its expression in the dorsal pancreas may be marking exocrine precursor cells that will subsequently express the other late differentiation markers as indicated above. As with other model systems, there is very little known about the development of the ductal system in Xenopus.
Fig. 4.

Exocrine marker expression in isolated liver and pancreas tissue. A–C: Elastase expression. D-G: Mist1 expression.
INDUCTION OF THE PANCREAS (NF11–30)
The pancreas, like organs such as the lungs, liver, stomach, and colon, develops from the endodermal germ layer. One of the first things to occur once the endoderm layer is formed is the specification of anterior and posterior regions of the endoderm. Although this occurs before the end of gastrulation, stable regional specification is not achieved until much later (Table 1) (Zeynali et al., 2000; Horb and Slack, 2001; McLin et al., 2007). Thereafter, during neurula and tail bud stages, these regions are further subdivided into discrete organ domains. It is only after stable regional specification occurs that specific organs, such as the pancreas, develop.
Early investigations into regional specification of the gut endoderm in amphibians were performed in Triturus pyrrhogaster (a urodele amphibian); these studies demonstrated that signals from the mesoderm were necessary and sufficient to specify regions of the gut endoderm (Okada, 1960). Initial molecular studies in Xenopus seemed to suggest otherwise, that endoderm differentiation did not require input from other cell layers (Gamer and Wright, 1995; Henry et al., 1996). These studies examined the differentiation of endoderm in vegetal explants from blastula stage embryos and found that at tail bud and tadpole stages, expression of pancreas and liver markers was detectable in the absence of axial mesoderm (Gamer and Wright, 1995). However, when these vegetal explants were examined for expression of other mesoderm markers, they were found to express more lateral mesoderm markers (Horb and Slack, 2001). The presence of mesoderm in these vegetal explants was due to the fact that at blastula stages, the mesoderm and endoderm are not cleanly separable. When endoderm explants were isolated at later neurula and early tail bud stages (NF15–25), endodermal differentiation did not occur; at this stage, the mesoderm is completely separate from the endoderm (Horb and Slack, 2001). Further evidence for the importance of mesoderm was provided by endoderm-mesoderm recombinations (i.e., anterior endoderm with posterior mesoderm) wherein the differentiation state of the endoderm (anterior vs. posterior) depends on the character of the mesoderm (Horb and Slack, 2001; McLin et al., 2007). Stable regional specification of the endoderm (independent of mesoderm signals) is not established until late tail bud stages, between NF28 and 32 (Zeynali et al., 2000; Horb and Slack, 2001; McLin et al., 2007). However, many of the important inductive signals involved in specifying anterior and posterior foregut occur prior to stable regional specification, during gastrula and neurula stages.
In the next section, we briefly review the data implicating that some of these signaling pathways are involved in early specification of the endoderm leading to a pancreatic cell fate. For more detail about these early signaling pathways and the similarities between Xenopus and mammals, we refer the reader to a recent review on this topic (Zorn and Wells, 2007).
RA Signaling
One of the earliest molecules required for regional specification of the endoderm leading to a pancreatic fate is retinoic acid (RA). Animal caps treated with high doses of Activin A contain both mesoderm and endoderm, but even at the highest doses of Activin A, pancreatic tissue is only rarely induced. (Animal cap tissue is naive ectoderm removed from blastula stage embryos that can be induced to form every tissue in the tadpole, and is similar to embryonic stem cells.) Addition of RA to the Activin-treated animal caps increased the amount of pancreatic tissue substantially (Moriya et al., 2000; Asashima et al., 2008). Interestingly, the timing of RA addition was found to be critical to the induction of pancreas; only when RA was added several hours after Activin A treatment was a large amount of pancreatic tissue induced in the animal caps. However, caution should be taken when interpreting data from in vitro induction of genes in animal caps or ES cells, as these in vitro events may not necessarily be representative of the in vivo reality. It is, therefore, important to note the effects of RA on pancreas development in the whole animal.
Indeed, whole animal experiments confirmed the animal cap results showing that RA is required for regional specification in vivo, although it has different effects on dorsal versus ventral pancreas. Inhibition of RA at gastrula stages causes a loss of both endocrine and exocrine marker expression later in development, whereas excess RA stimulates endocrine cell differentiation in the dorsal pancreas (at the expense of exocrine cells), and exocrine cell differentiation in the ventral pancreas (Chen et al., 2004). In agreement with the animal cap data, the timing of RA signaling is critical; treatments at gastrula stages (NF11–13) affect pancreas development, but in embryos treated after NF13, the pancreas develops normally.
It is interesting to note that RA only affects dorsal-anterior and not ventral-posterior endoderm (Pan et al., 2007). This differential sensitivity to RA signals between dorsal and ventral endoderm explants can be explained by the spatial restriction of certain RA receptors (RARs). RARγ2.1 has high expression in the dorsal mesoderm, but low expression in the ventral mesoderm, and overexpression of RARγ2.1 in ventral endoderm-mesoderm explants makes them responsive to exogenous RA (Pan et al., 2007). Another factor acting in opposition to RA is BMP; BMP signaling is high ventrally and low dorsally. When BMP signaling was inhibited in ventral endoderm-mesoderm explants, RA was then able to induce pancreatic tissue. Furthermore, the BMP inhibitor Noggin induces the transcription of RALDH2, a gene involved in the biosynthesis of RA (Pan et al., 2007). These results all show that RA is required for the specification of endoderm at early stages, but is not sufficient to promote pancreatic fates on its own.
Wnt Signaling
Following the requirement for RA signaling at late gastrula stages in specifying anterior endoderm, the next pathway implicated in regional specification is the Wnt signaling pathway. In contrast to the positive requirement for RA signaling in promoting development of the anterior endoderm, it is the inhibition of Wnt signaling at neurula stages that is essential for proper foregut development; in contrast, activation of Wnt signaling is necessary for posterior endoderm development. Ectopic activation of Wnt signaling in anterior endoderm (β-catenin) is sufficient to prevent foregut specification, while inhibition of Wnt signaling in posterior endoderm (Gsk3β) promotes ectopic foregut development (McLin et al., 2007). Furthermore, the temporal requirement for Wnt was found to differ for anterior versus posterior endoderm. Wnt signaling must be inhibited in anterior endoderm between NF11–20, whereas activation of Wnt signaling in posterior endoderm is required between NF11–13 (McLin et al., 2007). These studies, however, do not directly address whether Wnt signals act directly on the endoderm or whether they respecify mesoderm first.
To address the question of which germ layer Wnt is acting in, McLin et al. (2007) used the Einsteck method. In this procedure, a piece of labeled tissue is implanted into the blastocoelic cavity of a gastrula-stage embryo, and the final fate of the donor tissue is examined at a later stage. In this instance, the donor tissue was either anterior or posterior endoderm (expressing β-catenin or Gsk3β). In control conditions, anterior endoderm contributed to liver and stomach, while posterior endoderm contributed to intestine. However, when anterior endoderm expressing β-catenin was transplanted, the final fate of these cells was now intestine. In contrast, posterior endoderm expressing Gsk3β contributes to liver and stomach (McLin et al., 2007). In agreement with this, the Wnt antagonist secreted frizzled-related protein 5 (Sfrp5) is expressed in the anterior endoderm beginning at NF15, is required for proper foregut specification, and acts directly to prevent signaling by Wnt11 (Li et al., 2008).
TGF-B/BMP
Prior to gastrulation, TGF-β signaling plays an important role in endodermal germ layer induction (Henry et al., 1996; Zorn et al., 1999; Afouda et al., 2005), but its role in later events of endodermal specification has only recently been investigated. TGF-β induced factor 2 (TGIF2) was identified as a modifier of early endoderm, and required for pancreas development (Spagnoli and Brivanlou, 2008). TGIF2 was identified in a microarray screen for targets of GATA5, which acts downstream of TGF-β in promoting an endoderm fate (Weber et al., 2000; Spagnoli and Brivanlou, 2008). TGIF2, a BMP antagonist, was found to act as a modifier of the endoderm to promote more anterior fates; overexpression of TGIF2 in ventral vegetal explants is sufficient to activate pancreatic gene expression. This is consistent with the results of Pan et al. (2007); both studies demonstrated that inhibition of BMP signaling plays an important role in endodermal patterning, which leads to pancreas development. The exact stage and in what germ layer TGIF2 acts is currently unknown. In agreement with this, recent studies using activintreated animal caps have identified genes acting in the TGFβ pathway to effect endoderm regionalization (Weber et al., 2000; Afouda et al., 2005). In addition to the early role of TGFβ signaling in endoderm induction prior to gastrulation, these results implicate TGF-β signaling in later events of endodermal patterning and regionalization during neurula and tail bud stages.
Intracellular Factors
One of the benefits of Xenopus embryos is the ability to identify new genes (or known genes not previously implicated in pancreas development) without a priori knowledge of either them or their role in pancreas development through gain-of-function screens. For example, using an expression cloning screen and injecting pools of mRNAs into vegetal blastomeres and looking for ectopic insulin expression the 3′UTR of Shirin was found to be sufficient to induce ectopic insulin expression (Spagnoli and Brivanlou, 2006). Secondarily, they found that the RNA binding protein, Vg1RBP, bound the 3′UTR of shirin. (Vg1RBP is best known for being expressed early in embryogenesis and regulating Vg1 mRNA localization.) In the context of pancreas development, Vg1RBP was found to be essential for proper development of insulin-expressing cells, but its necessity for acinar cell development was not examined. Although on its own, Vg1RBP is unable to promote endoderm development, it respecifies more posterior-ventral endoderm to become more dorsal-anterior.
Regional Specification of the Endoderm: Summary
At the end of gastrulation, once the endoderm has been established, regionalization subdivides the endoderm into discrete anterior and posterior domains. To produce stable regional identity, continuous endoderm-mesoderm interactions are required until late tail bud stages. Distinct signaling pathways operate in subsequent stages to pattern the endoderm. Initially, RA signaling immediately at the end of gastrulation between NF11 and NF13 is critical for proper pancreas development. Subsequently, Wnt (and BMP) signaling must be inhibited between NF11 and NF20 for proper development of anterior endoderm; active Wnt signaling promotes posterior endoderm development. Thus, although the signals required for patterning the endoderm occur relatively early, pancreatic cell fates are not established until after NF30 when pancreas-specific genes are activated.
PANCREATIC SPECIFICATION (AFTER NF30)
As outlined above, the majority of research into Xenopus pancreas development has been focused on the early patterning and specification events that occur prior to NF30. The fact that Xenopus can be used to investigate the function of “late-acting” pancreas-specific genes has only recently become appreciated. From these studies, it is becoming clear that Xenopus is a valuable model system for elucidating the molecular mechanisms underlying pancreatic cell fate specification; including identifying new genes, determining their function, defining transcriptional regulatory networks, and more specifically identifying candidate genes for diabetes.
Ptf1a and Pdx1
Two important genes in pancreas development that have been studied in Xenopus include Pdx1 and Ptf1a. Both genes are expressed in pancreatic progenitors, and are necessary and sufficient for pancreas development (Horb et al., 2003; Afelik et al., 2006; Jarikji et al., 2007). In fact, ectopic expression of both simultaneously converts posterior endoderm into pancreatic tissue (Afelik et al., 2006). The bHLH pancreas-specific transcription factor 1a (PTF1a) is one of the earliest pancreas-specific markers. Loss of Ptf1a function in mice and humans results in the complete absence of acinar cells and a large reduction in insulin expression (Krapp et al., 1996; Kawaguchi et al., 2002; Sellick et al., 2004), whereas in zebrafish it is required mainly for exocrine pancreas development (Zecchin et al., 2004; Lin et al., 2004). In Xenopus, ptf1a is detected as early as NF27/30 in the developing dorsal and ventral pancreatic anlagen (Fig. 2A) (Afelik et al., 2006). It is expressed in the tadpole acinar cells until the climax of metamorphosis when the PTF1a gene is shut off. Its expression returns at the end of metamorphic climax and remains acinar-specific (Mukhi et al., 2008). This expression is identical to that seen in mice, where Ptf1a is expressed in all pancreatic progenitors at the earliest stages of pancreas development, but becomes localized to acinar cells shortly thereafter (Krapp et al., 1996; Kawaguchi et al., 2002).
Two studies examined the function of Xenopus Ptf1a in early pancreas development and found that Ptf1a was both necessary and sufficient for pancreas development. Both studies found that knockdown of Ptf1a resulted in a complete loss of acinar cells; however, phenotypic differences regarding endocrine cell development were found (Afelik et al., 2006; Jarikji et al., 2007). In the first study, a single morpholino was used to knockdown Ptf1a, and early expression of insulin at NF35 was unaffected, while the late expression of both insulin and glucagon was lost at NF48 (Afelik et al., 2006). The second study, using two morpholinos to target distinct regions of the Ptf1a transcript, found the exact opposite: knockdown of Ptf1a resulted in an absence of early insulin expression, while low levels of insulin were detected at later stages (Jarikji et al., 2007). The reason for this discrepancy is currently unclear. Nevertheless, these studies show that the function of Ptf1a in Xenopus pancreas development is quite similar to that observed in other vertebrates where Ptf1a is required for development of all acinar cells and a subset of endocrine cells.
The homeobox gene Pdx1 was originally cloned in Xenopus 20 years ago and called XlHbox8 (Wright et al., 1989). It is expressed in a broad domain in the anterior endoderm beginning at NF27, encompassing the developing dorsal and ventral pancreatic buds as well as parts of the stomach and duodenum (Wright et al., 1989; Afelik et al., 2006). Low levels of Pdx1 are detected by RNase protection analysis as early as NF12.5, and by RT-PCR at NF19/21 (Gamer and Wright, 1995; Horb and Slack, 2001). Morpholino knockdown of Pdx1 in Xenopus resulted in the complete loss of exocrine markers, but had little effect on insulin expression (Afelik et al., 2006). These results are in contrast to the defects observed in mice and humans, where lack of Pdx1 resulted in pancreas agenesis (Ohlsson et al., 1993; Jonsson et al., 1994; Stoffers et al., 1997; Schwitzgebel et al., 2003). The defect seen in Xenopus is similar to that observed in mice where although loss of Pdx1 leads to pancreatic agenesis, there is a small dorsal bud present that produces insulin and glucagon (Ahlgren et al., 1996).
One of the benefits of Xenopus is the relative ease in which gain-of-function studies can be performed. In addition to these loss-of-function studies showing that Ptf1a and Pdx1 are necessary for pancreas development, ectopic overexpression experiments revealed that both were also sufficient to promote ectopic pancreatic cell fates. Overexpression of Pdx1 in naïve endoderm or transgenic overexpression of Pdx1 in the liver after NF44 did not result in the activation of pancreatic differentiation markers (Horb et al., 2003; Afelik et al., 2006). However, it should be noted that some studies in mammals have found that Pdx1 alone is sufficient to promote ectopic pancreatic cell fates (Ferber et al., 2000; Ber et al., 2003; Sapir et al., 2005; Shternhall-Ron et al., 2007). In contrast, overexpression of Pdx1-VP16, an activated form of Pdx1, was sufficient to promote ectopic endocrine and acinar cell fates in the developing liver; no effect was seen in the intestine (Horb et al., 2003). The relevance to mammals was shown by the fact that this same transgene was able to convert mammalian HepG2 cells to pancreas (Horb et al., 2003; Li et al., 2005). Subsequent studies have confirmed these results. In particular, Pdx1-VP16 was found sufficient to convert rat liver cells into immature beta cells that were able to restore euglycemia in diabetic mice (Cao et al., 2004; Tang et al., 2006). The reasons for this difference between Pdx1 and Pdx1-VP16 are unclear, although it is reasonable to assume that the addition of the VP16 activation domain allows for interactions with other proteins creating a favorable environment in which Pdx1 can activate transcription of its downstream targets.
Overexpression of Ptf1a, on the other hand, was found sufficient to promote ectopic pancreatic cell fates. Injection of Ptf1a mRNA into the two dorso-vegetal blastomeres at the eight-cell stage (targeting the anterior endoderm) resulted in the conversion of stomach/duodenum to pancreas (Afelik et al., 2006; Jarikji et al., 2007). Using a hormone-inducible version (Ptf1a-GR), it was found that when Ptf1a was activated at NF27 or earlier, it was able to promote ectopic pancreatic cell fates, whereas when activated after NF36, it had no effect (Afelik et al., 2006). Activation of Ptf1a between NF30 and 35 did not convert stomach/duodenum to pancreas, but did result in an enlarged pancreas. In contrast, Ptf1a-VP16 was found to have greater activity than the unmodified Ptf1a in naive endoderm. In addition to being able to convert prospective stomach/duodenum to pancreas as seen with unmodified Ptf1a, Ptf1a-VP16 was also able to convert prospective liver to pancreas. However, only the acinar cell fate was promoted (Jarikji et al., 2007). No effect was seen with either Ptf1a or Ptf1a-VP16 when overexpressed in posterior endoderm. Similar results were also found when Ptf1a or Ptf1a-VP16 was ectopically expressed at later stages after the organs had formed. Transgenic overexpression of Ptf1a-VP16 in Xenopus tadpoles was sufficient to convert liver to acinar cells (but had no effect in the stomach or duodenum), while Ptf1a converted stomach/duodenum to endocrine and acinar cell fates (but had no effect in liver) (Jarikji et al., 2007). These results confirm earlier data in mice where it was shown that loss of Hes1 induces ectopic expression of Ptf1a in the stomach, duodenum, and common bile duct resulting in the production of ectopic patches of pancreatic tissue (Fukuda et al., 2006).
Other Factors
There are numerous other transcription factors that have been well studied in pancreas development in other species, but little is known about their role in Xenopus pancreas development. Many of these genes, however, have been cloned in Xenopus and below we briefly describe what is known about their expression patterns in the developing Xenopus pancreas. We also provide a short description of their function in pancreas development based on results from other animals.
HNF6
In mice, Hnf6 is one of the earliest markers of the developing anterior endoderm, and is expressed in a broad domain of the anterior endoderm marking the developing pancreas and liver (Jacquemin et al., 2003). In Hnf6 mutant mice, Pdx1 expression is delayed and the pancreas was hypoplastic (Jacquemin et al., 2000, 2003). We recently cloned Xenopus Hnf6 and found it to be expressed in a similar pattern as in mice (data not shown). At NF30, Hnf6 is expressed in a broaddomain in the anterior endoderm marking the developing pancreas and liver, while at later stages it is found exclusively in the liver.
Sox9
Sox9 is expressed in several different tissues and is implicated in the regulation of various stem/progenitor cells (Lynn et al., 2007; Seymour et al., 2007). In the pancreas, it is expressed in undifferentiated progenitor cells, and is involved in regulating their proliferation; loss of Sox9 in the pancreas results in hypoplastic pancreas (Seymour et al., 2007). In Xenopus, expression is first detected at NF25 in two regions of the undifferentiated endoderm: dorsally in the prospective foregut and ventrally on each side of the future liver diverticulum (Lee and Saint-Jeannet, 2003). By NF35, its expression is localized to the dorsal and ventral pancreatic buds, similar to Ptf1a; expression is also seen in the developing lung buds.
Hes1
Hes-1 is a member of the hairy and enhancer-of-split-like transcriptional repressors that acts in the Notch signaling pathway. In the pancreas, Hes1 is involved in antagonizing the function of B-class bHLH proteins such as Ngn3, and is expressed in undifferentiated precursor cells (Jorgensen et al., 2007). In Xenopus, Hes1 is known as hairy2b and it has been extensively studied in the context of neural development (Yamaguti et al., 2005; Murato et al., 2006). Within the pancreas, it is expressed in a punctate fashion from the earliest stages, most likely marking pancreatic progenitor cells (data not shown).
Ngn3
Neurogenin3 (ngn3) is a bHLH transcription factor that is the earliest marker of all pancreatic endocrine cells, marking a population of endocrine progenitor cells (Gradwohl et al., 2000; Schwitzgebel et al., 2000; Gu et al., 2002). Mice lacking ngn3 function fail to generate any pancreatic endocrine cells and die postnatally from diabetes (Gradwohl et al., 2000). In Xenopus, ngn3 displays a punctate pattern of expression throughout the gastrointestinal tract as seen in mice (Nieber et al., 2009). Within the pancreas, ngn3 is initially detected only in the dorsal pancreas in a speckled fashion, and at later stages expression extends into the ventral pancreas as well (data not shown). This pattern of expression is identical to Insm1.
Insm1
Insulinoma-associated protein 1, also known as IA-1, is a zinc finger transcription factor expressed in all endocrine cells, and is necessary for their differentiation (Gierl et al., 2006; Mellitzer et al., 2006). We recently cloned Xenopus Insm1 and found it to also be expressed in both pancreatic and enteroendocrine cells (Fig. 5A,B). Similar to the mouse knockouts, we found that Xenopus Insm1 was downstream of Ngn3 and was required for the differentiation of all endocrine cells (Horb et al., 2009).
Fig. 5.
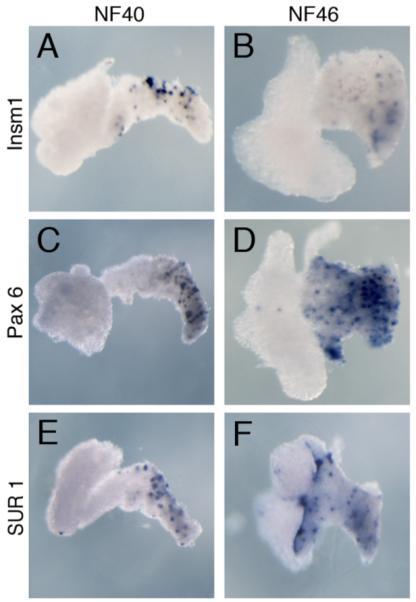
Expression of endocrine pancreatic factors in isolated liver and pancreas tissue. A,B: Pax6 expression. C,D: Insm1 expression. E,F: Sur1 expression.
NeuroD
NeuroD is a bHLH transcription factor that is expressed in all endocrine cells. NeuroD mutant mice have reduced numbers of all endocrine cells and develop diabetes, and mutations in NeuroD in humans are associated with maturity onset diabetes of the young (MODY6) (Liu et al., 2006). NeuroD is also possibly a susceptibility marker for type1 in a Japanese study (Iwata et al., 1999) and in a Danish study (Hansen et al., 2000). In Xenopus, NeuroD has been studied mainly in the context of eye and neural development (Lee et al., 1995; Chae et al., 2004). In the developing pancreas, Xenopus NeuroD is restricted to the dorsal rudiment at stage 40; expression then expands to the intestine as well by NF45 (Kelly and Melton, 2000).
Pax4 and Pax6
Pax4 and 6 genes contain both a paired box and a homeobox, and both are expressed in a subset of pancreatic endocrine cells. Pax4 is essential for β and δ cell development, whereas Pax6 is involved in α cell development (Sosa-Pineda et al., 1997). As with NeuroD, much of what we know about Pax6 in Xenopus is derived from studies on its function in eye and neural development (Hirsch and Harris, 1997; Chow et al., 1999; Zaghloul and Moody, 2007). In the developing pancreas, it is expressed in a punctate fashion in the dorsal pancreas at NF40 (Fig. 5C,D) (Kelly and Melton, 2000). It is also expressed in the developing intestinal tract marking endocrine cells. Pax4, on the other hand, has yet to be cloned in Xenopus.
Arx
Arx encodes a homeodomain protein that is expressed in endocrine pancreas progenitors, but is required only for development of the α cell lineage; Arx-deficient mice do not develop α cells, but have a concomitant increase in β and δ cells (Collombat et al., 2003). In contrast, overexpression of Arx in the pancreas converts β cells to α and PP cells (Collombat et al., 2007). In Xenopus, Arx has been cloned and studied in the context of forebrain development (El-Hodiri et al., 2003; Seufert et al., 2005). Its expression and function in the pancreas has not been examined.
Nkx2.2
In mice, Nkx2.2 is an early marker of all endocrine cells, but loss of Nkx2.2 only affects maturation of endocrine cells at the secondary transition (Sussel et al., 1998). In Xenopus, Nkx2.2 is first detected in the pancreas beginning at NF40, where it is expressed in both the dorsal and ventral pancreas (data not shown).
Nkx6.1
In rodents, Nkx6.1 is expressed early in pancreatic progenitors and at later stages is restricted to β cells (Jensen et al., 1996; Oster et al., 1998; Jorgensen et al., 2007; Hald et al., 2008). Although not essential for the initial specification of β cells, it is required for the increase in β-cell number that occurs at the secondary transition (Sander et al., 2000). Xenopus Nkx6.1 was only recently cloned; it is first detected in the anterior endoderm at NF36 in the developing pancreas and stomach (Zhao et al., 2007). At NF40, Nkx6.1 is found expressed in a punctate fashion only in the dorsal part of the pancreas (data not shown).
Islet1
Islet1 is a homeobox transcription factor that is expressed in the dorsal pancreatic mesenchyme at early stages and later in the pancreatic epithelium. Mice lacking islet1 do not develop a dorsal pancreas (Ahlgren et al., 1997). In Xenopus, expression of islet1 is detected in the lateral plate mesoderm extending from the dorsal pancreas down to the duodenum, but is absent from the ventral pancreatic region (Kelly and Melton, 2000).
MafA and MafB
The Maf family of proteins encode leucine zipper transcription factors. MafA is a β-cell-specific factor involved in regulating insulin gene transcription in the mature cell (Zhang et al., 2005). MafB, on the other hand, is expressed in both α and β cells early in development, becoming localized to only α cells later in development (Nishimura et al., 2006). In Xenopus, only MafB has been cloned, but its expression in the developing pancreas has not been examined.
SUR1
SUR1 (sulfonylurea receptor 1), encoded by the gene ABCC8, is the regulatory subunit of the KATP channel in β-cell membranes (Bryan et al., 2007). KATP channels are octamers consisting of four regulatory subunits (in this case four SUR1 subunits) and four pore-forming subunits (see Kir6.2 below). SUR1 is a target of the sulfonylurea diabetes treatments, which induce insulin release (Rafiq et al., 2008). In Xenopus, SUR1 is expressed in a punctate pattern in the dorsal pancreas; this pattern is consistent with expression in β cells (Fig. 5E,F).
Kir6.2
Kir6.2 is the pore-forming subunit of the KATP channel in the β-cell membrane, encoded by the gene KCNJ11. Kir6.2 is also a target for sulfonylurea diabetes treatments, and such treatments have successfully replaced insulin treatment in diabetics with mutations in KCNJ11 (Pearson et al., 2006). Xenopus KCNJ11 has been cloned but the expression pattern has not been examined.
Mist1
Mist1 is a bHLH transcription factor expressed in the exocrine cells late in development (Lemercier et al., 1997). In mice and zebrafish, Mist1 has been shown to be necessary for maturation and maintenance of exocrine cells (Johnson et al., 2004; Guo et al., 2007). In Xenopus, Mist1 is expressed throughout the pancreas by NF40 and expression is maintained as the pancreas develops (Fig. 4D–F).
Early Development Summary
In this first part of the review, we have attempted to provide a general overview of early pancreas development in Xenopus. These results demonstrate that patterning and specification of the endoderm into distinct anterior and posterior regions begins as early as NF11. However, this early patterning is labile, and stable regional specification is not achieved until late tail bud stages. We have also given a summary of various pancreatic transcription factors, with a particular focus on Ptf1a and Pdx1. These results demonstrate one of the strengths of the Xenopus model: the ability to determine the sufficiency of specific factors in promoting ectopic pancreatic cell fates, either in naive endoderm, through overexpression of mRNA in vegetal blastomeres at early stages, or in specific organs later in development, using transgenics.
PANCREAS DEVELOPMENT DURING METAMORPHOSIS (AFTER NF55)
Differences Between the Tadpole and Frog Exocrine Pancreas
The anuran pancreas undergoes a major change during metamorphosis (Fig. 6) (Janes, 1937; Bollin et al., 1973; Dodd and Dodd, 1976; Leone et al., 1976; Milano and Chimenti, 1995; Mukhi et al., 2008). The tadpole-to-adult remodeling process is in response to the surge of thyroid hormone (TH) during spontaneous metamorphosis (Leloup and Buscaglia, 1977). The tadpole pancreas is a large spongy, boggy tissue that grows proportionately with the tadpole up to the climax of metamorphosis (NF58). The exocrine tadpole pancreas is rich in acinar cells with typical zymogen granules (Fig. 1D) and expresses very high levels of digestive enzymes. One of the obvious histological differences between the tadpole and adult exocrine pancreas is the absence of a complex ductal arrangement in the tadpole (Mukhi et al., 2008). The ductal system in the frog pancreas comprises all of the types of ductal cells as described by Egerbacher and Bock (1997) in mammals including the intercalcated ducts, intra and extralobular ducts, and collecting ducts (Fig. 7). The rare tadpole ductal structures are single-cell epithelial tubes without fibrous sheaths surrounding them, morophologically resembling the lobular duct of an adult pancreas. It has been observed that the giant-tadpoles (lacking thyroid function) and tadpoles grown in methimazole (an inhibitor of thyroid hormone synthesis) for long periods of time also lack a complex ductal system (Mukhi et al., 2008).
Fig. 6.
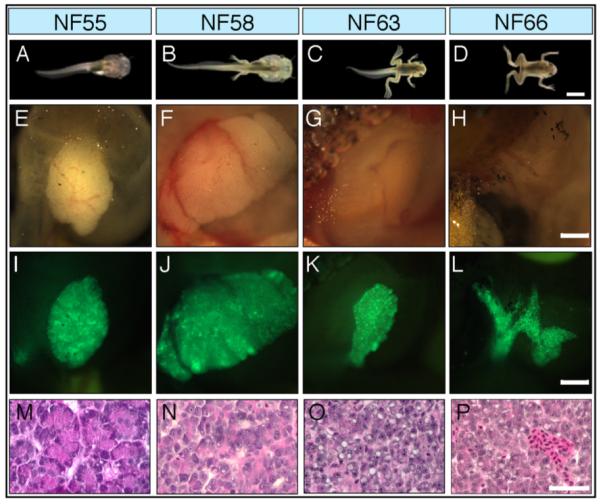
The development of the X. laevis pancreas through metamorphosis. A–D: Pictures of four development stages. E–H: The pancreases at each stage are below the pictures. I–L: Transgenic animals expressing GFP controlled by the rat elastase promoter. M–P: Histology of the pancreas at each stage stained with H and E. Scale bars = (A–D) 4 mm; (E–L) 1 mm; (M–P) 40 μm. Redrawn from Mukhi et al. (2008).
Fig. 7.

Diagram of a typical mature acinar assembly with its collecting ducts (after Egerbacher and Bock, 1997). Histological sections of (left) premetamorphic pancreas and (right) frog pancreas. The arrows point to ducts. Scale bars = 40 μm. Redrawn from Mukhi et al. (2008).
The frog exocrine pancreas synthesizes many of the same terminally differentiated enzymes as the tadpole pancreas; however, microarray analyses comparing the frog and tadpole pancreas reveal many extreme differences in gene expression. Out of a total of 33,098 microarray 60-mers (Agilent), more than 4,000 entries were up regulated significantly in the tadpole pancreas compared to the frog pancreas and vice versa. The most extreme expression differences revealed by the microarrays are the high activity of carboxyl ester lipases in the tadpole pancreas and trypsin-like serine protease (elastase) in the frog pancreas (Mukhi and Brown, unpublished data). Such huge differences in gene expression profiles and tissue structure (morphology and histology) between tadpole and frog pancreas suggest that the exocrine pancreas in the tadpole is not mature and requires the TH-dependent remodeling process. As we will see, the same can be said for the insulin secreting β cells.
Remodeling of the Exocrine Pancreas During Metamorphosis
Several authors (Janes, 1937; Race et al., 1966; Milano and Chimenti, 1995) have studied remodeling of the pancreas during metamorphosis in different species of frogs. The metamorphic changes have been described as “partial degeneration” followed by “regeneration” (Janes, 1937) and cellular “necrosis” and “dehydration” (Race et al., 1966; Leone et al., 1976). We have observed that at metamorphosis, remodeling begins with the dedifferentiation of the entire exocrine pancreas followed by a redifferentiation phase in the growing frog (Mukhi et al., 2008). One of the first changes in the tadpole exocrine pancreas at metamorphosis in response to the increase in thyroid hormone (TH) is the steady decline of mRNAs that encode the terminally differentiated enzymes (Shi and Brown, 1990; Mukhi et al., 2008). The levels of these mRNAs begin dropping at prometamorphosis (NF56), until they are almost totally absent by the middle of the metamorphic climax (NF62) (Figs. 8 and 9). These transformed exocrine cells have sparse cytoplasm and no zymogen granules. Two months after the end of metamorphosis, the frog’s exocrine pancreas once again contains acinar cells but now also a full set of ducts typical of an adult vertebrate pancreas (Fig. 7).
Fig. 8.

Developmental profile of trypsin mRNA (x) and endogenous T3 (triangles). The period called climax is bracketed. Redrawn from Shi and Brown (1990).
Fig. 9.
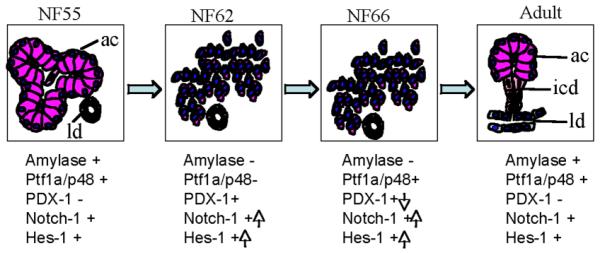
Diagram of exocrine pancreas remodeling during metamorphosis. The expression changes of key genes are indicated below each diagram. Reproduced from Mukhi et al. (2008) with permission of the publisher.
Thyroid Hormone (TH) Induces the Dedifferentiation of the Tadpole Pancreas
The sequence of dedifferentiation events in the Xenopus pancreas during climax of metamorphosis is similar to the dedifferentiation process observed in mammalian models. Pancreas acinar cells are considered to be highly plastic in mammals, because they can be induced to dedifferentiate. Exocrine pancreas dedifferentiation and plasticity have been observed in chronic pancreatitis (Tezel et al., 2004), upon exposure to the pharmacological agent, cerulein (Jensen et al., 2005; Strobel et al., 2007), and when the cells are placed in culture (Means et al., 2005). The uniqueness in Xenopus is that the changes occur as a part of the normal developmental process as a response to a hormone (Race et al., 1966). The changes to the acinar cells during the climax of metamorphosis result in the cells dedifferentiating and entering a progenitor state (Mukhi et al., 2008). This is evident by the transient upregulation of pancreatic progenitor markers (Pdx1, Notch-1, and Hes1) and the down-regulation of the acinarspecific transcription factor Ptf1a as well as exocrine-specific pancreatic enzyme mRNAs such as amylase and trypsin. The more stable proteins persist through climax. This proves that the surviving cells at climax are dedifferentiated acinar cells. In the redifferentiaion phase, the expression of Pdx1 and Notch-1 is down-regulated and the mRNAs encoding the pancreatic enzyme reappear. Activation of the Notch signaling pathway has been reported in mammalian pancreas that has been induced to dedifferentiate (Jensen et al., 2005; Rooman et al., 2006). The molecular events involved in this chemically induced dedifferentiation and subsequent regeneration of the mammalian pancreas was described as a “recapitulation of embryonic development” (Jensen et al., 2005). Amphibian metamorphosis provides a new model for pancreatic dedifferentiation and redifferentiation with the major advantage that it can be controlled by TH (Janes, 1937; Race et al., 1966; Milano and Chimenti, 1995; Mukhi et al., 2008). While the dedifferentiation of acinar cells is triggered by TH, the hormone does not seem to be involved in the redifferentiation of the exocrine pancreas that occurs after metamorphosis.
Transgenic tadpoles expressing a dominant-negative form of the thyroid hormone receptor alpha (TRDN) controlled by the rat elastase promoter do not dedifferentiate their exocrine pancreas at climax (Mukhi et al., 2008). The transient up-regulation of Pdx1 and Notch-1 and the down-regulation of amylase at climax were inhibited. This genetic interference in the spontaneous metamorphosis process inhibited the maturation of the tadpole to the frog pancreas. Even after 2 months of growth, the transgenic pancreas of these frogs has only a rudimentary ductal system. These abnormal pancreases have unusual inclusions that look nothing like pancreas cells. Despite the abnormal pancreas, the transgenic frogs are healthy and grow to sexual maturity. This is not surprising since pancreatectomy in frogs has been reported not to be lethal (Frye, 1964). A summary of the changes that occur in the exocrine pancreas at metamorphosis is presented in Figure 9.
Remodeling of the Endocrine Pancreas at Metamorphosis
In the adult Xenopus pancreas, the islets consist of only insulin-producing β cells; the other endocrine cells are scattered around in the pancreas as single cells sometimes loosely associated with the islets (Maake et al., 1998; Horb and Slack, 2002). The shrinkage of the pancreas that occurs at climax brings the various endocrine cells closer together (Maake et al., 1998). In addition, a “maturation” of the pancreatic system has been proposed to occur at climax (Maake et al., 1998; Accordi and Chimenti, 2001), which involves limited cell death and changes in insulin expression. In the tadpole, β cells are present in small clusters (Kelly and Melton, 2000), but during the eight days of metamorphic climax, while the exocrine pancreas dedifferentiates, these clusters are induced by TH to become larger (Fig. 10). The reason for this increase in islet size during climax is due to the aggregation of pre-existing β cells (Mukhi et al., 2009). During climax, β cells do not replicate, and the absolute number of β cells does not change. In addition, acinar to β cell transdifferentiation was not observed. In the months following metamorphosis, the islets grow larger at least in part by replication of existing β cells.
Fig. 10.
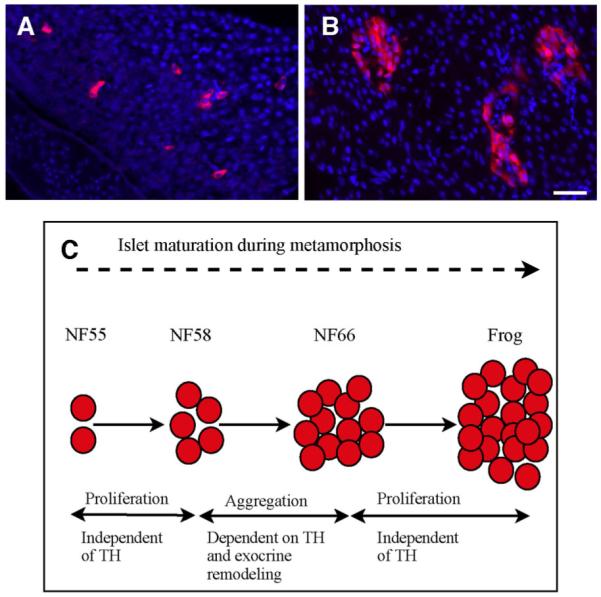
Islet size in the pancreases of (A) premetamorphic tadpoles and (B) NF66 froglets at the end of climax. Scale bars = 40 μm. C: A summary of remodeling b-cells.
Unlike the gene expression changes seen in the exocrine pancreas, insulin protein is detected in the β cells throughout metamorphic climax. However, there is a brief loss of insulin mRNA during early climax (NF62). Insulin mRNA is synthesized again several days later at the end of climax (NF66). No known β cell progenitor genes have been found to be activated when the β cells stop synthesizing insulin mRNA, leaving no explanation for this brief down-regulation of insulin mRNA (Mukhi et al., 2009).
This aggregation of β cells at climax is not cell-autonomous but requires interactions with the surrounding dedifferentiated acinar cells. In transgenic elastase-TRDN animals, where acinar cells do not dedifferentiate, there is no aggregation of β cells. However, once metamorphosis is complete, the small (nonaggregated) islets in these transgenic frogs begin to replicate so that in two months they are equal in size to the control islets (Mukhi et al., 2009). This finding adds evidence that the role of TH in pancreas remodeling ends when metamorphosis is complete and does not account for the redifferentiation process. In contrast, the down-regulation of insulin gene expression in beta cells is a cell-autonomous response to TH. In transgenic pIns-TRDN animals, insulin expression is not down-regulated, while the aggregation of β cells occurs normally (Mukhi et al., 2009). We conclude that changes in β cells during climax result from two separate TH-controlled programs. The aggregation of β cells during climax requires the concomitant dedifferentiation of the exocrine cells, whereas the transient down-regulation of insulin gene expression is a direct response of TH.
Metamorphosis Summary
The remarkable TH-induced changes in the tadpole pancreas are a normal part of the animal’s life cycle. Although definitive lineage studies have not been carried out, it is likely that the frog’s mature ductal system as well as the new acinar cells derived from dedifferentiated acinar cells at climax. The first islets formed at climax are derived by aggregation of preexisting β cells. By analogy, mammalian islets are generated in a multistep process involving differentiation, migration, and aggregation of progenitor cells into islets (Deltour et al., 1991; Kim and Hebrok, 2001). The mechanisms that control these processes are unknown.
The pancreas is just one of many amphibian organs and cell types that remodel during metamorphosis. The tadpole intestine undergoes extensive shortening, and each of its cell types change during metamorphic climax (McAvoy and Dixon, 1978a,b). The brain remodels by essentially rewiring its connections (Alley and Barnes, 1983). Tadpole red cells undergo globin switching (Weber, 1996); the B and T-cells are renewed (Du Pasquier et al., 2000). The liver (Paik and Cohen, 1960) and the skeleton (Berry et al., 1998) also remodel. Each of these changes is controlled by thyroid hormone. Organs like the pancreas can have more than one TH-controlled program. Some of these are cell autonomous, others require the influence of an adjacent cell type just as the aggregation of β cells depends upon the dedifferentiation of the exocrine cells.
SUMMARY
We have covered many of the most important aspects of pancreas development, including regional specification, early morphological events, induction of pancreas, and the changes that occur during metamorphosis. What is obvious from this review is that although early pancreas development and pancreatic remodeling during metamorphosis are quite distinct events, they have much in common, and the same signaling molecules that promote early pancreas development are re-expressed during metamorphosis as the cells dedifferentiate.
In this review, we have attempted to provide a broad overview of pancreas development that would be beneficial not only to the Xenopus community, but also researchers studying pancreas development in other animals. Although some morphological events are unique to Xenopus, it is becoming increasingly obvious that the early stages of Xenopus pancreas development are quite similar to mammalian pancreas development. The same genes that control major developmental decisions in the developing pancreas of a frog also control them in a mouse or indeed a human. And although metamorphosis is not found in mammals, the changes that occur in the Xenopus pancreas at metamorphosis can be studied to understand how remodeling of a “mature” pancreas occurs.
ACKNOWLEDGMENTS
We thank everyone in the Horb lab for help during the writing of this review, and especially Lori Dawn Horb for taking pictures of the in situs and her assistance with the figures. The Horb lab is supported in part by grants from the National Institutes of Health (DK077197) and the Juvenile Diabetes Research Foundation. Marko E. Horb is a junior 2 research scholar of the Fonds de la recherche en santédu Québec (FRSQ). The Brown lab is supported by grants from the National Institutes of Health (2 R01 GM022395-33) and the G. Harold and Leila Y. Mathers Charitable Trust.
Grant sponsor: National Institute of Diabetes and Digestive and Kidney Diseases, NIH; Grant numbers: 5R01DK77197-02 and 2 R01 GM022395-33; Grant sponsor: Juvenile Diabetes Research Foundation; Grant number: 6-2007-910; Grant sponsor: G. Harold and Leila Y. Mathers Charitable Trust.
REFERENCES
- Accordi F, Chimenti C. Programmed cell death in the pancreas of Bufo bufo during metamorphosis. J Anat. 2001;199:419–427. doi: 10.1046/j.1469-7580.2001.19940419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afelik S, Chen Y, Pieler T. Pancreatic protein disulfide isomerase (XPDIp) is an early marker for the exocrine lineage of the developing pancreas in Xenopus laevis embryos. Gene Expr Patterns. 2004;4:71–76. doi: 10.1016/s1567-133x(03)00150-9. [DOI] [PubMed] [Google Scholar]
- Afelik S, Chen Y, Pieler T. Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 2006;20:1441–1446. doi: 10.1101/gad.378706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afouda BA, Ciau-Uitz A, Patient R. GATA4, 5 and 6 mediate TGFbeta maintenance of endodermal gene expression in Xenopus embryos. Development. 2005;132:763–774. doi: 10.1242/dev.01647. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Alley KE, Barnes MD. Birth dates of trigeminal motoneurons and metamorphic reorganization of the jaw myoneural system in frogs. J Comp Neurol. 1983;218:395–405. doi: 10.1002/cne.902180404. [DOI] [PubMed] [Google Scholar]
- Asashima M, Michiue T, Kurisaki A. Elucidation of the role of activin in organogenesis using a multiple organ induction system with amphibian and mouse undifferentiated cells in vitro. Dev Growth Differ. 2008;50(Suppl 1):S35–45. doi: 10.1111/j.1440-169X.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278:31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- Berry DL, Rose CS, Remo BF, Brown DD. The expression pattern of thyroid hormone response genes in remodeling tadpole tissues defines distinct growth and resorption gene expression programs. Dev Biol. 1998;203:24–35. doi: 10.1006/dbio.1998.8975. [DOI] [PubMed] [Google Scholar]
- Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Andelfinger G, Horb ME. Germ layers to organs: using Xenopus to study “later” development. Semin Cell Dev Biol. 2006;17:133–145. doi: 10.1016/j.semcdb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Bollin E, Jr., Carlson CA, Kim KH. Anuran pancreas development during thyroxine-induced metamorphosis of Rana catesbeiana: RNA metabolism during the regressive phase of pancreas development. Dev Biol. 1973;31:185–191. doi: 10.1016/0012-1606(73)90329-1. [DOI] [PubMed] [Google Scholar]
- Bryan J, Munoz A, Zhang X, Dufer M, Drews G, Krippeit-Drews P, Aguilar-Bryan L. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 2007;453:703–718. doi: 10.1007/s00424-006-0116-z. [DOI] [PubMed] [Google Scholar]
- Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132:745–762. doi: 10.1053/j.gastro.2006.12.054. [DOI] [PubMed] [Google Scholar]
- Cao LZ, Tang DQ, Horb ME, Li SW, Yang LJ. High glucose is necessary for complete maturation of Pdx1-VP16-expressing hepatic cells into functional insulin-producing cells. Diabetes. 2004;53:3168–3178. doi: 10.2337/diabetes.53.12.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae JH, Stein GH, Lee JE. NeuroD: the predicted and the surprising. Mol Cells. 2004;18:271–288. [PubMed] [Google Scholar]
- Chalmers AD, Slack JM. Development of the gut in Xenopus laevis. Dev Dyn. 1998;212:509–521. doi: 10.1002/(SICI)1097-0177(199808)212:4<509::AID-AJA4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature β cells acquire α and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117:961–970. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltour L, Leduque P, Paldi A, Ripoche MA, Dubois P, Jami J. Polyclonal origin of pancreatic islets in aggregation mouse chimaeras. Development. 1991;112:1115–1121. doi: 10.1242/dev.112.4.1115. [DOI] [PubMed] [Google Scholar]
- Dodd MHI, Dodd JM. The biology of metamorphosis. In: Lofts B, editor. Physiology of the amphibia. Academic Press; New York: 1976. pp. 467–599. [Google Scholar]
- Du Pasquier L, Robert J, Couret M, Mussmann R. B-cell development in the amphibian Xenopus. Immunol Rev. 2000;175:201–213. doi: 10.1111/j.1600-065x.2000.imr017501.x. [DOI] [PubMed] [Google Scholar]
- Egerbacher M, Bock P. Morphology of the pancreatic duct system in mammals. Microsc Res Tech. 1997;37:407–417. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<407::AID-JEMT5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- El-Hodiri HM, Qi XL, Seufert DW. The Xenopus arx gene is expressed in the developing rostral forebrain. Dev Genes Evol. 2003;212:608–612. doi: 10.1007/s00427-002-0282-8. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- Frye BE. Metamorphic changes in the blood sugar and the pancreatic islets of the frog, Rana clamitans. J Exp Zool. 1964;155:215–223. doi: 10.1002/jez.1401550208. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R, Kageyama R, Wright CV, Chiba T. Ectopic pancreas formation in Hes1-knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116:1484–1493. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer LW, Wright CV. Autonomous endodermal determination in Xenopus: regulation of expression of the pancreatic gene XlHbox 8. Dev Biol. 1995;171:240–251. doi: 10.1006/dbio.1995.1275. [DOI] [PubMed] [Google Scholar]
- Gierl MS, Karoulias N, Wende H, Strehle M, Birchmeier C. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic β cells and intestinal endocrine cells. Genes Dev. 2006;20:2465–2478. doi: 10.1101/gad.381806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes GK. Developmental biology of the pancreas: A comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guo X, Cheng L, Liu Y, Fan W, Lu D. Cloning, expression, and functional characterization of zebrafish Mist1. Biochem Biophys Res Commun. 2007;359:20–26. doi: 10.1016/j.bbrc.2007.05.055. [DOI] [PubMed] [Google Scholar]
- Hald J, Sprinkel AE, Ray M, Serup P, Wright C, Madsen OD. Generation and characterization of Ptf1a antiserum and localization of Ptf1a in relation to Nkx6.1 and Pdx1 during the earliest stages of mouse pancreas development. J Histochem Cytochem. 2008;56:587–595. doi: 10.1369/jhc.2008.950675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L, Jensen JN, Urioste S, Petersen HV, Pociot F, Eiberg H, Kristiansen OP, Hansen T, Serup P, Nerup J, Pedersen O, Danish Study Group of Diabetes in Childhood. the Danish IDDM Epidemiology and Genetics Group NeuroD/BETA2 gene variability and diabetes: no associations to late-onset type 2 diabetes but an A45 allele may represent a susceptibility marker for type 1 diabetes among Danes. Diabetes. 2000;49:876–878. doi: 10.2337/diabetes.49.5.876. [DOI] [PubMed] [Google Scholar]
- Henry GL, Brivanlou IH, Kessler DS, Hemmati-Brivanlou A, Melton DA. TGF-β signals and a pattern in Xenopus laevis endodermal development. Development. 1996;122:1007–1015. doi: 10.1242/dev.122.3.1007. [DOI] [PubMed] [Google Scholar]
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- Hirsch N, Harris WA. Xenopus Pax-6 and retinal development. J Neurobiol. 1997;32:45–61. [PubMed] [Google Scholar]
- Horb ME, Slack JM. Endoderm specification and differentiation in Xenopus embryos. Dev Biol. 2001;236:330–343. doi: 10.1006/dbio.2001.0347. [DOI] [PubMed] [Google Scholar]
- Horb ME, Slack JM. Expression of amylase and other pancreatic genes in Xenopus. Mech Dev. 2002;113:153–157. doi: 10.1016/s0925-4773(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- Iwata I, Nagafuchi S, Nakashima H, Kondo S, Koga T, Yokogawa Y, Akashi T, Shibuya T, Umeno Y, Okeda T, Shibata S, Kono S, Yasunami M, Ohkubo H, Niho Y. Association of polymorphism in the NeuroD/BETA2 gene with type 1 diabetes in the Japanese. Diabetes. 1999;48:416–419. doi: 10.2337/diabetes.48.2.416. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Durviaux SM, Jensen J, Godfraind C, Gradwohl G, Guillemot F, Madsen OD, Carmeliet P, Dewerchin M, Collen D, Rousseau GG, Lemaigre FP. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol. 2000;20:4445–4454. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin P, Lemaigre FP, Rousseau GG. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev Biol. 2003;258:105–116. doi: 10.1016/s0012-1606(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Janes RG. Studies on the amphibian digestive system II. Comparative histology of the pancreas, following early larval development, in certain species of anura. J Morphol. 1937;61:581–611. [Google Scholar]
- Jarikji ZH, Vanamala S, Beck CW, Wright CV, Leach SD, Horb ME. Differential ability of Ptf1a and Ptf1a-VP16 to convert stomach, duodenum and liver to pancreas. Dev Biol. 2007;304:786–799. doi: 10.1016/j.ydbio.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- Jensen J, Serup P, Karlsen C, Nielsen TF, Madsen OD. mRNA profiling of rat islet tumors reveals Nkx 6.1 as a β-cell-specific homeodomain transcription factor. J Biol Chem. 1996;271:18749–18758. doi: 10.1074/jbc.271.31.18749. [DOI] [PubMed] [Google Scholar]
- Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Kowalik AS, Rajakumar N, Pin CL. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech Dev. 2004;121:261–272. doi: 10.1016/j.mod.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Melton DA. Development of the pancreas in Xenopus laevis. Dev Dyn. 2000;218:615–627. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1027>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111–127. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997a;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Melton DA. Pancreas development in the chick embryo. Cold Spring Harb Symp Quant Biol. 1997b;62:377–383. [PubMed] [Google Scholar]
- Krapp A, Knofler M, Frutiger S, Hughes GJ, Hagenbuchle O, Wellauer PK. The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. EMBO J. 1996;15:4317–4329. [PMC free article] [PubMed] [Google Scholar]
- Kume S. The molecular basis and prospects in pancreatic development. Dev Growth Differ. 2005;47:367–374. doi: 10.1111/j.1440-169X.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Lee YH, Saint-Jeannet JP. Sox9, a novel pancreatic marker in Xenopus. Int J Dev Biol. 2003;47:459–462. [PubMed] [Google Scholar]
- Leloup J, Buscaglia M. La triiodothyronine: hormone de lametamorphose des amphibiens. C R Acad Sci. 1977;284:2261–2263. [Google Scholar]
- Lemercier C, To RQ, Swanson BJ, Lyons GE, Konieczny SF. Mist1: a novel basic helix-loop-helix transcription factor exhibits a developmentally regulated expression pattern. Dev Biol. 1997;182:101–113. doi: 10.1006/dbio.1996.8454. [DOI] [PubMed] [Google Scholar]
- Leone F, Lambert-Gardini S, Sartori C, Scapin S. Ultrastructural analysis of some functional aspects of Xenopus laevis pancreas during development and metamorphosis. J Embryol Exp Morphol. 1976;36:711–724. [PubMed] [Google Scholar]
- Li WC, Horb ME, Tosh D, Slack JM. In vitro transdifferentiation of hepatoma cells into functional pancreatic cells. Mech Dev. 2005;122:835–847. doi: 10.1016/j.mod.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Pack MA, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Dev Biol. 2004;274(2):491–503. doi: 10.1016/j.ydbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Liu L, Jia W, Zheng T, Li M, Lu H, Xiang K. Ala45Thr variation in neuroD1 gene is associated with early-onset type 2 diabetes with or without diabetic pedigree in Chinese. Mol Cell Biochem. 2006;290:199–204. doi: 10.1007/s11010-006-9217-4. [DOI] [PubMed] [Google Scholar]
- Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci USA. 2007;104:10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maake C, Hanke W, Reinecke M. An immunohistochemical and morphometric analysis of insulin, insulin-like growth factor I, glucagon, somatostatin, and PP in the development of the gastroentero-pancreatic system of Xenopus laevis. Gen Comp Endocrinol. 1998;110:182–195. doi: 10.1006/gcen.1998.7064. [DOI] [PubMed] [Google Scholar]
- Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy JW, Dixon KE. Cell specialization in the small intestinal epithelium of adult Xenopus laevis: functional aspects. J Anat. 1978a;125:237–245. [PMC free article] [PubMed] [Google Scholar]
- McAvoy JW, Dixon KE. Cell specialization in the small intestinal epithelium of adult Xenopus laevis: structural aspects. J Anat. 1978b;125:155–169. [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr., Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- Mellitzer G, Bonne S, Luco RF, Van De CM, Lenne-Samuel N, Collombat P, Mansouri A, Lee J, Lan M, Pipeleers D, Nielsen FC, Ferrer J, Gradwohl G, Heimberg H. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J. 2006;25:1344–1352. doi: 10.1038/sj.emboj.7601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano EG, Chimenti C. Morphogenesis of the pancreas of Bufo bufo during metamorphosis. Gen Comp Endocrinol. 1995;97:239–249. doi: 10.1006/gcen.1995.1023. [DOI] [PubMed] [Google Scholar]
- Moriya N, Komazaki S, Takahashi S, Yokota C, Asashima M. In vitro pancreas formation from Xenopus ectoderm treated with activin and retinoic acid. Dev Growth Differ. 2000;42:593–602. doi: 10.1046/j.1440-169x.2000.00542.x. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Mao J, Brown DD. Remodeling the exocrine pancreas at metamorphosis in Xenopus laevis. Proc Natl Acad Sci USA. 2008;105:8962–8967. doi: 10.1073/pnas.0803569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhi S, Horb ME, Brown DD. Remodeling of insulin producing β-cells during Xenopus laevis metamorphosis. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.01.038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murato Y, Yamaguti M, Katamura M, Cho KW, Hashimoto C. Two modes of action by which Xenopus hairy2b establishes tissue demarcation in the Spemann-Mangold organizer. Int J Dev Biol. 2006;50:463–471. doi: 10.1387/ijdb.052106ym. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC. Pancreas and betacell development: from the actual to the possible. Development. 2007;134:427–438. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- Nair RJ, Lawler L, Miller MR. Chronic pancreatitis. Am Fam Physician. 2007;76:1679–1688. [PubMed] [Google Scholar]
- Nieber F, Pieler T, Henningfeld KA. Comparative expression analysis of the neurogenins in Xenopus tropicalis and Xenopus laevis. Dev Dyn. 2009;238:451–458. doi: 10.1002/dvdy.21845. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Garland; New York: 1967. reprinted in 1994. [Google Scholar]
- Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic β-cells. Dev Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober EA, Field HA, Stainier DY. From endoderm formation to liver and pancreas development in zebrafish. Mech Dev. 2003;120:5–18. doi: 10.1016/s0925-4773(02)00327-1. [DOI] [PubMed] [Google Scholar]
- Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada TS. Epithelio-mesenchymal relationships in the regional differentiation of the digestive tract in the amphibian embryo. Roux Arch Entwicklungsmech. 1960;152:1–21. doi: 10.1007/BF00575217. [DOI] [PubMed] [Google Scholar]
- Oster A, Jensen J, Serup P, Galante P, Madsen OD, Larsson LI. Rat endocrine pancreatic development in relation to two homeobox gene products (Pdx-1 and Nkx 6.1) J Histochem Cytochem. 1998;46:707–715. doi: 10.1177/002215549804600602. [DOI] [PubMed] [Google Scholar]
- Paik WK, Cohen PP. Biochemical studies on amphibian metamorphosis. I. The effect of thyroxine on protein synthesis in the tadpole. J Gen Physiol. 1960;43:683–696. doi: 10.1085/jgp.43.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Chen Y, Bayha E, Pieler T. Retinoic acid-mediated patterning of the pre-pancreatic endoderm in Xenopus operates via direct and indirect mechanisms. Mech Dev. 2007;124:518–531. doi: 10.1016/j.mod.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Pearl EJ, Horb ME. Promoting ectopic pancreatic fates: pancreas development and future diabetes therapies. Clin Gen. 2008;74:316–324. doi: 10.1111/j.1399-0004.2008.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Sovik O, Polak M, Hattersley AT. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- Pieler T, Chen Y. Forgotten and novel aspects in pancreas development. Biol Cell. 2006;98:79–88. doi: 10.1042/BC20050069. [DOI] [PubMed] [Google Scholar]
- Race JJ, Robinson C, Terry RJ. The influence of thyroxine on the normal development of the pancreas in Rana pipens larvae. J Exp Zool. 1966;162:181–192. [Google Scholar]
- Rafiq M, Flanagan SE, Patch AM, Shields BM, Ellard S, Hattersley AT. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31:204–209. doi: 10.2337/dc07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooman I, De Medts N, Baeyens L, Lardon J, De Breuck S, Heimberg H, Bouwens L. Expression of the Notch signaling pathway and effect on exocrine cell proliferation in adult rat pancreas. Am J Pathol. 2006;169:1206–1214. doi: 10.2353/ajpath.2006.050926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela CF, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of β-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I, Goldberg I, Pri-Chen S, Ben Dor L, Polak-Charcon S, Karasik A, Shimon I, Mor E, Ferber S. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci USA. 2005;102:7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Mamin A, Brun T, Ritz-Laser B, Zaiko M, Maret A, Jornayvaz FR, Theintz GE, Michielin O, Melloul D, Philippe J. Agenesis of human pancreas due to decreased half-life of insulin promoter factor 1. J Clin Endocrinol Metab. 2003;88:4398–4406. doi: 10.1210/jc.2003-030046. [DOI] [PubMed] [Google Scholar]
- Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, Gloyn AL, Edghill EL, Hattersley AT, Wellauer PK, Goodwin G, Houlston RS. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat.Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- Seufert DW, Prescott NL, El-Hodiri HM. Xenopus aristaless-related homeobox (xARX) gene product functions as both a transcriptional activator and repressor in forebrain development. Dev Dyn. 2005;232:313–324. doi: 10.1002/dvdy.20234. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YB, Brown DD. Developmental and thyroid hormone-dependent regulation of pancreatic genes in Xenopus laevis. Genes Dev. 1990;4:1107–1113. doi: 10.1101/gad.4.7.1107. [DOI] [PubMed] [Google Scholar]
- Shternhall-Ron K, Quintana FJ, Perl S, Meivar-Levy I, Barshack I, Cohen IR, Ferber S. Ectopic PDX-1 expression in liver ameliorates type 1 diabetes. J Autoimmun. 2007;28:134–142. doi: 10.1016/j.jaut.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulinproducing β cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Spagnoli FM. From endoderm to pancreas: a multistep journey. Cell Mol Life Sci. 2007;64:2378–2390. doi: 10.1007/s00018-007-7184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli FM, Brivanlou AH. The RNA-binding protein, Vg1RBP, is required for pancreatic fate specification. Dev Biol. 2006;292:442–456. doi: 10.1016/j.ydbio.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Spagnoli FM, Brivanlou AH. The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm. Development. 2008;135:451–461. doi: 10.1242/dev.008458. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat.Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Strobel O, Dor Y, Stirman A, Trainor A, Fernandez-del Castillo C, Warshaw AL, Thayer SP. β cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo. Proc Natl Acad Sci USA. 2007;104:4419–4424. doi: 10.1073/pnas.0605248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic β cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Tang DQ, Lu S, Sun YP, Rodrigues E, Chou W, Yang C, Cao LZ, Chang LJ, Yang LJ. Reprogramming liverstem WB cells into functional insulinproducing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab Invest. 2006;86:83–93. doi: 10.1038/labinvest.3700368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel E, Nagasaka T, Tezel G, Kaneko T, Takasawa S, Okamoto H, Nakao A. REG I as a marker for human pancreatic acinoductular cells. Hepatogastroenterology. 2004;51:91–96. [PubMed] [Google Scholar]
- Weber H, Symes CE, Walmsley ME, Rodaway AR, Patient RK. A role for GATA5 in Xenopus endoderm specification. Development. 2000;127:4345–4360. doi: 10.1242/dev.127.20.4345. [DOI] [PubMed] [Google Scholar]
- Weber R. Switching of globin genes during anuran metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis. Academic Press; New York: 1996. pp. 567–597. [Google Scholar]
- Wright CV, Schnegelsberg P, De Robertis EM. XlHbox 8: a novel Xenopus homeo protein restricted to a narrow band of endoderm. Development. 1989;105:787–794. doi: 10.1242/dev.105.4.787. [DOI] [PubMed] [Google Scholar]
- Yamaguti M, Cho KW, Hashimoto C. Xenopus hairy2b specifies anterior prechordal mesoderm identity within Spemann’s organizer. Dev Dyn. 2005;234:102–113. doi: 10.1002/dvdy.20523. [DOI] [PubMed] [Google Scholar]
- Zaghloul NA, Moody SA. Changes in Rx1 and Pax6 activity at eye field stages differentially alter the production of amacrine neurotransmitter subtypes in Xenopus. Mol Vis. 2007;13:86–95. [PMC free article] [PubMed] [Google Scholar]
- Zecchin E, Mavropoulos A, Devos N, Filippi A, Tiso N, Meyer D, Peers B, Bortolussi M, Argenton F. Evolutionary conserved role of ptf1a in the specification of exocrine pancreatic fates. Dev Biol. 2004;268:174–184. doi: 10.1016/j.ydbio.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Zeynali B, Kalionis B, Dixon KE. Determination of anterior endoderm in Xenopus embryos. Dev Dyn. 2000;218:531–536. doi: 10.1002/1097-0177(200007)218:3<531::AID-DVDY1010>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Jiang H, Wang W, Mao B. Cloning and developmental expression of the Xenopus Nkx6 genes. Dev Genes Evol. 2007;217:477–483. doi: 10.1007/s00427-007-0155-2. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Molecular basis of vertebrate endoderm development. Int Rev Cytol. 2007;259:49–111. doi: 10.1016/S0074-7696(06)59002-3. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in Xenopus by Wnt/β-catenin and TGF-β signalling pathways. Dev Biol. 1999;209:282–297. doi: 10.1006/dbio.1999.9257. [DOI] [PubMed] [Google Scholar]


