Abstract
Tissue oxygen plays a crucial role in maintaining tissue viability and in various diseases, including responses to therapy. Useful knowledge has been gained by methods that can give limited snapshots of tissue oxygen (e.g., oxygen electrodes) or evidence of a history of tissue hypoxia (e.g., EF5) or even indirect evidence by monitoring oxygen availability in the circulatory system (e.g., NMR methods). Each of these methods has advantages and significant limitations. EPR oximetry is a technique for direct measurement of tissue pO2, which has several advantages over the other existing methods for applications in which the parameter of interest is the pO2 of tissues, and information is needed over a time course of minutes to hours, and/or for repetitive measurements over days or weeks or years. The aim of this article is to provide an overview of EPR oximetry using particulates to readers who are not familiar with this technique and its potential in vivo and clinical applications. The data presented here are from the experiments currently being carried out in our laboratory. We are confident that in vivo EPR oximetry will play a crucial role in the understanding and clinical management of various pathologies in the years to come.
RATIONALE FOR THE USE OF EPR OXIMETRY FOR TISSUE pO2 MEASUREMENTS
The amount of oxygen plays an important role in the pathophysiology of many diseases and in their response to treatment, including cancer, ischemia–reperfusion injury, and wound healing (7, 22, 32, 63, 66, 67). Over the past several years, considerable time and effort have been invested in developing techniques to measure effectively and reliably tissue oxygen (reported as the concentration [O2] or the partial pressure, pO2). Currently available techniques can be broadly classified in two categories (a) direct methods (invasive and non-invasive) that measure the [O2] or pO2, and (b) indirect methods that measure a parameter related to them, such as the saturation of hemoglobin. Of the direct methods, the Eppendorf pO2 histograph is considered the “gold standard,” even though it has several limitations (46, 51). Other useful direct methods include fluorescence quenching, O2 binding to myoglobin, phosphorescence quenching, Overhauser imaging, and 19F-NMR spectroscopy, but these have limitations for making continuous and repeated measurements (2, 31, 39, 69). Therefore, a need exists for methods that can make the desired continuous and repeated measurements.
In the last two decades, our laboratory has actively contributed to the development of in vivo electron paramagnetic resonance (EPR) oximetry for direct tissue pO2 measurements using particulates. Our rationale for the development of this type of oximetry is to provide a method to measure tissue pO2 directly and repeatedly to facilitate the study of the mechanisms and modifications of various physiologic and pathologic processes that occur in intact animals, including human subjects. All the factors that affect these processes are fully active in living subjects as compared with studies in model systems or even isolated organs, where systemic factors, effects from other organ systems, etc., do not occur.
INTRODUCTION TO EPR OXIMETRY
The basis of EPR oximetry is the paramagnetic nature of molecular oxygen, which therefore affects the EPR spectra of other paramagnetic species in its vicinity by altering their relaxation rates and, possibly, by other mechanisms as well. The magnitudes of the effects are directly related to the amount of oxygen that is present in the environment of the paramagnetic materials. The placement of the paramagnetic material in the tissue of interest is minimally invasive (it usually requires an insertion via a 23- to 26-gauge needle, or direct implantation during a surgical procedure required for other reasons), but all subsequent measurements are entirely noninvasive (10, 57, 59). Two types of oxygen-sensitive paramagnetic materials are used in EPR oximetry: particulates [such as lithium phthalocyanine (LiPc) crystals] and soluble probes (such as nitroxides and trityl radicals). The particulates are materials with unpaired electrons distributed over complex arrays of atoms and crystalline materials, such as in LiPc. The useful properties of the particulates for EPR oximetry are their stability and strong response of their spectra to the presence of oxygen. These are metabolically inert and are very sensitive for measuring low levels of oxygen. Once introduced in the tissue of interest, they allow repeated measurement of pO2 at the same site for up to years after implantation (37, 38, 61). The region of the sample that is measured directly is that immediately surrounding the paramagnetic material. If the paramagnetic material is a macroscopic particle (such as chars, LiPc), it reflects the pO2 in the tissue that is in contact with the surface of the particle. If the paramagnetic material is a slurry of small particles (such as ink), then it reports the average pO2 of the sum of the surfaces that are in contact with the individual components of the slurry. EPR oximetry using particulates, under appropriate conditions, can measure pO2 levels in tissues over a wide range from the extremes of very low (<0.1%) to very high (≤100%) oxygen levels.
To measure pO2 using EPR oximetry, a detector (also referred to as a resonator) is placed on the surface of the skin above the tissue injected with the paramagnetic material, and through the application of an appropriate combination of an electromagnetic field (excitation frequency near 1,200 MHz in our studies) and a magnetic field (~400 gauss), the scanning of the magnetic field produces a characteristic resonance signal (10, 57, 59). Typical EPR signals recorded in applications in vivo in animals (from LiPc) and in human subjects (from India ink) are shown subsequently. Using an appropriate calibration curve, the line width of the EPR signal provides a sensitive measurement of tissue oxygen. This technique has been used to study the tissue pO2pO2 in a wide range of experimental systems, including muscle (18; 23), heart (11; 16; 17), brain (26; 27; 28; 41; 62), kidney (33; 34), liver (35; 44; 64), skin (1) and tumors (29; 30; 47; 49; 50).
Tissue pO2 reported by EPR oximetry has been compared directly with the Eppendorf system in the same animals (49). The average pO2 values in the rat brain cortex measured by the two methods were similar, but the EPR oximetry method reported a statistically significantly higher average pO2. These differences in the average pO2 measured by the two methods were consistent with other reports (3, 43) and suggest that the Eppendorf electrode may report a lower than expected pO2, likely because of local perturbations caused by this method. We also have directly compared the absolute pO2 and the changes in rat brain pO2 measured using EPR oximetry during hyperoxic treatment with the pO2 reported by the OxyLite method at adjacent locations in the same animals (48). The pO2 values reported by the two methods were similar but not identical. Both methods can record a baseline and rapid changes in pO2. The changes in pO2 induced by increasing FiO2 from 26% (balanced with N2) to carbogen (95% O2 + 5% CO2) were similar by the two methods. It should be noted that OxyLite can measure pO2 only up to 200 mm Hg, but no such limits exist with EPR oximetry as long as a reasonable EPR spectrum can be recorded under hyperoxic conditions.
EPR oximetry with particulates has features that often complement those available with other methods and are summarized here:
The measurements are made directly in the tissues at the sites of interest (as compared with indirect techniques such as BOLD NMR and NIR that make measurements in the vascular system).
The measurements provide quantitative data on the pO2 at the measurement sites (as compared with techniques such as labeled ATSM or misonidazole, which provide information on the occurrence of hypoxia but cannot provide quantitative information on the pO2).
The measurements can be made continuously and repeatedly as desired.
The placement of the paramagnetic material is minimally invasive (usually requiring insertion via a 23- to 26-gauge needle, or direct insertion if a surgical procedure is required for other reasons), and all subsequent measurements are entirely noninvasive.
The oxygen probes used in EPR oximetry are metabolically inert and are very sensitive for measuring low levels of oxygen.
The oxygen-sensitive particulate is placed in one or more sites in the tissue, where it then resides permanently, providing an EPR signal that can be repeatedly and selectively measured, reporting pO2 from a well-characterized site.
The technique can use materials that already are accepted for use in human subjects.
Measurements can be made at several sites simultaneously and quickly (within a minute).
Feasibility has been demonstrated in human subjects, as illustrated in the data from measurements of oxygen in the human foot and in tumors, as summarized in the clinical section.
No other available techniques can make such measurements.
Systematic reviews of EPR oximetry with more-extensive information on the principles, methods, and technical aspects are available in recent reports and are not covered here (56, 58, 60). An important recent advance in EPR oximetry is the capability to obtain simultaneous pO2pO2 measurements from several sites (19, 20, 26, 55, 68). The High Spatial Resolution Multi-Site (HSR-MS) EPR oximetry technique can be used to provide pO2 estimates from multiple LiPc implants with greatly increased spatial resolution (19). This technique uses two EPR spectra that have been acquired with magnetic field gradients, and an analytic relation that occurs between the spectra is used to estimate the line width and hence tissue pO2 for each implant site. Very useful results have been obtained with this approach, which allows multiple measurements within an area of pathophysiology and simultaneous measurements at a control site (68). We recently refined this technique and achieved improved oximetric sensitivity through the application of overmodulation during EPR detection (68). The present resolution of this technique is approximately 1 mm center-to-center, which has allowed us to measure pO2 simultaneously at three sites in an intracranial tumor and at one site in the contralateral normal brain (see subsequent sections). The following sections provide an overview of the in vivo and clinical applications of EPR oximetry using particulates that are currently being pursued in our laboratory.
IN VIVO APPLICATIONS OF EPR OXIMETRY IN EXPERIMENTAL ANIMALS
Measurement of tissue pO2 in tumors
The level of oxygen in tumors at the time of radiotherapy plays a crucial role in the response to the radiation, with a decrease in responsiveness by up to a factor of three when the tumor pO2 decreases from radiobiologically oxic levels (>15–20 mm Hg) to significant radiobiologic hypoxia (pO2 < 10 mm Hg) (21). The presence of hypoxic cells in tumors, and their impact on the ability of radiotherapy to control tumor growth, has been demonstrated in numerous studies over the past decades (5, 14, 15, 21, 25, 45). The region covered by EPR oximetry spans the intercapillary distance of normal and tumor tissue. Normal rat brain is reported to have an intercapillary distance of about 45 μm (42) and a capillary density of between 150 and 300 per mm2 (6, 13), depending on the study or the method of measurement. EPR oximetry therefore samples a region that includes, at minimum, scores of capillaries and potentially a region that spans the heterogeneous tumor structure.
The ability to follow the tumor pO2 over the course of therapy could provide the crucial information needed to optimize the effectiveness of hypoxia-modifying procedures and radiotherapy. The need for such measurements has increased further because of recent developments in radiation oncology, in which conformal therapy, alternative fractionation schemes, and variations in dose rates are increasingly used. This information potentially then could be used to understand, optimize, and individualize the treatment strategies, including its use in combined treatments with other modalities, such as chemotherapy, immunotherapy, and surgery. These treatment strategies can significantly affect the extent and the kinetics of changes in tumor oxygenation and therefore may significantly alter the therapeutic outcome. Tumor pO2 is an independent prognostic indicator in cancer because it influences tumor progression and treatment outcomes (51). Given the importance of tumor pO2 in therapy and in predicting disease progression, it is imperative that reliable and technically simple methods should be developed for repetitive noninvasive tumor pO2 measurements.
Measurement of tissue pO2 in intracranial tumors
HSR-MS EPR oximetry currently is being used in our laboratory to measure tumor pO2 at more than one site in the tumor and to investigate the effect of hyperoxia (such as breathing carbogen) and the effect of irradiation on intracranial tumors. This technique is being used to study the intracranial tumors at their orthotopic position, as compared with other methods that have been used to study intracranial tumor models grown subcutaneously in the flanks because of technical limitations. The treatment strategies developed using subcutaneous tumors may not show the same efficacy in orthotopic tumors, as the oxygenation in the brain is regulated by factors such as perfusion and local metabolism.
The changes in tumor pO2 observed in a rat with 9L-intracranial tumor (three LiPc implants in the tumor, and one LiPc implant in the contralateral normal brain) when allowed to breathe carbogen, are shown in Fig. 1a, and the typical EPR spectra obtained from these experiments are shown in Fig. 1b. Results indicate that the tumor pO2 was <10 mm Hg for the lateral and medial sites of the tumor, and the pO2 of the center site was ~12 mm Hg in a rat breathing 26% O2 (balance with N2). Carbogen breathing resulted in a significant increase in pO2 in the lateral and center tumor sites, as well as in the contralateral normal brain. These results illustrate the capability of multisite EPR oximetry to monitor the dynamic changes in tissue pO2 simultaneously from three sites in the tumor along with one site in the contralateral brain. This information can be used to deliver radiotherapy at the time of optimal tumor oxygenation to enhance the radiotherapeutic efficacy.
FIG. 1. (a) Intracranial 9L-tumor and contralateral normal brain pO2 in anesthetized (1.5% isoflurane) rat breathing 26% O2 and during carbogen breathing.
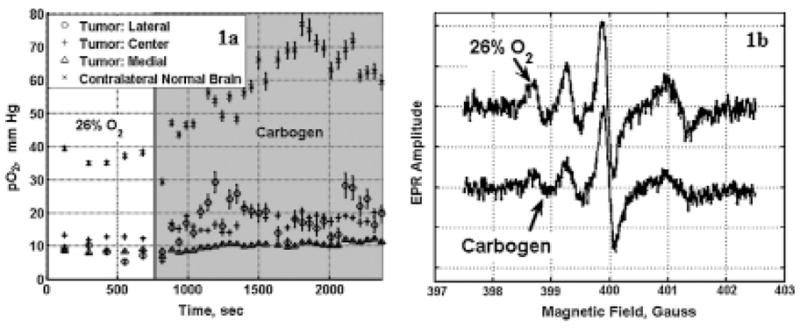
EPR spectra were averaged over 2 min at each time point. For the HSR-MS oximetry, spectra were collected with magnetic field gradients of 8 G/cm and 12.5 G/cm, and the modulation amplitude was scaled in proportion to the applied gradient. The pO2 values are expressed as the mean ± SEM. (b) Typical EPR spectra obtained from the three LiPc implants in the tumor and one LiPc implant in the contralateral normal brain of rat breathing 26% O2 (upper tracing) and after 25 min of carbogen breathing (lower tracing). The lowest field EPR signal (farthest left) is from the lateral LiPc implant of the tumor; the next signal is from the center LiPc implant of the tumor; and the third one is from the medial LiPc implant of the tumor. The highest field EPR signal is from the LiPc implanted in the contralateral normal brain. The carbogen breathing resulted in the broadening of the EPR signals (i.e., increase in tissue pO2).
The effect of irradiation (9.3 Gy) on the tissue pO2 of the tumor also was investigated using HSR-MS EPR oximetry, Fig. 2. The tumors were irradiated on day 0 after baseline pO2 measurements, and changes in tissue pO2 were followed up for 6 consecutive days. The results indicate that HSR-MS EPR oximetry could resolve different pO2 values at the three sites in the tumor and that all sites had a similar pattern of oxygen changes after irradiation. The pO2 initially decreased at the 24-h time point, and this was followed by reoxygenation at the 48-h time point. The tumor pO2 then gradually decreased during the next 4–6 days. The pattern of the pO2 changes in the contralateral normal brain was different; the pO2 decreased on days 4–6, which may be the effect of increased tumor volume and intracranial pressure. The different baseline tumor pO2 observed in Figs. 1a and 2, in our experience, is typical for variations between tumors in this model; and this further supports the value of making direct measurements of pO2 in individual tumors.
FIG. 2. Changes in intracranial 9L tumors and contralateral normal brain pO2 before and after a single dose of irradiation (9.3 Gy).
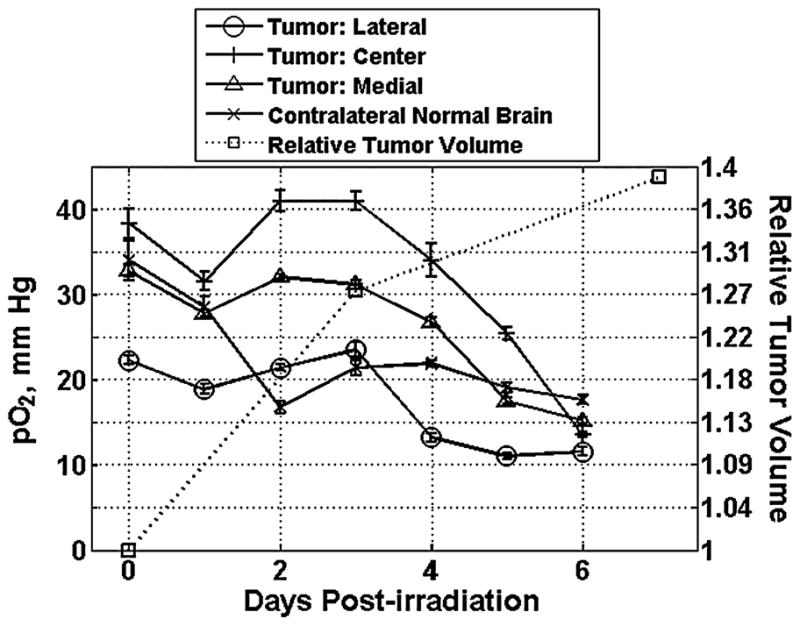
The tumor volume was measured using MRI on days 0, 3, and 7. The rats were anesthetized with 1.5% isoflurane with 26% FiO2, and the body temperature was maintained at 37°C using a warm-water pad. At each time point, the data were collected for 30 min and averaged. The pO2 values are expressed as the mean ± SEM.
These results highlight the capability of EPR oximetry to follow the time course of tumor pO2 levels over the course of hyperoxia or radiotherapy. We believe that EPR oximetry will provide novel, detailed information on the changes in the oxygen status of tumors in response to a treatment.
Measurement of tissue pO2 of subcutaneous tumors
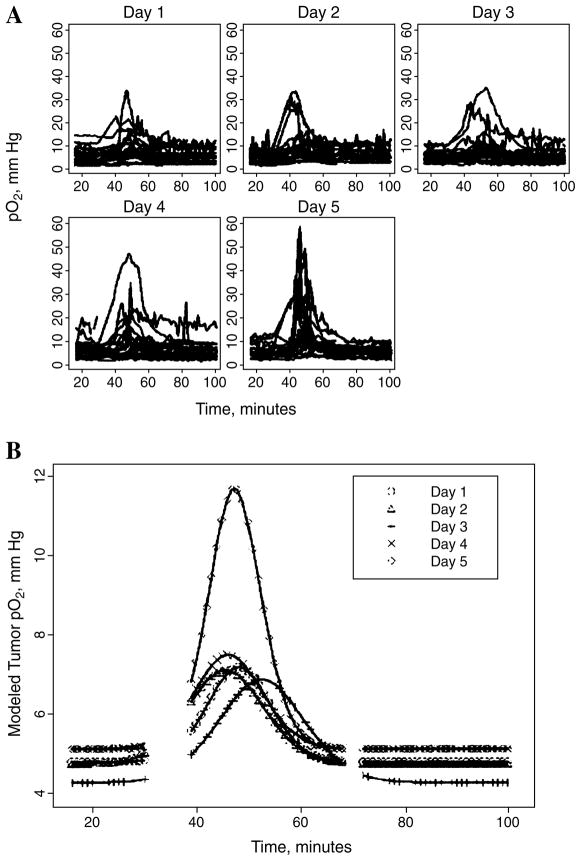
Multisite EPR oximetry has been successfully used noninvasively to monitor pO2 at two sites in subcutaneously grown RIF-1 tumors in the flanks of C3H mice (30). Here, we describe the pO2 results obtained immediately after each dose during a fractionated radiotherapy (five repeated daily doses at 4 Gy), Fig. 3, and Table 1. Both a traditional t test and more-advanced dynamic models confirm that statistically significant changes in tumor pO2 are found shortly after irradiation (9). No significant difference was noted in the pO2 measured from the two sites in the tumors, and also no significant difference was found in the baseline pO2 between the irradiation and the sham-irradiation groups.
FIG. 3. Dynamic changes in the individual (A) and mean (B) RIF-1 tumor pO2 after a daily dose of 4 Gy of ionizing radiation on 5 consecutive days (n = 20–22).
The mice were anesthetized using 1.5% Isoflurane with 26% FiO2, and the body temperature was maintained at 37°C. For each day, the pO2 values averaged across all animals were fit using a gaussian distribution, as shown in (B), to characterize the average changes in pO2. Baseline EPR measurements were made for 30 min, and then at 35 min, a 4-Gy fraction of radiation was delivered, the animals were returned to the EPR spectrometer, and the pO2 in the tumor was monitored for another 60 min.
Table 1.
Time Course and Extent of Maximum Increase in pO2 in Tumors from Irradiation (4 Gy/day) on 5 Consecutive Days
| Days | N | pO2,base (torr) | Tmax (min) | pO2,max (torr) | Tbase (min) |
|---|---|---|---|---|---|
| 1 | 22 | 5.5 (0.7) | 18.2 (0.2) | 7.2 (1.0)* | 9.2 (0.3) |
| 2 | 22 | 5.0 (0.5) | 15.6 (0.3) | 7.1 (1.1)* | 11.1 (0.6) |
| 3 | 20 | 5.0 (0.5) | 22.5 (0.2) | 6.9 (1.0)* | 12.7 (0.4) |
| 4 | 20 | 6.1 (0.8) | 16.1 (0.3) | 7.5 (1.3)* | 9.9 (0.5) |
| 5 | 20 | 5.3 (0.7) | 17.5 (0.2) | 11.7 (2.0)*† | 8.2 (0.3) |
pO2,base, baseline pO2; Tmax, time to reach maximal pO2; pO2,max, maximal pO2; Tbase, time required to come back to the baseline pO2 from maximal pO2. Standard error is shown in parentheses.
p < 0.01, compared with the baseline pO2;
p < 0.01, compared with the pO2 max from day 1 to day 5.
N, number of implants on which measurements were made (two implants per mouse).
The pO2 increase reached a maximum at 15.2–22.5 min (average, 20 min) after irradiation, and the average time required to return to the baseline pO2 was 8.2–12.7 min. The average maximum tumor pO2 achieved after irradiation from days 1 through 5 ranged from 7.1–11.7 mm Hg. No significant increase in tumor pO2 was observed in the sham-irradiation (control) group. The average maximum increases in tumor pO2 after irradiation treatment on day 5 were significantly higher than the maximum tumor pO2 for days 1–4 (Table 1).
These results demonstrate the value of direct monitoring of the dynamics of pO2 in tumors to detect and characterize quantitatively the time course of changes in tumor oxygenation. It is likely that with this knowledge, treatments such as those proposed here can be used more optimally so that even changes that are quantitatively modest may have large radiobiologic effects if they can be delivered at appropriate times.
Application of EPR oximetry to study the effect of preexisting vessels and gene therapy on tissue oxygenation of the hindlimbs in response to ischemia
The role of hypoxia in the regions of collateral arterial growth in femoral artery ligation models or in patients with peripheral vascular disease has been intensely debated (24, 54, 65). We have used EPR oximetry to investigate the role of preexisting collateral vessels and the effect of gene therapy on tissue pO2 in response to ischemia due to blockage of the femoral artery of the hindlimbs in mice.
A wood char was used as an oximetry probe to measure tissue pO2 in the hindlimb muscle before and after femoral artery ligation in two strains of mice (C57BL/6 and BALB/c) with different degrees of innate collateral circulation. The response of tissue pO2 of muscle distal to the site of occlusion (gastrocnemius muscle) and of the medial thigh (adductor muscle) was different in the two mice strains. This study provided the first conclusive evidence that the preexistent collateral vessels play an important role in mouse strain ependent differences. The remodeling of the preexisting interarterial collateral connections into mature collateral arteries is potentially the most important process for the restoration of blood flow after femoral artery ligation in mice (23).
We also investigated the effect of an inflammatory mediator CD154 on the response to hypoxia of the gastrocnemius muscle in mice (Fig. 4). The procedures for ligation in the right hindlimb (RHL) were similar to that described by Helisch et al. (23). Tissue pO2 was measured on day 1 before ligation, and the effect of ischemic challenge was followed up for 4 weeks. Results indicate a profound ischemia from day 0 to day 3 after ligation, followed by a slow recovery of tissue pO2. However, no significant differences in the recovery of tissue pO2 between the control and knockout groups were observed. No significant changes in the tissue pO2 of the left hind limb (LHL, nonligated) of the control and knockout groups were observed. These results indicate that CD154 likely does not play a significant role in collateral development to normalize blood flow after ischemic challenge. Nevertheless, these results highlight the capability of EPR oximetry to monitor and detect changes in tissue pO2 over time for several weeks in mice and the effect of genetic manipulations on tissue response to ischemia.
FIG. 4. Tissue pO2 of gastrocnemius muscle of control (WT) and CD154 knockout mice in response to femoral artery ligation in the right hindlimb (RHL).
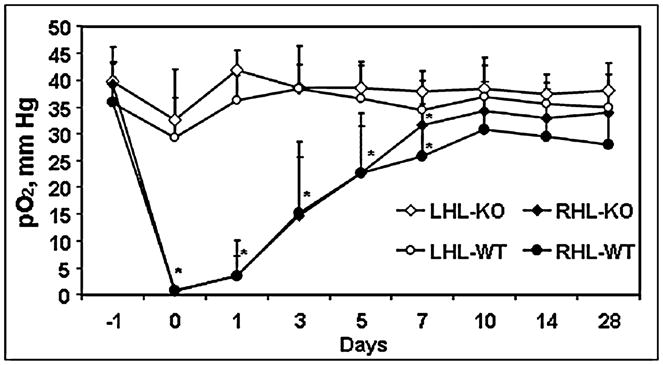
No ligation was performed in the left hind limb (LHL), and it served as a control in each mouse. n = 10, mean ± SD. *p < 0.05 vs. LHL. This study was carried out in collaboration with Dr. DeMuinck and Dr. Helisch from Dartmouth-Hitchcock Medical Center, Lebanon, NH.
Application of EPR oximetry to study the response of heart tissue to ischemic insult
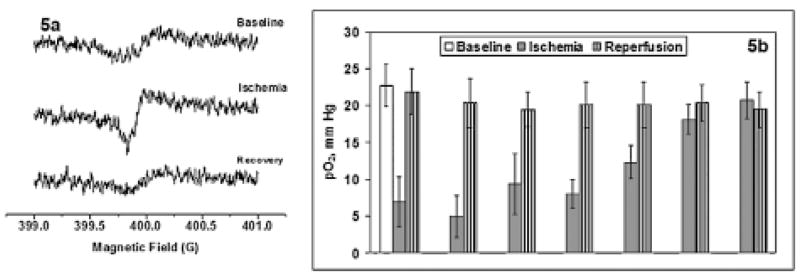
These experiments were designed to investigate the myocardial tissue pO2 during ischemia and reperfusion and the effect of collateral growth during ischemic challenges to the heart. EPR oximetry was successfully used in this study for repeated measurement of the myocardial tissue pO2 before, during, and after the left anterior descending coronary artery (LAD) occlusion. These experiments were carried out in collaboration with Dr. Chilian group, Louisiana State University Health Sciences Center (LSU). The rats were implanted with a pneumatic occluder over the LAD by the procedure developed at LSU. During the surgery, LiPc crystals (40 μg) were injected into the left ventricular myocardial wall, and the rats were allowed to recover for 1 week. The tissue pO2 was continuously measured, and repeated ischemia (5-min cycles) was induced by inflating the balloon (thus occluding the LAD) using an externalized occluder catheter (PE-50 tubing). This occlusion procedure allowed continuous measurement of tissue pO2 during repetitive, transient occlusions in anesthetized animals. Representative EPR spectra obtained during baseline, ischemia, and reperfusion are shown in Fig. 5a, and the tissue pO2, in Fig. 5b. These results indicate a significant decrease in the tissue pO2 during an ischemic challenge, and then the pO2 recovered to baseline value during the reperfusion cycle. However, after several cycles of repetitive ischemic insults, no further decrease in tissue pO2 during ischemia was observed. These results also highlight the ability of in vivo EPR oximetry to follow up tissue oxygen in beating hearts of anesthetized animals over time. Currently, we are developing implantable resonators, which can be used to study myocardial pO2 repetitively for several weeks. The initial implantation procedure will be invasive, but rest of the oximetry measurements will be entirely noninvasive.
FIG. 5. (a) In vivo EPR spectra acquired from the LiPc crystals injected in the left ventricular myocardial tissue of an anesthetized rat during baseline, ischemia, and reperfusion.
Optimized spectrometer settings were used with a scan time of 10 s, P = 10 mW, and the modulation amplitude was one third of the line width. (b) Tissue pO2 of the left ventricular myocardial tissue of an anesthetized rat during baseline (10 min), repetitive cycles of ischemia (LAD occlusion, 5 min each), and reperfusion (5 min). The rat was anesthetized with 1.5% isoflurane with 100% FiO2 through a nose cone. After a baseline pO2 measurement, the ischemia eperfusion cycles were repeated using an LAD occluder.
Application of in vivo EPR oximetry to follow up tissue pO2 of wounds
Oxygen is a critical nutritional substrate and perhaps the most important variable in the healing of wounds. An adequate supply of oxygen facilitates cell replication, deposition of collagen, angiogenesis, epithelialization, and resistance to infection (32, 63). Oxygen tensions in wounds have previously been measured using oxygen electrodes by methods such as aspiration of fluid from the wound dead space, or from an implanted oxygen-permeable tube. However, the electrodes often are unstable, requiring frequent calibration, and cannot be used for repeated measurements in the same tube.
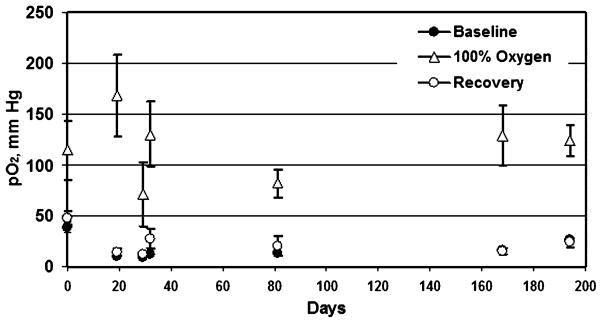
EPR oximetry can make noninvasive, accurate, and repeated measurements of tissue pO2 in wounds. These experiments were carried out in collaboration with Dr. Hopf, University of Utah. We placed two aggregates of LiPc crystals (total, ~200 μg, 10 mm apart) into oxygen-permeable Teflon tubing, and the tubing was fixed in the Silastic cylinders. These cylinders were then implanted in the space created by longitudinal dorsal mid-line incisions. Each incision was used to place two cylinders in the animals, one on each side, for a total of four cylinders per animal. This approach enabled us to measure oxygen levels in the wound dead space at several defined sites and time points. We also tested the response of the wound pO2 to changes in the breathing gas (Fig. 6).
FIG. 6. Mean pO2 measured within the wound cylinders for 11 weeks after implantation.
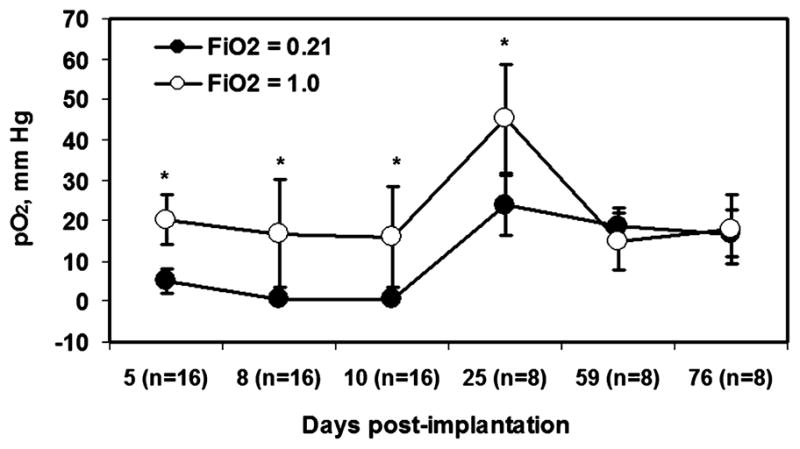
*A significant increase in wound pO2 when FiO2 was changed from 21% to 100%. The pO2 values are expressed as mean ± SD, n = 2. Four cylinders were implanted in each rat, and pO2 measurements were made from two LiPc implants in each cylinder.
A significant difference in wound pO2 was observed when the breathing gas was changed from 21% oxygen to 100% oxygen on days 5–25, but no significant difference was observed on days 59–76. A gross examination of Silastic cylinders and the surrounding tissues on days 10 and 76 after implantation revealed a connective tissue layer around the tubes. We speculate that the decrease in the response of tissue pO2 of older wounds (days 59–76) during 100% oxygen breathing is probably due to the formation of more scar tissue.
These results indicate the feasibility of using this approach for repeated pO2 measurements in a dorsal wound from a few days to weeks. In principle, this approach also could be used to calculate the gradients from the center to the edge of the cylinder in the wound dead space.
Application of in vivo EPR oximetry for pO2 measurement in deeper tissue using implantable resonators
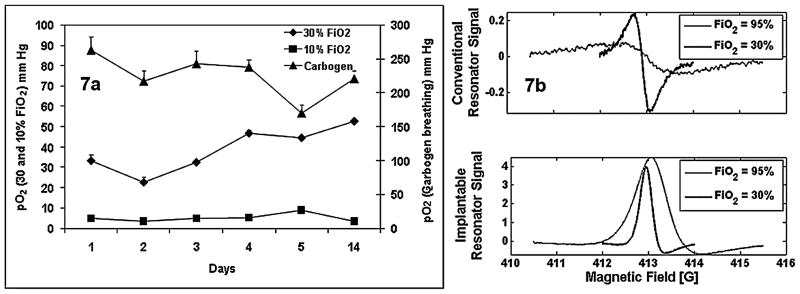
The currently available EPR oximetry (at 1,200 MHz) technique can be used for pO2 measurements in tissues up to a depth of 8–10 mm from the surface. The rationale for using an implantable resonator is to overcome this depth limitation so that the measurements can be made in the deeper tissues repetitively. The implantable resonator is composed of three parts: a small loop (~1 mm) with the oxygen-sensing materials in a biocompatible film at the site to be measured, a larger loop (~10 mm) placed just under the skin for magnetic coupling to the external surface resonator, and a wire to bridge the two loops. Operation of an implantable resonator is based on the well-known properties of a transmission line that is short-circuited at one end (40). In vitro calibration of the resonators indicated a similar response of line width to perfused gases as that of the LiPc crystals used in the resonators. We have begun a systematic study to test the feasibility of this approach in animal models. The calibrated resonators were implanted into the spinalis thoracis muscle of the rats, and tissue pO2 measurements were carried out for 2 weeks (Fig. 7a). A stable baseline tissue pO2 was observed in measurements done for 2 weeks, and the pO2 increased significantly during carbogen breathing. A significant decrease in tissue pO2 was observed when the FiO2 was changed to 10% oxygen. These results are encouraging and suggest good stability in vivo and good sensitivity of the implantable resonators to oxygen changes in the perfused gases. Another advantage of implantable resonator is a remarkable increase in the SNR (signal-to-noise ratio) as compared with conventional methods. In vivo EPR spectra acquired using an implantable resonator were compared with a spectrum acquired using the conventional technique with a rat breathing 30% O2 and carbogen (Fig. 7b). In this comparison, 300 μg of LiPc (an amount 10 times larger than the amount used in the implantable resonator) was inserted into the biceps femoris muscle at depth of ~3 mm, which is the same depth as the implantable resonator. The SNR values of the spectrum obtained with implantable resonator were 3–8 times larger than those observed with conventional detection. This technique has considerable promise for clinical use, with the resonators being implanted at the time of biopsy or surgery of tumors and then removed at the end of the treatment regimen several weeks later. Removal can occur via a small incision in the skin overlying the large loop.
FIG. 7. (a) In vivo measurement of the rat muscle pO2 using an implantable resonator.
The rat was anesthetized using 1.5% isoflurane, and the body temperature was maintained at 37°C using a warm-water pad. The pO2 data are expressed as mean ± SD. (b) EPR spectrum acquired from the injected LiPc crystals and from the implantable resonator under similar conditions in muscle of a rat breathing 30% O2 and carbogen. Measurements were made with the automatic frequency-control circuit in operation, which, at this state of development, leads to a significant amount of dispersion in the signal recorded with the implantable resonator. Recognizing the fact that the phases where not equal and that the pO2 at the implantable resonator was slightly lower (51 vs. 60 mm Hg, breathing 30% O2), a significant increase in S/N of the EPR signal acquired from the implantable resonator was observed.
CLINICAL APPLICATIONS OF IN VIVO EPR OXIMETRY
This section describes the potential application of in vivo EPR oximetry to follow up tissue pO2 in clinical settings. Our goal is to provide a method that can be used to measure and follow up tissue pO2 to provide information that can be used to determine either the status of a disease or the effect of various therapeutic interventions during treatments. All the major technical challenges to making measurements in human subjects have been met, and in vivo EPR oximetry now is being applied to monitor tissue oxygen in peripheral tumors and in the feet of normal volunteers, which is the first step toward application with peripheral vascular disease (PVD). We believe that once in vivo EPR oximetry is successfully introduced in the clinic as a valuable tool for tissue pO2 measurements in PVD and radiation therapy, it is quite likely that its use will be extended to other diseases that are directly or indirectly linked with tissue pO2.
Before initiating the clinical studies, we thoroughly examined the potential hazards of in vivo EPR for use in human subjects. Because of the low magnitude of the magnetic field and the absence of substantial gradients, the primary concern was the possibility of local heating (usually referred to as specific absorption rate, SAR). Results indicate that the SAR measured in our clinical setup is well below the established limits (53). We have initiated the clinical studies using India ink as an oximetry probe, which is sensitive to tissue pO2, is very stable in tissue, and already has been approved for use in human subjects (8, 37, 38, 61). This ink is prepared using components that are already in use in various pharmaceutical preparations. The reproducibility of this ink and its sensitivity to oxygen has been thoroughly examined and is found to be very satisfactory (8).
The following data were collected using the clinical L-band (1.2 GHz) EPR spectrometer that was specifically designed at our center for use with human subjects (52). This spectrometer is located at the Dartmouth-Hitchcock Medical Center adjacent to the Department of Radiation Oncology. The close proximity of the spectrometer to the clinical radiation therapy units enables EPR oximetry to be performed quickly before and after the treatment. The magnet has a pole separation of 50 cm, which allows the comfortable positioning of a patient in the magnet. A customized nonferrous stretcher and chair are available for positioning the patient. In combination with a variety of surface-loop resonators, EPR measurements can be made at virtually any location on the body surface (52).
Application of EPR oximetry for pO2 measurements in patients with peripheral vascular disease
Poor tissue perfusion to the feet and legs is an important and common problem, especially in diabetics (12). Foot ulcers are estimated to occur in 10–15% of people with diabetes, and more than half of the lower-limb amputations occur in diabetic patients (4). Tissue oxygen appears to be the most relevant parameter for PVD, but it is not measured in clinical practice because of a lack of any direct and noninvasive tissue oximetry technique. Transcutaneous oximetry (TcO2) provides a measure of local soft-tissue oxygen content, but this measurement is indirect and has several limitations (36), and it is not clear how the measurements with the TcO2 technique relate to the actual tissue oxygenation. It measures the amount of oxygen available during local hyperthermia (local heating of tissue at 42–44°C), which is dependent on several factors such as systolic arterial blood pressure, arterial oxygen content, local blood flow, local oxygen consumption, and diffusion. All these factors are influenced by commonly occurring pathophysiologies such as local alterations in the vasculature, heart failure, respiratory diseases, infection, edema, and skin thickness. Because of the dependence on skin thickness, TcO2 measurements are generally made at prescribed locations on the dorsal surface of the foot, although ulcers form primarily on the plantar pressure-bearing surfaces. Direct measurements of the tissue pO2 at various sites within the affected areas can provide very useful information to the clinician, and this will facilitate the choice of therapy and the subsequent adjustments of the therapy, based on the pO2 data obtained with EPR oximetry.
Before initiating clinical measurements in patients with compromised tissues, we have been performing measurements in normal volunteers to demonstrate the feasibility of the oximetry approach and the ability of EPR to monitor tissue pO2 for prolonged durations. These measurements are being performed using India ink as the oximetry probe (8, 37, 38, 61). India ink is sensitive to changes in pO2, has a stable and predictable response to these changes, and is already used in the clinic as a fiducial marker for radiation therapy and to delineate lymphatics, and so on, in surgical procedures. The ink remains permanently in the tissue and can be used to make repeated pO2 measurements in the same tissue over time and throughout the course of treatments. Before measurements, the ink is sterilized using an autoclave, and a small volume (~20 μl) is injected into the tissue using a narrow-gauge needle (20–23 gauge). For these measurements, the volunteer is seated in a customized reclining chair that is equipped with a fixture for comfortably immobilizing the foot, centering the injection site with respect to the modulation coils, and holding the surface-loop resonator in position. All measurements are performed using a surface-loop resonator with a 10-mm detection loop. An adjustable warm-air system is used to maintain the surface temperature of the foot at 37°C. Data are typically collected for 5–10 min to measure the pO2 of the tissue, using sets of 10-s scans that can be either averaged to provide a mean value or used individually to characterize the dynamics. The observed line widths of the collected spectra are related directly to pO2 through an established calibration curve.
Measurements have been made with the normal volunteers at the metatarsal head on the plantar surface of the foot, a common location of diabetic ulcers, and also at the first interosseous space on the dorsal surface of the foot, the site where TcO2 measurements are often performed clinically. Measurements have been made in five volunteers to date, with measurements ongoing for each and the longest set of measurements carried out successfully over the last 4 years. In addition to monitoring the pO2 under normal conditions, we have used two perturbations to assess how the tissue responds to acute hyperoxygenation and hypoxia. Hyperoxygenation is initiated by increasing the fraction of inhaled oxygen to 100%. Acute hypoxia is established by impeding the flow of blood to the foot by compressing the thigh with a standard blood pressure cuff. The response of the tissue to such interventions may be useful in clinical application, as the pO2 response may be used to characterize the status of blood supply and O2 consumption in the tissue. Figures 8 and 9 show results for one of the volunteers, with ink injected at the metatarsal head, where measurements have been under way since March 2005. Figure 8 shows baseline pO2 levels and the response to compression. At each time point, compression has led to near anoxia, followed immediately by full recovery to the baseline pO2 level. In this volunteer, variation in the baseline level was observed over the total measurement period. It is possible that these variations could be due to actual acute and gradual changes in the tissue pO2, but we cannot rule out that the variations could be related to instability in the material calibration. We are currently investigating this issue through the collection of data with a more-extended population of volunteers. Figure 9 shows similar data for this volunteer, where 100% inhaled oxygen was applied. Again, baseline and recovery values are consistent, and a marked increase in tissue pO2 during the perturbation is observed.
FIG. 8. India ink was used to monitor the tissue pO2 at the metatarsal head on the plantar surface of the foot of a healthy volunteer.
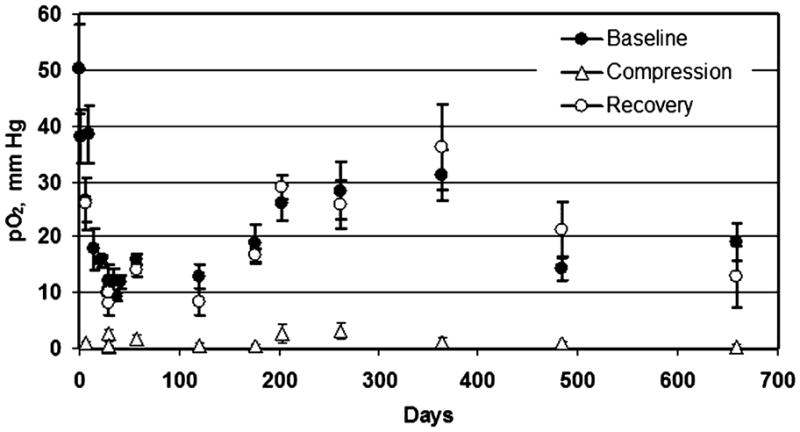
Baseline pO2 was measured for 10 min, followed by measurements during a 5-min period during which blood circulation to the foot was restricted and a 10-min recovery period. The foot temperature was maintained at 37°C with a warm-air blower in all the measurements. The pO2 values are shown as mean ± SD.
FIG. 9. The response of tissue pO2 to an increase in the fraction of inhaled oxygen was monitored using India ink injected at the metatarsal head on the plantar surface of the foot of a healthy volunteer.
Tissue pO2 was measured during a 10-min baseline period with the subject breathing room air, followed by a 10-min period during which the fraction of inhaled O2 was increased to 100%, and then a 10-min recovery period. The foot temperature was maintained at 37°C with a warm-air blower in all the measurements. The pO2 values are shown as mean ± SD.
These results indicate that EPR oximetry is a feasible way to measure and track peripheral tissue oxygenation. Using EPR oximetry, clinicians will be able to directly assess the status of these tissues in disease and monitor the effects of therapeutic intervention. After further testing with additional normal volunteers, and initial studies with diabetic patients, it is expected that this technique may be used to provide clinicians with direct and useful information to better manage peripheral vascular disease.
Clinical application of EPR oximetry for pO2 measurements in tumors
In view of the critical role that pO2 has in the response and prognosis of tumors, considerable research has been carried out to develop techniques that might be suitable for the measurement of tumor oxygenation or related parameters in patients (43, 61). However, the application of many of these methods for making repeated measurements is problematic. Studies in animal tumor models using EPR oximetry, however, demonstrated the feasibility of making such repeated measurements of pO2 during the course of radiotherapy. We recently initiated a tumor oximetry study in patients with tumors at depths of ≤1 cm from the surface. We also are at an advanced stage of planning for the use of implantable resonators, to increase the depth at which oxygen measurements can be made.
Data on changes in tumor pO2 over the course of radio- or chemotherapy would provide important clinical information on the probability that human tumors will respond to combined treatments using radiation or chemotherapy or both or antiangiogenic and vascular targeting approaches or surgery, or a combination of these, which could lead to changes of oxygen that would then affect the response to the subsequent therapy or therapies.
To allow the capabilities of EPR oximetry to be used in clinical studies of human tumors, it is necessary to demonstrate that the measurements be made successfully in tumors under clinically applicable conditions, and that the data are consistent with the expectation that such measurements will be clinically useful. Ongoing studies focus on demonstrating the ability to make repeated measurements with EPR oximetry to measure changes from “baseline” tumor pO2, following up this parameter over time within individual patients, and across groups of patients. Along with the absolute value of the baseline pO2, the measurements of time-dependent changes are likely to be very productive parameters for clinical decision making, providing information on the progression of tumors and the effects of treatment.
In such a feasibility study, it would be premature and perhaps limiting to make measurements and refinements of the technique in a restricted type of patients (e.g., with a specific tumor type in specific stages), so we have instead used patients who have tumors of which the geometry is suitable for the most straightforward measurements (i.e., within 10 mm of the surface), regardless of the prescribed treatment plans. Tumors suitable for this study include, but not are limited to, skin cancers, head and neck tumors (especially those with nodal metastases), lymphomas, sarcomas, and metastases to the skin from visceral sites. We are making measurements in these volunteers to demonstrate the feasibility of making repeated measurements both continuously over short intervals and intermittently through an entire course of therapy. These ongoing studies will provide some of the first systematic data on tumor oxygen levels during the course of clinical radiation-therapy protocols. The initial clinical studies have been performed in two general groups of patients. The first group comprises patients with advanced melanoma with cutaneous lesions, where the measurements are relatively simple and the lesions are geometrically similar. Treatments for these patients have generally included systemic chemotherapy and surgical resection. The second group of patients includes those with superficial tumors who are undergoing radiation therapy, where the effects of radiation and tumor growth on tumor pO2 can be monitored.
In these clinical studies, between 10 and 20 μl of India ink (8) is injected into the tumor tissue at the sites of interest. The ink is biologically inert and sterilized before injection by autoclaving. The ink is injected using a 23-gauge needle and microsyringe under sterile conditions, with optional local anesthesia. In the studies involving melanoma lesions, the ink is deposited in the tumor tissue at a depth of ~2–3 mm. In the studies of other superficial tumors, where a range of tumor types and depths will be studied, the ink can be deposited at depths of up to 10 mm below the skin surface.
The data-acquisition procedures for EPR tumor oximetry are similar to those used successfully in our prior clinical studies (37, 61). Volunteers are positioned centrally in the clinical EPR magnet in either a seated or a lying position, dependent on the site of the measurement. Additional pillows and supports are used to maximize patient comfort, immobilize the tissue, and isolate the measurement site from diaphragmatic motion. Care is taken to provide optimal instrumental access to the measurement site. A surface-loop resonator is placed on the surface of the tumor with the detection loop centered above the ink deposit of interest. The surface temperature of the patient at the measurement site is monitored and maintained using warm air. The resonator is externally supported using an assortment of holders to prevent perturbation of blood flow and tissue pO2pO2 by excess pressure of the resonator on the tumor surface. Continuous-wave (CW) EPR spectra are recorded using instrumental settings that are optimized for the observed physiologic and anatomic conditions. Typically, sets of spectra are collected, with periods of 3–10 s, over a 5- to 10-min period, making it feasible to obtain data on the time course of changes or more-precise estimates of the average pO2 or both. Spectra are analyzed using least-squares spectral fitting with a double-lorentzian spectral model, and the observed peak-to-peak line width is converted to pO2 according to an oxygen-sensitivity calibration curve (8, 37).
To date, measurements have been performed in five volunteers, involving two melanoma tumors, one soft tissue sarcoma, one superficial lymphoma, and normal tissue within the irradiated tissue volume. A description of our first human tumor measurement, which was performed in a melanoma, has been published (38). Figure 10 shows a set of spectra acquired with a different volunteer, in which ink was injected in the tissue of a subcutaneous lymphoma tumor.
FIG. 10. Response of tumor pO2 to breathing 100% oxygen in the volunteer with a subcutaneous lymphoma.
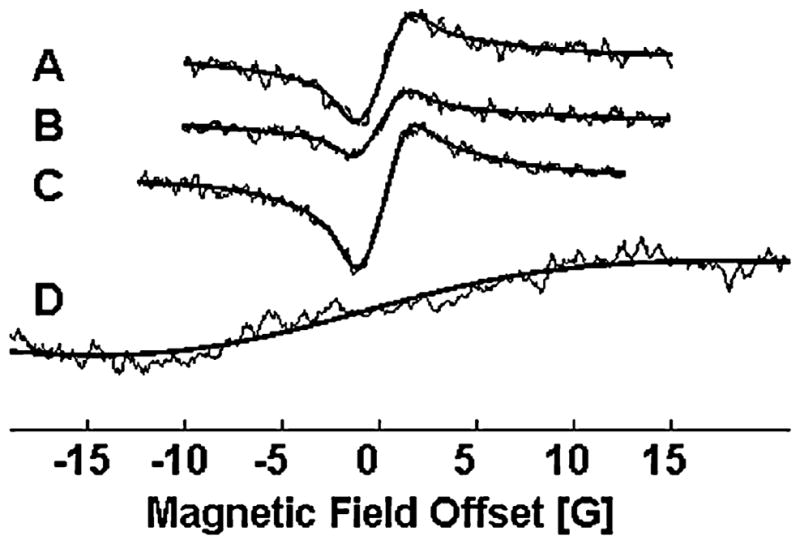
Spectra A–C are consecutive averages of 60 scans (4 s each) with the volunteer breathing room air (pO2 = 4.1 ± 0.1 mm Hg). These were followed by another acquisition of 60 4-s scans with the volunteer breathing 100% O2, after a 10-min equilibration period, during which the pO2 increased to ~150 mm Hg.
This tumor was located centrally on the posterior surface of the right thigh, and formed an open lesion with a necrotic core surrounded by viable tumor tissue. Ink was deposited in tumor tissue on the medial rim of the lesion. For these measurements, the volunteer was in a prone position with the tumor centered in the magnet volume. Sets of spectra were collected under baseline conditions, while the volunteer breathed room air, and then repeated after the volunteer had acclimated to breathing 100% O2. Each data set was acquired over a 4-min period, with 60 separate sweeps of the magnetic field. A stable baseline pO2 of 4.1 mm Hg was observed, which increased significantly when the patient was breathing 100% O2. These results, and similar results measured in other volunteers, indicate that it is likely that EPR oximetry can be used successfully in the clinical setting to measure tumor pO2 in humans.
CONCLUSIONS
Our extensive animal studies highlight the applicability and usefulness of EPR oximetry for repeated measurements of oxygen in various tissues. The unique advantage of this approach is its capability to make repeated measurements of oxygen from the same sites with little or no invasion.
The most exciting and promising area for the application of EPR oximetry is in clinical applications. It now has been demonstrated that measurements can be made successfully in human subjects. It will be essential to demonstrate that such measurements can provide clinically useful information. This can occur both by use of the present capabilities and by enhancement of the capabilities through additional technical developments. Ample reason exists for optimism in both regards.
The present capabilities appear to be very suitable for a number of significant applications, where the targets of interest are within a few millimeters of the surface, and it is reasonable to place paramagnetic India ink directly into the tissue. One of the areas of immediate promise is for the use of in vivo EPR to obtain crucial data on acute or chronic ischemic diseases. The largest and most feasible application is to monitor the status of peripheral vascular disease in diabetic patients. The oxygenation of the tissues of the foot and leg is the critical parameter that determines the viability of the tissues and their susceptibility to infection and tendency to necrosis. Currently no other methods provide direct measurements of the pO2 in the tissues at risk, whereas in vivo EPR oximetry has this potential. In principle, the measurement of oxygen at selected sites in the foot and leg could be done routinely after the placement of India ink at these sites. It also should be feasible to obtain indications of functional status at the sites by simple maneuvers such as breathing in oxygen-enriched gas mixtures. These data would enable clinicians to have an objective measure of the efficacy of therapeutic regimes as well as providing indications of deteriorating conditions in critical tissues.
Another area of immediate application is pO2 measurements in suitably located tumors. Such measurements provide an opportunity to obtain unique and clinically important information: the level of oxygen in the tumor and how it varies over time, especially during the course of treatment. When the treatment is radiation therapy only, this capability makes it feasible to tailor the delivery of the radiation so that the doses are given at times of optimal oxygenation. In vivo EPR has the capability to do this with each patient, enabling the treating physician to schedule the delivery of radiation at the times that will provide the best therapeutic efficacy. This capability also should enhance the ability to develop optimal modifications of standard therapy, where the dose per fraction or the intervals between fractions is varied (or both); such modifications are being increasingly used to enhance therapeutic effectiveness or for greater convenience of patients, especially for a palliative therapy. This may be very important because, although a vast amount of empiric evidence exists on the efficacy of standard fractionation schemes, no such data base is available for the new fractionation schemes that are increasingly being used. Similar considerations apply to the development of new therapeutic approaches that combine radiation therapy with chemotherapy and/or surgery. Each modality has the potential both to have its efficacy affected by the level of oxygen and to affect those levels. Using the information available with in vivo EPR oximetry, the timing and sequence of the treatments could be optimized to obtain the best therapeutic ratio.
The third area of early clinical applications is likely to be monitoring wound healing. This is another area where the local oxygenation is a crucial parameter that provides an indication as to whether the wound will heal effectively and could be used to monitor the effectiveness of therapeutic interventions.
Although a number of potential significant enhancements of the capabilities of in vivo EPR are known, the one that is likely to have the greatest impact at an early date is the development of the implantable resonator. This will enable measurements to be made at essentially any depth, and after the treatment regimen is completed in several weeks or months, the implanted resonator can be readily removed through a small incision. We are preparing to initiate the first such studies to measure oxygen in deeper head and neck tumors.
Another technical advance that is likely to become available in the near future includes the use of oximetric probes with enhanced sensitivity that can be used in human subjects by encapsulating them in biocompatible containers that can be removed. With the recent development of a whole-body electromagnet that can accommodate human subjects (the current clinical studies at Dartmouth use a permanent magnet) with a large gap with a large region of high magnetic homogeneity, it should become feasible to use various other frequencies that may be optimal for particular applications.
Acknowledgments
We thank our collaborators, Dr. Ebo D. DeMuinck, Dr. Armin Helisch from Angiogenesis Research Center, Dartmouth-Hitchcock Medical Center, Lebanon, NH; Dr. Harriet W. Hopf, Dr. Noah Rosen, Department of Anesthesiology, University of Utah; Dr. William M. Chilian, Dr. Petra Rocic, and Dr. Barry Potter, Louisiana State University Health Sciences Center for the collaborative results included in this article. This study was supported by NIH grants CA120919, CA118069, DK072112, and CA121593 and used the facilities of The EPR Center for the Study of Viable Systems (P41 EB002032).
ABBREVIATIONS
- ATSM
Diacetyl-bis(N4-methylthiosemicarbazone)
- BOLD imaging
blood oxygen level ependent imaging
- EPR spectroscopy
electron paramagnetic resonance spectroscopy
- HSR-MS
high spatial resolution–multi site
- LAD
left anterior descending
- LHL
Left hindlimb
- LiPc
lithium phthalocyanine
- NIR
near infrared
- NMR
nuclear magnetic resonance
- PVD
peripheral vascular disease
- RHL
right hindlimb
- RIF
radiation-induced fibrosarcoma
- SAR
specific absorption rate
- TcO2
transcutaneous oximetry
- WT
wild type
Footnotes
EPR Center for Viable Systems, Dartmouth Medical School, Hanover, NH.
References
- 1.Abramovic Z, Sentjurc M, Kristl J, Khan N, Hou H, Swartz HM. Influence of different anesthetics on skin oxygenation studied by electron paramagnetic resonance in vivo. Skin Pharmacol Physiol. 2006;20:77–84. doi: 10.1159/000097654. [DOI] [PubMed] [Google Scholar]
- 2.Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med. 2002;48:980–986. doi: 10.1002/mrm.10318. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartl H, Zimelka W, Lubbers DW. Evaluation of pO2 profiles to describe the oxygen pressure field within the tissue. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:75–85. doi: 10.1016/s1095-6433(01)00532-3. [DOI] [PubMed] [Google Scholar]
- 4.Borssen B, Bergenheim T, Lithner F. The epidemiology of foot lesions in diabetic patients aged 15–50 years. Diabetes Med. 1990;7:438–444. doi: 10.1111/j.1464-5491.1990.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 5.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 6.Cavaglia M, Dombrowski SM, Drazba J, Vasanji A, Bokesch PM, Janigro D. Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 2001;910:81–93. doi: 10.1016/s0006-8993(01)02637-3. [DOI] [PubMed] [Google Scholar]
- 7.Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003;420:217–221. doi: 10.1016/j.abb.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Charlier N, Beghein N, Gallez B. Development and evaluation of biocompatible inks for the local measurement of oxygen using in vivo EPR. NMR Biomed. 2004;17:303–310. doi: 10.1002/nbm.902. [DOI] [PubMed] [Google Scholar]
- 9.Demidenko EZ. Mixed Models: Theory and Applications. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- 10.Dunn JF, Swartz HM. In vivo electron paramagnetic resonance oximetry with particulate materials. Methods. 2003;30:159–166. doi: 10.1016/s1046-2023(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 11.Friedman BJ, Grinberg OY, Isaacs KA, Ruuge EK, Swartz HM. Effect of repetitive ischemia on myocardial oxygen tension in isolated perfused and hypoperfused rat hearts. Magn Reson Med. 1996;35:214–220. doi: 10.1002/mrm.1910350213. [DOI] [PubMed] [Google Scholar]
- 12.Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin dependent diabetes. In: Harris M, Cowie C, Stern M, Boyko E, Reiber G, Bennett P, editors. Diabetes in America. 1985. pp. 233–258. [Google Scholar]
- 13.Gobel U, Theilen H, Kuschinsky W. Congruence of total and perfused capillary network in rat brains. Circ Res. 1990;66:271–281. doi: 10.1161/01.res.66.2.271. [DOI] [PubMed] [Google Scholar]
- 14.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature. 1996;379 doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 15.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OCA. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 16.Grinberg OY, Friedman BJ, Swartz HM. Intramyocardial pO2 measured by EPR. Adv Exp Med Biol. 1997;428:261–268. doi: 10.1007/978-1-4615-5399-1_36. [DOI] [PubMed] [Google Scholar]
- 17.Grinberg OY, Grinberg SA, Friedman BJ, Swartz HM. Myocardial oxygen tension and capillary density in the isolated perfused rat heart during pharmacological intervention. Adv Exp Med Biol. 1997;411:171–181. doi: 10.1007/978-1-4615-5865-1_21. [DOI] [PubMed] [Google Scholar]
- 18.Grinberg OY, Hou H, Grinberg SA, Moodie KL, Demidenko E, Friedman BJ, Post MJ, Swartz HM. pO2 and regional blood flow in a rabbit model of limb ischemia. Physiol Meas. 2004;25:659. doi: 10.1088/0967-3334/25/3/006. [DOI] [PubMed] [Google Scholar]
- 19.Grinberg OY, Smirnov AI, Swartz HM. High spatial resolution multi-site EPR oximetry: the use of convolution-based fitting method. J Magn Reson. 2001;152:247–258. doi: 10.1006/jmre.2001.2408. [DOI] [PubMed] [Google Scholar]
- 20.Grinberg VO, Smirnov AI, Grinberg OY, Grinberg SA, O’Hara JA, Swartz HM. Practical experimental conditions and limitations for high-spatial-resolution multisite EPR oximetry. Appl Magn Reson. 2005;28:69–78. [Google Scholar]
- 21.Hall EJ. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 22.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 23.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 24.Hershey JC, Baskin EP, Glass JD, Hartman HA, Gilberto DB, Rogers IT, Cook JJ. Revascularization in the rabbit hindlimb: dissociation between capillary sprouting and arteriogenesis. Cardiovasc Res. 2001;49:618–625. doi: 10.1016/s0008-6363(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 25.Hockel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, Knapstein PG, Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 26.Hou H, Grinberg O, Grinberg S, Swartz H. Cerebral tissue oxygenation in reversible focal ischemia in rats: multi-site EPR oximetry measurements. Physiol Meas. 2005;26 doi: 10.1088/0967-3334/26/1/012. [DOI] [PubMed] [Google Scholar]
- 27.Hou H, Grinberg OY, Grinberg SA, Khan N, Dunn JF, Swartz HM. Cerebral PtO2, acute hypoxia, and volatile anesthetics in the rat brain. Adv Exp Med Biol. 2005;566:179–185. doi: 10.1007/0-387-26206-7_25. [DOI] [PubMed] [Google Scholar]
- 28.Hou H, Grinberg OY, Taie S, Leichtweis S, Miyake M, Grinberg S, Xie H, Csete M, Swartz HM. Electron paramagnetic resonance assessment of brain tissue oxygen tension in anesthetized rats. Anesth Analg. 2003;96:1467–1472. doi: 10.1213/01.ANE.0000055648.41152.63. [DOI] [PubMed] [Google Scholar]
- 29.Hou H, Khan N, O’Hara JA, Grinberg OY, Dunn JF, Abajian MA, Wilmot CM, Demidenko E, Lu S, Steffen RP, Swartz HM. Increased oxygenation of intracranial tumors by EFAPROXYN (efaproxiral), an allosteric hemoglobin modifier: in vivo EPR oximetry study. Int J Radiat Oncol Biol Phys. 2005;61:1503–1509. doi: 10.1016/j.ijrobp.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 30.Hou H, Khan N, O’Hara JA, Grinberg OY, Dunn JF, Abajian MA, Wilmot CM, Makki M, Demidenko E, Lu S, Steffen RP, Swartz HM. Effect of RSR13, an allosteric hemoglobin modifier, on oxygenation in murine tumors: an in vivo electron paramagnetic resonance oximetry and BOLD MRI study. Int J Radiat Oncol Biol Phys. 2004;59:834–843. doi: 10.1016/j.ijrobp.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 31.Hunjan S, Zhao D, Constantinescu A, Hahn EW, Antich PP, Mason RP. Tumor oximetry: demonstration of an enhanced dynamic mapping procedure using fluorine-19 echo planar magnetic resonance imaging in the dunning prostate r3327-AT1 rat tumor. Int J Radiat Oncol Biol Phys. 2001;49:1097–1108. doi: 10.1016/s0360-3016(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 32.Hunt TK, Ellison EC, Sen CK. Oxygen: at the foundation of wound healing: introduction. World J Surg. 2004;28:291–293. doi: 10.1007/s00268-003-7405-x. [DOI] [PubMed] [Google Scholar]
- 33.James PE, Bacic G, Grinberg OY, Goda F, Dunn JF, Jackson SK, Swartz HM. Endotoxin-induced changes in intrarenal pO2, measured by in vivo electron paramagnetic resonance oximetry and magnetic resonance imaging. Free Radic Biol Med. 1996;21:25–34. doi: 10.1016/0891-5849(95)02221-x. [DOI] [PubMed] [Google Scholar]
- 34.James PE, Goda F, Grinberg OY, Szybinski KG, Swartz HM. Intrarenal pO2 measured by EPR oximetry and the effects of bacterial endotoxin. Adv Exp Med Biol. 1997;411:557–568. doi: 10.1007/978-1-4615-5865-1_69. [DOI] [PubMed] [Google Scholar]
- 35.Jiang J, Nakashima T, Liu KJ, Goda F, Shima T, Swartz HM. Measurement of pO2 in liver using EPR oximetry. J Appl Physiol Respir Environ Exerc Physiol. 1996;80:552–558. doi: 10.1152/jappl.1996.80.2.552. [DOI] [PubMed] [Google Scholar]
- 36.Jorneskog G, Djavani K, Brismar K. Day-to-day variability of transcutaneous oxygen tension in patients with diabetes mellitus and peripheral arterial occlusive disease. J Vasc Surg. 2001;34:277–282. doi: 10.1067/mva.2001.115799. [DOI] [PubMed] [Google Scholar]
- 37.Khan N, Hou H, Hein P, Comi RJ, Buckey JC, Grinberg O, Salikhov I, Lu SY, Wallach H, Swartz HM. Black magic and EPR oximetry: from lab to initial clinical trials. Adv Exp Med Biol. 2005;566:119–126. doi: 10.1007/0-387-26206-7_17. [DOI] [PubMed] [Google Scholar]
- 38.Khan N, Williams BB, Swartz HM. Clinical applications of in vivo EPR: rationale and initial results. Appl Magn Reson. 2006;30:185–199. [Google Scholar]
- 39.Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, Cook JA, Golman K, Ardenkjaer P, Larsen JH, Subramanian S, Mitchell JB. Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A. 2002;99:2216–2221. doi: 10.1073/pnas.042671399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lance AL. Introduction to Microwave Theory and Measurements. New York: McGraw-Hill; 1964. [Google Scholar]
- 41.Lei H, Grinberg O, Nwaigwe CI, Hou HG, Williams H, Swartz HM, Dunn JF. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 2001;913:174–179. doi: 10.1016/s0006-8993(01)02786-x. [DOI] [PubMed] [Google Scholar]
- 42.Lierse W. Capillary density in the vertebrate brain. Acta Anat (Basel) 1963;54:1–31. [PubMed] [Google Scholar]
- 43.Lubbers DW, Baumgartl H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kidney Int. 1997;51:372–380. doi: 10.1038/ki.1997.49. [DOI] [PubMed] [Google Scholar]
- 44.Madhani M, Barchowsky A, Klei L, Ross CR, Jackson SK, Swartz HM, James PE. Antibacterial peptide PR-39 affects local nitric oxide and preserves tissue oxygenation in the liver during septic shock. Biochim Biophys Acta. 2002;1588:232–240. doi: 10.1016/s0925-4439(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 45.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 46.Nozue M, Lee I, Yuan F, Teicher BA, Brizel DM, Dewhirst MW, Milross CG, Milas L, Song CW, Thomas CD, Guichard M, Evans SM, Koch CJ, Lord EM, Jain RK, Suit HD. Interlaboratory variation in oxygen tension measurement by Eppendorf “histograph” and comparison with hypoxic marker. J Surg Oncol. 1997;66:30–38. doi: 10.1002/(sici)1096-9098(199709)66:1<30::aid-jso7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 47.O’Hara JA, Blumenthal RD, Grinberg OY, Demidenko E, Grinberg S, Wilmot CM, Taylor AM, Goldenberg DM, Swartz HM. Response to radioimmunotherapy correlates with tumor pO2 measured by EPR oximetry in human tumor xenografts. Radiat Res. 2001;155:466–473. doi: 10.1667/0033-7587(2001)155[0466:rtrcwt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 48.O’Hara JA, Hou H, Demidenko E, Springett RJ, Khan N, Swartz HM. Simultaneous measurement of rat brain cortex PtO2 using EPR oximetry and a fluorescence fiber-optic sensor during normoxia and hyperoxia. Physiol Meas. 2005;26:203–213. doi: 10.1088/0967-3334/26/3/006. [DOI] [PubMed] [Google Scholar]
- 49.O’Hara JA, Khan N, Hou H, Wilmo CM, Demidenko E, Dunn JF, Swartz HM. Comparison of EPR oximetry and Eppendorf polarographic electrode assessments of rat brain PtO2. Physiol Meas. 2004;25:1413–1423. doi: 10.1088/0967-3334/25/6/007. [DOI] [PubMed] [Google Scholar]
- 50.Pogue BW, O’Hara JA, Goodwin IA, Wilmot CJ, Fournier GP, Akay AR, Swartz H. Tumor pO2 changes during photodynamic therapy depend upon photosensitizer type and time after injection. Comp Biochem Physiol Part a, Mol Integr Physiol. 2002;132:177–184. doi: 10.1016/s1095-6433(01)00545-1. [DOI] [PubMed] [Google Scholar]
- 51.Rudat V, Stadler P, Becker A, Vanselow B, Dietz A, Wannenmacher M, Molls M, Dunst J, Feldmann HJ. Predictive value of the tumor oxygenation by means of pO2 histography in patients with advanced head and neck cancer. Strahlenther Onkol. 2001;177:462–468. doi: 10.1007/pl00002427. [DOI] [PubMed] [Google Scholar]
- 52.Salikhov I, Walczak T, Lesniewski P, Khan N, Iwasaki A, Comi R, Buckey J, Swartz HM. EPR spectrometer for clinical applications. Magn Reson Med. 2005;54:1317–1320. doi: 10.1002/mrm.20689. [DOI] [PubMed] [Google Scholar]
- 53.Salikhov IK, Swartz HM. Measurement of specific absorption rate for clinical EPR at 1200 MHz. Appl Magn Reson. 2005;28:287–291. [Google Scholar]
- 54.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 55.Smirnov AI, Norby SW, Clarkson RB, Walczak T, Swartz HM. Simultaneous multi-site EPR spectroscopy in vivo. Magn Reson Med. 1993;30:213–220. doi: 10.1002/mrm.1910300210. [DOI] [PubMed] [Google Scholar]
- 56.Swartz HM. The measurement of oxygen in vivo using EPR techniques. In: Berliner LJ, editor. Biological Magnetic Resonance, Volume 18: In Vivo EPR (ESR): Theory and Applications. New York: Plenum Publishing; 2003. pp. 403–440. [Google Scholar]
- 57.Swartz HM. Potential medical (clinical!!) applications of EPR: overview and perspectives. In: Berliner LJ, editor. Biological Magnetic Resonance, Volume 18: In Vivo EPR (ESR): Theory and Applications. New York: Plenum Publishing; 2003. pp. 599–621. [Google Scholar]
- 58.Swartz HM, Berliner LJ. In: In vivo EPR: Foundations of Modern EPR. Eaton SS, Eaton GR, Salikhov KM, editors. New Jersey: World Scientific Publishing; 1998. pp. 361–378. [Google Scholar]
- 59.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Phys Med Biol. 1998;43:1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 60.Swartz HM, Halpern H. EPR studies of living animals and related model systems (in vivo EPR) Biol Magn Reson. 1998;14:367–404. [Google Scholar]
- 61.Swartz HM, Khan N, Buckey J, Comi R, Gould L, Grinberg O, Hartford A, Hopf H, Hou H, Hug E, Iwasaki A, Lesniewski P, Salikhov I, Walczak T. Clinical applications of EPR: overview and perspectives. NMR Biomed. 2004;17:335–351. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- 62.Swartz HM, Taie S, Miyake M, Grinberg OY, Hou H, el-Kadi H, Dunn JF. The effects of anesthesia on cerebral tissue oxygen tension: use of EPR oximetry to make repeated measurements. Adv Exp Med Biol. 2003;530:569–575. doi: 10.1007/978-1-4615-0075-9_55. [DOI] [PubMed] [Google Scholar]
- 63.Tandara AA, Mustoe TA. Oxygen in wound healing: more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 64.Towner RA, Sturgeon SA, Khan N, Hou H, Swartz HM. In vivo assessment of nodularin-induced hepatotoxicity in the rat using magnetic resonance techniques (MRI, MRS and EPR oximetry) Chemico-Biol Interact. 2002;139:231–250. doi: 10.1016/s0009-2797(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 65.Unthank JL, Nixon JC, Lash JM. Early adaptations in collateral and microvascular resistances after ligation of the rat femoral artery. J Appl Physiol. 1995;79:73–82. doi: 10.1152/jappl.1995.79.1.73. [DOI] [PubMed] [Google Scholar]
- 66.Vaupel P, Mayer A. Hypoxia and anemia: effects on tumor biology and treatment resistance. Transfus Clin Biol. 2005;12:5–10. doi: 10.1016/j.tracli.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Warnholtz A, Wendt M, August M, Munzel T. Clinical aspects of reactive oxygen and nitrogen species. Biochem Soc Symp. 2004:121–133. doi: 10.1042/bss0710121. [DOI] [PubMed] [Google Scholar]
- 68.Williams BB, Hou H, Grinberg O, Demidenko E, Swartz H. High spatial resolution multi-site EPR oximetry of transient focal cerebral ischemia in the rat. Antioxid Redox Signaling. doi: 10.1089/ars.2007.1723. in press. [DOI] [PubMed] [Google Scholar]
- 69.Young WK, Vojnovic B, Wardman P. Measurement of oxygen tension in tumours by time-resolved fluorescence. Br J Cancer Suppl. 1996;27:S256–S259. [PMC free article] [PubMed] [Google Scholar]