Abstract
Bile salts and phospholipids are both required to solubilize biliary cholesterol. Since interruption of the enterohepatic circulation (EHC) depletes bile of bile salts, we have examined in the rhesus monkey the effects of controlled interruption of the EHC on biliary secretion of bile salt, phospholipid, and cholesterol and on the relative proportions of these components in bile.
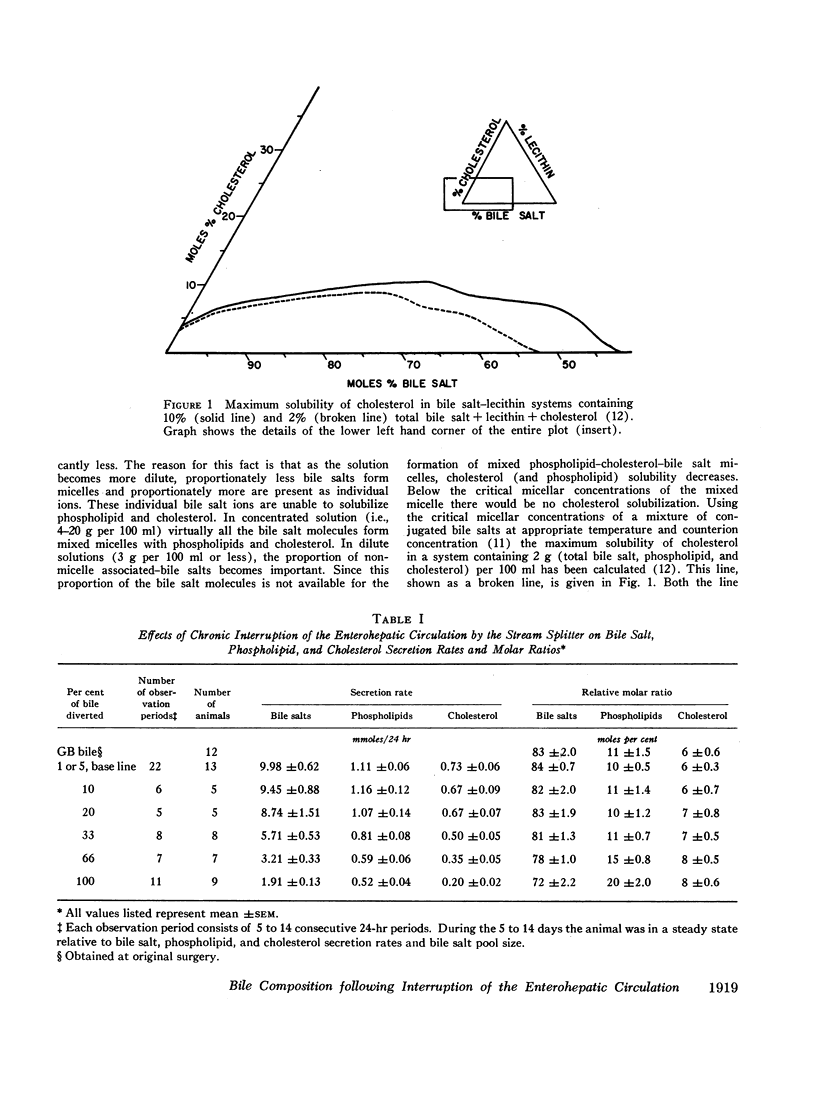
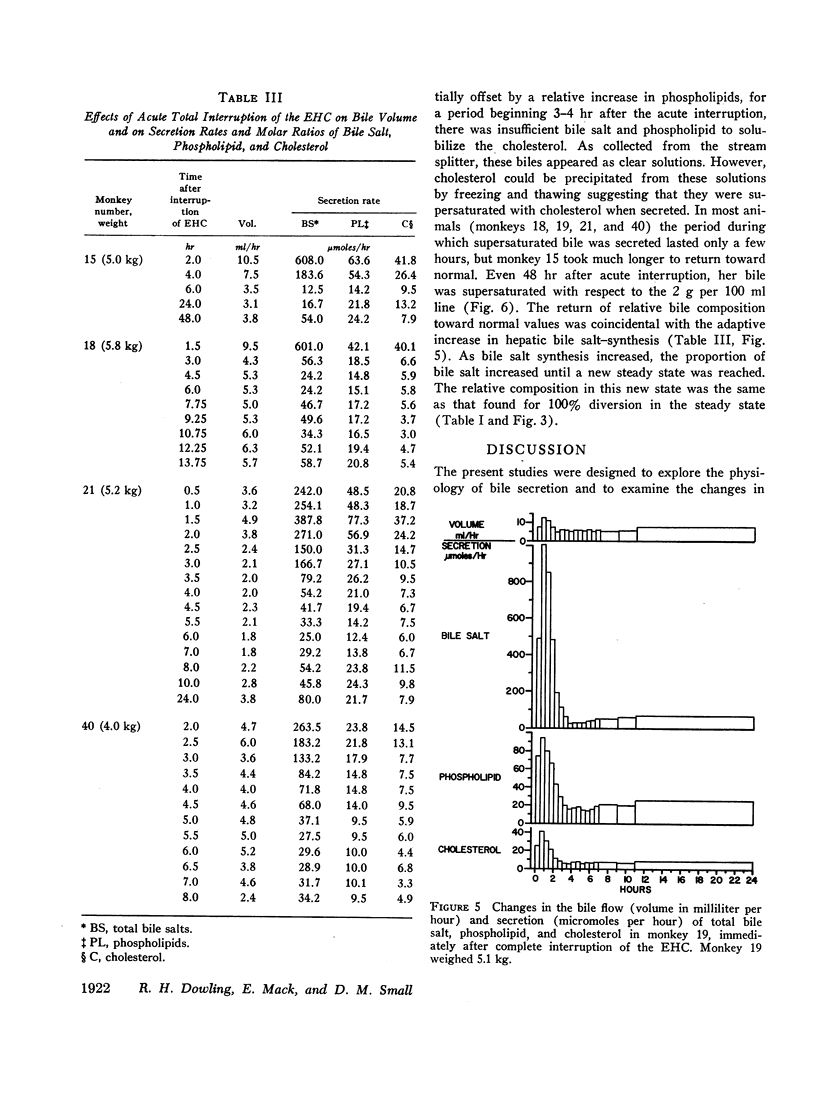
Immediately after complete interruption of the EHC, bile secretion and bile composition remained normal for 2-3 hr. During the next 3 hr, however, secretion of all components decreased. Bile salt decreased to a greater extent than phospholipid and cholesterol, and the bile was now supersaturated with cholesterol. 12-24 hr after interruption of the EHC, a new steady state was reached in which there was a relative deficiency of bile salt and a relative increase in phospholipid and cholesterol. The resulting bile, although somewhat more saturated with cholesterol, was not supersaturated with cholesterol but was stable with respect to cholesterol solubility. Thus, bile instability conducive to gallstone formation occurs transiently within hours after interruption of the EHC. Prolonged large interruptions in the steady state animal also produce a relative bile salt deficiency, but in this situation cholesterol remains soluble in the bile of these animals because there occurs a concomitant relative increase in phospholipid.
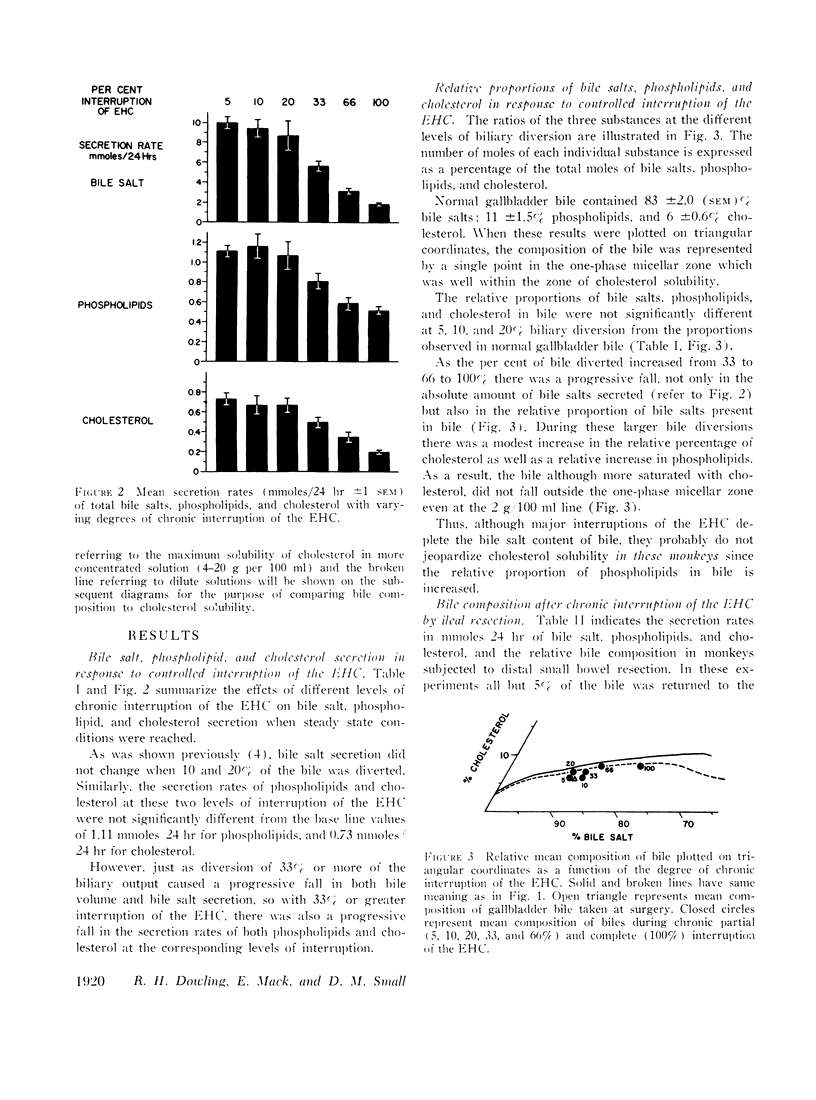
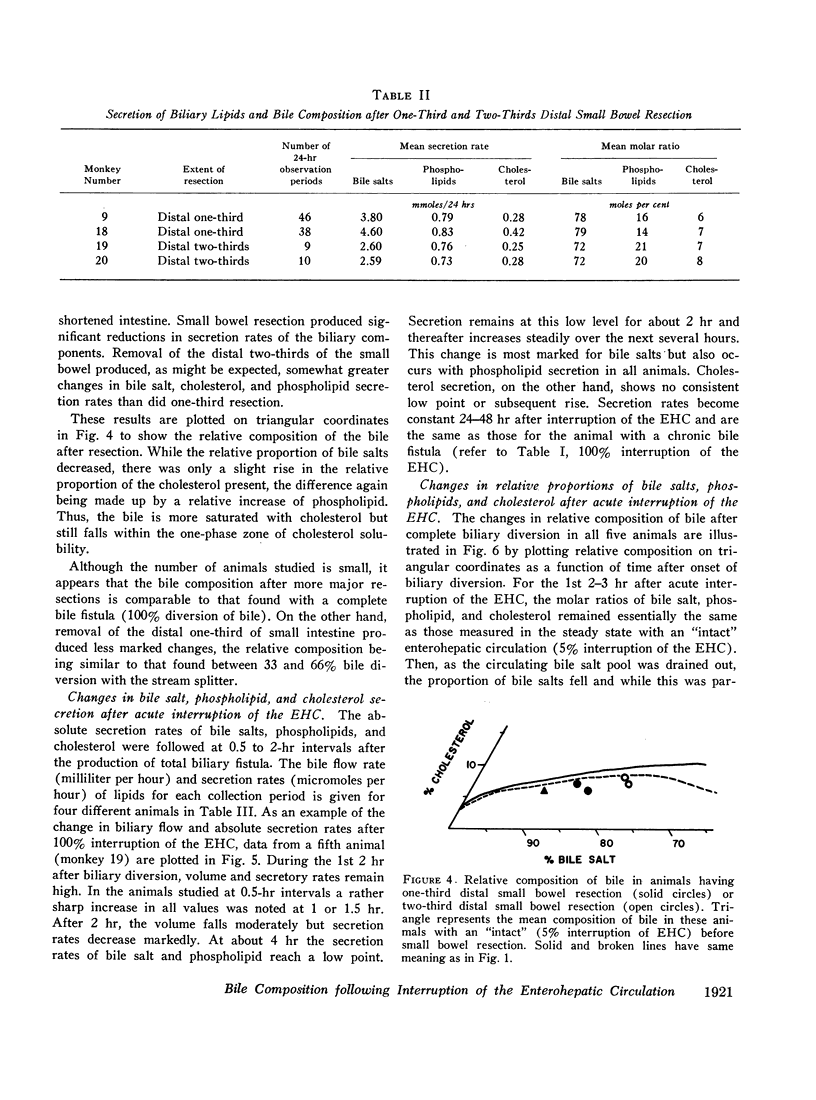
When the EHC was only partially interrupted, secretion rates and the relative concentration of bile salt, phospholipid, and cholesterol did not change significantly from control values until more than 20% of the bile was diverted. Modest changes in the relative composition of bile occurred when 33 and 66% of the bile was diverted, and these changes were very similar to those produced by resection of the distal small bowel.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bergman F., van der Linden W., Sjövall J. Biliary bile acids and hepatic ultra-structure in hamsters fed gallstone-inducing and -dissolving diets. Acta Physiol Scand. 1968 Nov;74(3):480–491. doi: 10.1111/j.1748-1716.1968.tb04256.x. [DOI] [PubMed] [Google Scholar]
- Bourgès M., Small D. M., Dervichian D. G. Biophysics of lipid associations. 3. The quaternary systems lecithin-bile salt-cholesterol-water. Biochim Biophys Acta. 1967 Oct 2;144(2):189–201. [PubMed] [Google Scholar]
- CARR J. J., DREKTER I. J. Simplified rapid technic for the extraction and determination of serum cholesterol without saponification. Clin Chem. 1956 Oct;2(5):353–368. [PubMed] [Google Scholar]
- Carey M. C., Small D. M. Micellar properties of dihydroxy and trihydroxy bile salts: effects of counterion and temperature. J Colloid Interface Sci. 1969 Nov;31(3):382–396. doi: 10.1016/0021-9797(69)90181-7. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kpplan M., Gottlieb L., Patterson J. Liver disease and gallstones in regional enteritis. Gastroenterology. 1971 Feb;60(2):237–245. [PubMed] [Google Scholar]
- Comess L. J., Bennett P. H., Burch T. A. Clinical gallbladder disease in Pima Indians. Its high prevalence in contrast to Framingham, Massachusetts. N Engl J Med. 1967 Oct 26;277(17):894–898. doi: 10.1056/NEJM196710262771702. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Mack E., Picott J., Berger J., Small D. M. Experimental model for the study of the enterohepatic circulation of bile in rhesus monkeys. J Lab Clin Med. 1968 Jul;72(1):169–176. [PubMed] [Google Scholar]
- Dowling R. H., Mack E., Small D. M. Effects of controlled interruption of the enterohepatic circulation of bile salts by biliary diversion and by ileal resection on bile salt secretion, synthesis, and pool size in the rhesus monkey. J Clin Invest. 1970 Feb;49(2):232–242. doi: 10.1172/JCI106232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSSON S. Biliary excretion of bile acids and cholesterol in bile fistula rats; bile acids and steroids. Proc Soc Exp Biol Med. 1957 Mar;94(3):578–582. doi: 10.3181/00379727-94-23018. [DOI] [PubMed] [Google Scholar]
- Heaton K. W., Read A. E. Gall stones in patients with disorders of the terminal ileum and disturbed bile salt metabolism. Br Med J. 1969 Aug 30;3(5669):494–496. doi: 10.1136/bmj.3.5669.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F. The syndrome of ileal disease and the broken enterohepatic circulation: cholerheic enteropathy. Gastroenterology. 1967 Apr;52(4):752–757. [PubMed] [Google Scholar]
- KAY R. E., ENTENMAN C. Stimulation of taurocholic acid synthesis and biliary excretion of lipids. Am J Physiol. 1961 Apr;200:855–859. doi: 10.1152/ajplegacy.1961.200.4.855. [DOI] [PubMed] [Google Scholar]
- LIGHT H. G., WITMER C., VARS H. M. Interruption of the enterohepatic cirucaltion and its effect on rat bile. Am J Physiol. 1959 Dec;197:1330–1332. doi: 10.1152/ajplegacy.1959.197.6.1330. [DOI] [PubMed] [Google Scholar]
- Nilsson S., Scherstén T. Importance of bile acids for phospholipid secretion into human hepatic bile. Gastroenterology. 1969 Nov;57(5):525–532. [PubMed] [Google Scholar]
- Sampliner R. E., Bennett P. H., Comess L. J., Rose F. A., Burch T. A. Gallbladder disease in pima indians. Demonstration of high prevalence and early onset by cholecystography. N Engl J Med. 1970 Dec 17;283(25):1358–1364. doi: 10.1056/NEJM197012172832502. [DOI] [PubMed] [Google Scholar]
- Schoenfield L. J., Sjövall J. Bile acids and cholesterol in guinea pigs with induced gallstones. Am J Physiol. 1966 Nov;211(5):1069–1074. doi: 10.1152/ajplegacy.1966.211.5.1069. [DOI] [PubMed] [Google Scholar]
- Small D. M., Bourgès M., Dervichian D. G. Ternary and quaternary aqueous systems containing bile salt, lecithin, and cholesterol. Nature. 1966 Aug 20;211(5051):816–818. doi: 10.1038/211816a0. [DOI] [PubMed] [Google Scholar]
- Small D. M., Rapo S. Source of abnormal bile in patients with cholesterol gallstones. N Engl J Med. 1970 Jul 9;283(2):53–57. doi: 10.1056/NEJM197007092830201. [DOI] [PubMed] [Google Scholar]
- Small D. M. The formation of gallstones. Adv Intern Med. 1970;16:243–264. [PubMed] [Google Scholar]
- Swell L., Bell C. C., Jr, Entenman C. Bile acids and lipid metabolism. 3. Influence of bile acids on phospholipids in liver and bile of the isolated perfused dog liver. Biochim Biophys Acta. 1968 Oct 22;164(2):278–284. [PubMed] [Google Scholar]
- TALALAY P. Enzymic analysis of steroid hormones. Methods Biochem Anal. 1960;8:119–143. doi: 10.1002/9780470110249.ch3. [DOI] [PubMed] [Google Scholar]
- THOMPSON J. C., VARS H. M. Biliary excretion of cholic acid and cholesterol in hyper-, hypo-, and euthyroid rats. Proc Soc Exp Biol Med. 1953 Jun;83(2):246–248. doi: 10.3181/00379727-83-20320. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Buhac I., Farrar J. T., Swell L. Diminished bile acid pool size in patients with gallstones. Gastroenterology. 1970 Aug;59(2):165–173. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C., Jr, Swell L. Significance of the liver in the production of lithogenic bile in man. Gastroenterology. 1970 Jul;59(1):62–69. [PubMed] [Google Scholar]


