Abstract
Altered concentrations of dopamine transporter and D2/D3 receptors have been observed in the amygdaloid complex of subjects with major depression. These findings are suggestive of neurochemical abnormalities in the limbic dopamine system in depression. Monoamine oxidase-B (MAO-B) is a key enzyme in the catabolism of biogenic amines, including dopamine, and alterations in this enzyme may underlie dopaminergic abnormalities associated with depression. The specific binding of [3H]lazabemide to MAO-B was measured in the right amygdaloid complex of 15 major depressive subjects and 16 psychiatrically normal controls. Subjects of the two study groups were matched as close as possible for age, sex, and postmortem interval. Examination of the regional distribution of MAO-B revealed lower [3H]lazabemide binding to MAO-B in the lateral and basal nuclei of the amygdala and higher binding in the medial nucleus. A modest elevation in binding to MAO-B observed in all amygdaloid nuclei in major depressive subjects as compared to control subjects failed to reach statistical significance. A significant decrease in binding to MAO-B was observed when cigarette smokers were compared to nonsmoking subjects. The amount of MAO-B binding positively correlated with the age of subjects in all nuclei investigated. A decreased amount of MAO-B in smokers further validates the pharmacological effect of tobacco smoke on this enzyme.
Keywords: Monoamine oxidase B, Major depression, Amygdala, Postmortem, Smoking, Dopamine, Catecholamine
1. Introduction
The amygdaloid complex is a limbic brain region that lies at the junction of the cerebral cortex and basal forebrain. The amygdala receives innervation from three monoamines (dopamine, serotonin, and norepinephrine) that have been traditionally held to be relevant to the biology of depression. A considerable amount of research has accumulated indicating that the amygdala plays a role in the pathobiology of depressive disorders [1,4,8,9,39,52]. Recently, evidence of dopaminergic abnormalities in the amygdala of subjects diagnosed with major depression was reported. Specifically, lower levels of dopamine transporter (DAT) and elevated levels of D2/D3 receptors were observed in the central and basal nuclei of the amygdaloid complex in subjects with major depression as compared to psychiatrically normal control subjects [21]. It was speculated that these findings may represent adaptive changes in response to low or deficient levels of dopamine. Hence, amygdala dysfunction in depression may result, in part, from deficient mesoamygdalar dopamine transmission.
Deficient dopaminergic transmission in depression could result from enhanced dopamine metabolism. Dopamine is metabolized along N-oxidative and O-methylation pathways. The N-oxidative pathway employs the enzyme monoamine oxidase (MAO). There are two isoforms of MAO (A and B) [44] coded by distinct genes [2,7,40,54]. Although both enzymes can metabolize dopamine, MAO-B is considered a key enzyme in dopamine metabolism in the human central nervous system [16,45]. Dopamine is also metabolized by O-methylation by the enzyme, catechol-O-methyl transferase (COMT) [35]. MAO isoforms differ in their localization in the central nervous system. MAO-A is found in noradrenergic and adrenergic neurons and MAO-B is found in dopaminergic, serotonergic, and histaminergic cells, as well as in astrocytes [18,23,24,26,30,50].
Inhibitors of MAO-B have clinical efficacy in the treatment of Parkinson’s disease. A mild to moderate antidepressant effect of l-deprenyl, a selective MAO-B inhibitor, has also been reported [27,29]. Given the physiological role of MAO-B, it is tempting to speculate that dysfunction of MAO-B could contribute to dopamine pathobiology in major depression. The present study was designed to examine the amount of MAO-B in the amygdale of major depressive subjects with previously reported abnormalities in DAT and D2/D3 receptors (see Klimek et al. [21]). In order to facilitate anatomically accurate measurement of MAO-B binding, histological staining of the sections taken from multiple levels along the anterior–posterior axis of the amygdala axis was performed. [3H]lazabemide binding to MAO-B was measured at approximately midportion of the amygdala, where the lateral, basal, basolateral–ventromedial, accessory basal, central, medial, and cortical nuclei were clearly identified. The amount of MAO-B was measured in 15 major depressive subjects and 16 matched controls. Additionally, the effect of potentially confounding variables, such as age of subjects, postmortem delay, and cigarette smoking, on the amount of MAO-B in postmortem material was carefully evaluated. Brain tissue was collected from carefully screened subjects (postmortem) who were diagnosed retrospectively with major depression at the time of death and from subjects who lacked any major (Axis I) psychiatric disorders (controls).
2. Materials and methods
2.1. Subjects
Tissues from 15 depressed subjects and 16 psychiatrically normal control subjects were obtained at autopsy at the Coroner’s Office of Cuyahoga County, Cleveland, OH, USA. An ethical protocol approved by the Institutional Review Board of the University Hospitals of Cleveland was used and informed written consent was obtained from the next-of-kin for all subjects. Blood and urine samples from all subjects were examined by the coroner’s office for psychotropic medications and substances of abuse.
Retrospective, informant-based psychiatric assessments were performed for all depressed and control subjects. See Table 1 for information on all subjects. A trained interviewer administered the Schedule for Affective Disorders and Schizophrenia: lifetime version (SADS-L) to knowledgeable next-of-kin of 12 depressed subjects. The Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID-IV) was administered to next-of-kin of the three remaining depressed subjects [12]. Axis I psychopathology was assessed and consensus diagnoses were reached in conference using information from interviews and medical records. The deaths of 13 of the 15 depressed subjects were ruled a suicide by the coroner. No antidepressant drugs were detected in the postmortem toxicology screening of subjects in the present study (Table 1), as the presence of such drugs was a criterion of exclusion from the study. The control subjects did not meet criteria for an Axis I disorder at the time of their deaths.
Table 1.
Vital data of subjects
| Subject | Age (year) |
Sex | PMI (h) |
Toxicology | Cause of death |
Diagnosis |
|---|---|---|---|---|---|---|
| A | 44 | M | 6 | Ephedrine, chlorpheniramine |
Myocardial infarction |
Cont |
| F | 70 | M | 20 | Clean | Pulmonary embolism |
Cont, S |
| G | 71 | M | 23 | Chlorpheniramine | Heart disease, scleritis |
Cont |
| I | 39 | F | 18 | Lidocaine | Heart disease | Cont |
| N | 58 | F | 12 | Codeine, lidocaine, cyclobenzaprine |
Heart disease | Cont, S |
| O | 78 | F | 11 | Clean | Heart disease, diabetes |
Cont |
| R | 73 | M | 22 | Clean | Myocardial infarction |
Cont |
| S | 67 | F | 28 | Clean | Heart disease | Cont, S |
| 2B | 50 | F | 27 | Clean | Heart disease | Cont, S |
| 2C | 77 | M | 24 | Clean | Heart disease | Cont |
| 2D | 69 | M | 18 | Clean | Heart disease | Cont |
| K | 26 | M | 13 | Clean | Gun shot | Cont |
| A1 | 54 | M | 19 | Lidocaine | Heart disease | Cont, S |
| B1 | 27 | M | 17 | Clean | Gun shot | Cont, S |
| C1 | 50 | M | 12 | Glucose | Heart disease | Cont |
| D1 | 59 | M | 6 | Lidocaine | Heart disease | Cont, S |
| B | 43 | M | 21 | Clean | Hanging | MD |
| E | 68 | M | 4 | Clean | CO poisoning | MD |
| H | 73 | M | 17.5 | Diazepam, codeine | Gun shot | MD |
| M | 63 | F | 18 | Lidocaine | Heart disease | MD, S |
| P | 75 | F | 30 | Clean | CO poisoning | MD, S |
| T | 77 | F | 32 | Propoxyphene | Heart disease | MD |
| U | 39 | M | 24 | Clean | Hanging | MD |
| 1B | 50 | F | 23 | Clean | Hanging | MD, S |
| 1D | 78 | F | 25 | Clean | Blunt force trauma |
MD |
| D | 47 | M | 11 | Clean | Gun shot | MD |
| 1K | 34 | F | 24 | Clean | CO poisoning | MD |
| A2 | 65 | M | 30 | Codeine | Gun shot | MD, S |
| B2 | 30 | M | 18 | Ethanol | Gun shot | MD, S |
| C2 | 52 | M | 17 | CO | CO poisoning | MD |
| D2 | 60 | M | 20 | Ethanol | Gun shot | MD, S |
PMI, postmortem interval; M, male; F, female; MD, major depression; Cont, psychiatrically healthy control; S, smoker.
Information on smoking was also collected in the interview. In the present study, 6 major depressive subjects and 7 control subjects were active smokers (smoked 20 or more cigarettes daily up to death). Nine depressed and 9 controls were nonsmokers (never smoked).
Tissue blocks were sectioned serially in the coronal plane along the anterior–posterior length of the amygdala, beginning at its anterior extent. Sections (30 µm) for acetylcholinesterase histochemistry, followed by a series of consecutive 20 µm sections for MAO-B autoradiography, were collected at 1 mm intervals by thaw-mounting onto gelatin-coated glass slides. Sections for autoradiography were vacuum-dried overnight at 4 °C and stored in an ultra-cold freezer (−83 °C) until assayed.
2.2. Histochemical staining
Histochemical localization of acetylcholinesterase was used to create anatomical templates for anatomical positioning of nuclei and for the study of radioligand binding by quantitative autoradiography. The histochemical staining of acetylcholinesterase was performed as described by Biegon and Wolff [3]. Briefly, 30 µm sections were incubated in an acetate buffer (pH 5.5) containing acetylthiocholine iodide (4.2 mmol/L), a butyrylcholinesterase blocker (ethopropazine, 10 µmol/L), 2 mmol/L CuSO4, and 10 mol/L glycine for 2 h at 37 °C. After incubation, sections were quickly dipped 4 times in distilled water. The stain was developed in 1.25% Na2S (pH 7.2) for 1 min then enhanced by 1 min in 0.125% AgNO3, followed by a water rinse (2 × 1 min). Stained sections were dehydrated through graded alcohols and fixed in xylene.
2.3. Radioligand autoradiography
After confirming anatomically equivalent levels of amygdaloid complex for all subjects, duplicate sections for total and non-specific binding were used in the experiments. [3H]lazabemide (6 nM; 10 Ci/mmol; gift kindly provided by F. Hoffmann-La Roche LTD, Basil, Switzerland) was used to label MAO-B. This ligand has an affinity for MAO-B that is 3000-fold higher than its affinity for MAO-A [37]. Slide-mounted sections were incubated for 90 min at room temperature with 6 nM [3H]lazabemide in 50 mM Tris HCl buffer (pH 7.4) containing 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 0.5 mM EGTA. Non-specific binding was determined in the presence of 1 µM deprenyl. Following incubations, sections were washed at 4 °C in the same buffer 2 times for 30 s and once for 60 s, then rinsed briefly (2 s) in ice-cold water before drying.
Sections and [3H] standards (American Radiolabeled Chemicals, Inc., St. Louis, MO) were apposed to [3H]-Hyperfilm (Amersham, Piscataway, NJ) and exposed in X-ray cassettes at room temperature for 4 weeks. Films were processed with GBX developer and fixer (Eastman Kodak, Rochester, NY, U.S.A.) at room temperature. After autoradiography, the same sections were stained for acetylcholinesterase and were used to create anatomical templates for measuring the density of radioligand binding. Densitometric measurements of autoradiograms were made using the Microcomputer Controlled Imaging Device (MCID Elite, Amersham Biosciences, Buckinghamshire, England). Amygdala autoradiograms were analyzed by simultaneously overlaying the image of the autoradiogram with the image of the same, acetylcholinesterase-stained section. The amygdaloid nuclei were outlined and the density of binding was measured. Specific binding was defined as the difference between total and non-specific binding.
2.4. Statistical analysis
Duplicate binding values of the amygdala were averaged for each amygdaloid nucleus for each subject. Mean values of specific binding in amygdaloid nuclei for groups of depressed and control subjects, as well as for groups of smokers and nonsmokers, were compared statistically using a two-way ANOVA followed by Bonferroni post tests. A P value <0.05 was considered statistically significant. All data are presented as means ± SEM. Linear regression analysis was used to compute potential correlations between binding densities and age, as well as between binding densities and postmortem delay for both groups of subjects.
3. Results
3.1. Anatomical positioning for MAO-B autoradiography
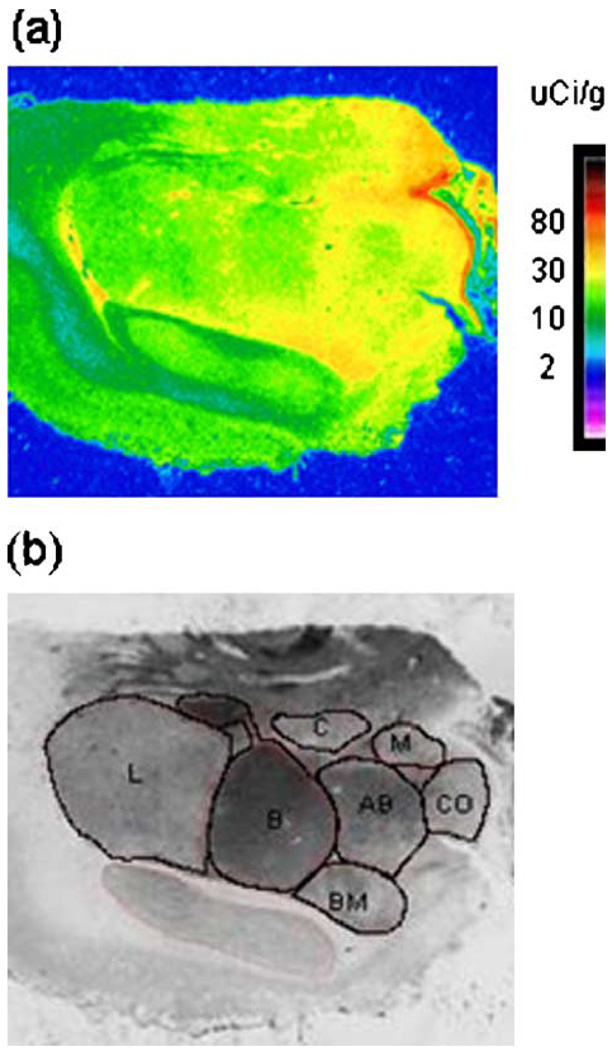
Each block of amygdala (16 controls and 15 major depressive subjects) varied in the length of the rostro-caudal axis. Thus, anatomical positioning of sections taken for these studies varied accordingly, with nuclei being defined anatomically using the atlas by Sims and Williams [41]. Therefore, in order to establish comparable anatomical resolution of MAO-B binding for each subject, tissue sections from each interval representing the 1 mm of axis was processed for acetylcholinesterase preparation. Anatomical position was established as close as possible to the level where basal nucleus appeared bipartite, approximately midway to the caudal end, and where all nuclei of interest were clearly identified: lateral (L), basal (B), accessory basal (AB), central (C), medial (M), cortical (CO), and basolateral–ventromedial (BM) (Fig. 1).
Fig. 1.
A digital autoradiogram of the specific binding of [3H]lazabemide to MAO-B in a coronal section of the amygdala (a) and a digital image of the same section stained histochemically for acetylcholinesterase (b).
3.2. MAO-B autoradiography
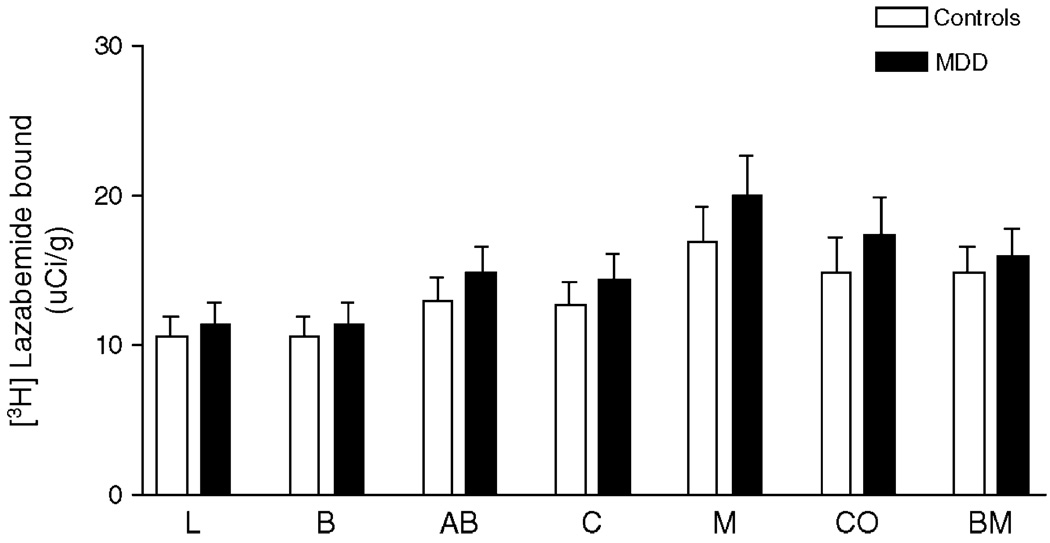
Examination of the regional distribution of MAO-B in all subjects revealed lower [3H]lazabemide binding to MAO-B in the lateral and basal nuclei of the amygdala and higher binding in the medial nucleus. There was no significant difference in [3H]lazabemide binding between subjects diagnosed with major depression and control subjects in any of the amygdaloid nuclei (F1,169 = 3.418, P = 0.07; Fig. 2). However, it is noteworthy that there was a trend towards elevation of the amount of MAO-B in subjects diagnosed with major depression as compared to controls consistently observed in all examined nuclei, ranging from 7% in basolateral–ventromedial to 18% in medial nucleus comparing depressives to control subjects.
Fig. 2.
The specific binding of [3H]lazabemide to MAO-B measured in psychiatrically normal control subjects (white bars, n = 10–16) and in subjects diagnosed with major depression (black bars, n = 8–15). Each bar represents the mean ± SEM. Abbreviations are as described in Fig. 1. There were no significant differences in [3H]lazabemide binding comparing major depressive to control subjects.
3.3. Postmortem delay and age
Postmortem intervals were not statistically different between major depressive and control subjects (Tables 1 and 2). There were no positive correlations between postmortem interval and the amount of [3H]lazabemide binding in any nuclei that were investigated (data not shown).
Table 2.
Summary of demographics
| Parameter | Control | Major depression |
|---|---|---|
| Age range | 26–78 y | 30–78 y |
| Age (mean ± SEM | 57 ± 4 y | 57 ± 4 y |
| PMI range | 6–28 h | 4–32 h |
| PMI (mean ± SEM) | 17 ± 2 h | 21 ± 2 h |
| Female:male | 5:11 | 6:9 |
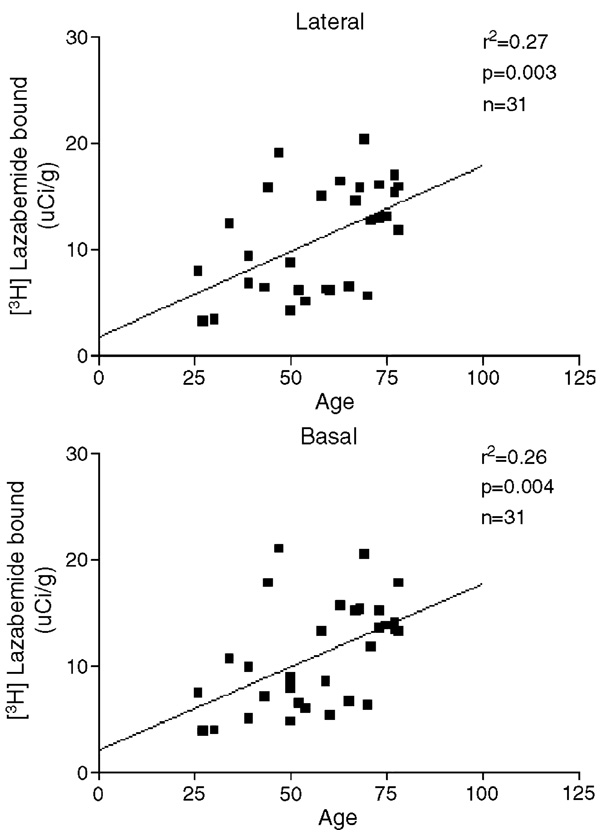
Average age was not different between major depressive and control subjects (Tables 1 and 2). However, there was a positive correlation between the [3H]lazabemide binding and the age of all subjects in the lateral (r2 = 0.27; P = 0.003; Fig. 3), basal (r2 = 0.26; P = 0.004; Fig. 3), accessory basal (r2 = 0.30; P = 0.003), central (r2 = 0.23; P = 0.014), medial (r2 = 0.27; P = 0.022), cortical (r2 = 0.31; P = 0.006), and basolateral–ventromedial (r2 = 0.25; P = 0.006) nuclei of the amygdaloid complex. When measured separately in control subjects, correlations between [3H]lazabemide binding and age reached statistic significance only in the lateral (r2 = 0.28; P = 0.036) and basal (r2 = 0.29; P = 0.032) nuclei (data not shown). As for depressed subjects alone, correlations between [3H]lazabemide binding and age reached statistic significance only in the accessory basal nucleus (r2 = 0.38, P = 0.026, data not shown).
Fig. 3.
The relationship between [3H]lazabemide binding and age of all subjects in two amygdaloid nuclei.
3.4. Cigarette smoking and MAO-B
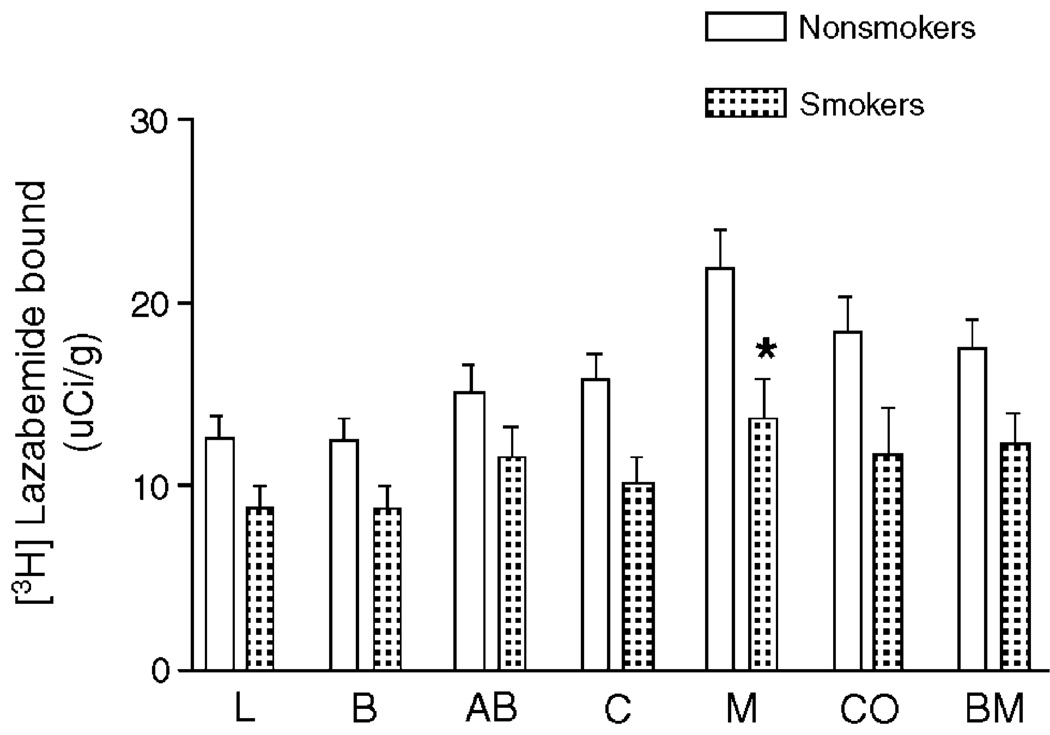
Since 7 depressives and 6 control subjects were cigarette smokers, the effect of smoking on radioligand binding to MAO-B was evaluated. However, the sample size of depressive smokers and control smokers was far too small to statistically evaluate the effect of smoking on these two groups separately. Therefore, the amount of MAO-B was compared between all smokers (depressive and controls, n = 13) and all nonsmokers (depressive and controls, n = 18). This was a reasonable alternative to evaluate potential effects of smoking since no statistically significant difference in MAO-B was observed between control and depressive subjects. Two-way ANOVA of the data revealed that MAO-B binding was significantly lower in smokers as compared to nonsmokers (F1,169 = 36.69; P < 0.0001; Fig. 4). A Bonferroni post-hoc test for individual comparisons revealed a significant decrease in MAO-B binding only in the medial nucleus of smokers as compared to nonsmokers, although MAO-B binding was modestly and consistently lower in smokers as compared to nonsmokers in all investigated nuclei.
Fig. 4.
The specific binding of [3H]lazabemide to MAO-B measured in nonsmokers (white bars, n = 10–18) and in chronic cigarette smokers (dotted bars, n = 8–13). Each bar represents the mean ± SEM. Abbreviations are as described in Fig. 1. Overall, [3H]lazabemide binding to MAO-B was significantly lower in smokers (P < 0.0001; 2-way ANOVA). Bonferroni post-hoc tests revealed a significant difference in [3H]lazabemide binding in medial nucleus comparing smokers to nonsmokers (P < 0.05).
4. Discussion
The present study is the first anatomically detailed examination of [3H]lazabemide binding to MAO-B in the human amygdaloid complex. The distribution of the binding of [3H]lazabemide to MAO-B in amygdala was homogenous in lateral, basal, and accessory basal nuclei and was moderately higher in the medial nucleus (Figs. 1, 2). Histological staining of the entire amygdala allowed us to establish anatomical positioning for [3H]lazabemide binding experiments, so that equivalent anatomical levels of the amygdala were compared in depressives and matched control subjects. This report demonstrates that the amount of MAO-B is not statistically different in major depressive subjects as compared to psychiatrically normal controls. Thus, dopaminergic pathobiology in the amygdaloid complex, as previously described by Klimek et al. [21], may not involve abnormalities in oxidative degradation of dopamine. Although major depression was the common diagnosis in the psychiatric group, 13 out of 15 major depressive subjects in this study died as a result of suicide. It is worth noting that two major depressive subjects that died of natural causes did not appear to differ from the suicide subjects and had amounts of MAO-B comparable to their matched controls.
The present study utilized the MAO-B inhibitor, [3H]lazabemide, to measure MAO-B protein distribution and density. The in vitro binding characteristics of [3H]lazabemide (kinetics, pharmacology) were previously reported by Saura et al. [37]. The binding of [3H]lazabemide is high affinity, reversible, saturable, and selective for MAO-B. In fact, [3H]lazabemide has an affinity for MAO-B 3000-fold higher than its affinity for MAO-A. Thus, this radioligand selectively labels MAO-B and can be used to investigate potential changes in MAO-B protein amount. However, a change in the activity of the enzyme cannot be detected using this radioligand binding method.
The present study is in agreement with previous observations of normal amounts of MAOs (A and B) in brainstem nuclei of depressed subjects. The specific binding of [3H]Ro41-1049 to MAO-A and [3H]lazabemide to MAO-B was also found unchanged in the locus coeruleus and raphe nuclei in subjects diagnosed with major depression [22,31]. Moreover, Mann and Stanley [28] reported that MAO enzyme kinetics is normal in the frontal cortex of suicide victims. In contrast, other studies demonstrate both lower brain MAO activity of suicide victims [17] and higher platelet MAO activity in unmedicated depressed patients as compared to controls [32]. Together, the present and previous studies do not convincingly demonstrate that MAOs are involved in the pathology associated with depression.
Dopamine in the central nervous system is catabolized along two different routes. Dopamine undergoes oxidative deamination (oxidative pathway) mediated by intracellular MAO and is O-methylated (O-methylation pathway) by extracellular catechol-O-methyltransferase (COMT). The products of both routes, 3,4-dihydroxyphenylacetic acid (DOPAC) and 3-methoxytyramine (3MT), undergo further metabolism to yield homovanillic acid (HVA) as the final product. Lower concentrations of HVA have been observed in cerebrospinal fluid of patients with depression, providing support for decreased dopamine turnover [20,33,36,48]. These findings indicate that potential abnormalities in dopamine synthesis and/or degradation might occur in major depression. The present study provides little evidence for a depression-related disruption of the oxidative pathway for dopamine metabolism. A modest increase in ligand binding to MAO-B in depressives failed to reach statistical significance. However, it is worth noting that the activity of the enzyme could be altered and the present study is not able to address this possibility.
The extensive in situ hybridization study by Saura et al. [38] revealed that MAO-B transcripts are predominantly found in cells of the dorsal and median raphe, lateral hypothalamic nuclei and in granule cells of the dentate gyrus. Konradi and coworkers [23,24] have observed moderate immunostaining for MAO-B in the ventral tegmental area, but no detectable level of MAO-B immunoreactivity was observed in dopaminergic neurons of the substantia nigra. Damier et al. [6], using a double immunostaining method, demonstrated the presence of MAO-B positive dopaminergic neurons in all dopaminergic groups of human midbrain including substantia nigra pars compacta. In previous autoradiographic study, high binding of [3H]deprenyl to MAO-B was observed in human caudate nucleus and putamen [19]. Richards et al. [34] demonstrated evidence that, in human substantia nigra, MAO-B protein was more abundant than MAO-A; MAO-B was localized in the reticular zone while MAO-A was found in the compact zone. In addition to a neuronal location, there is consistent and convincing data showing localization of MAO-B in glial cells throughout the human brain in areas which are known to contain catecholaminergic neurotransmitters as well as in the other brain regions [23,38,50,51]. Together, these findings imply that [3H]lazabemide binding measured in the amygdaloid complex in the present study could be to MAO-B in dopaminergic and non-dopaminergic neurons, as well as in glia.
A role of MAO-B in the physiology of normal aging and in neurological conditions has been established. MAO-B mRNA, protein, and enzymatic activity increases with age and increases in certain neurodegenerative diseases [11,15,25,42,43,46,49]. Age-related increases in cortical MAO-B concentrations in both controls and suicide victims have been reported [28]. The increase of MAO-B with age may reflect age-associated increases in glial cells [47]. The present findings demonstrate a positive correlation between [3H]lazabemide binding and age of all subjects included in the study. Since MAO-B is found in glial cells, particularly in astrocytes [10,24,26], the increase in the amount of MAO-B with age may be due to an increase in the MAO-B protein expression and/or in the number of astrocytes. Whether the age-related increase of MAO-B observed in the present study represents changes in neurons or glial cells will require double-labeling immunochemistry to resolve.
Present observation on the decreased amount of [3H]lazabemide binding to MAO-B in amygdala of chronic cigarette smokers, as compared to nonsmokers, is consistent with a large body of evidence showing that one of the biological actions of tobacco is inhibition of MAOs (A and B). Interestingly, the activities of MAO-A and MAO-B are inhibited when rats are exposed to tobacco smoke, but not when exposed to nicotine alone [5]. Similarly, an aqueous extract of cigarette smoke, or saliva obtained after smoking, can irreversibly inhibit the action of MAO on a variety of the enzyme’s substrates in rat lung tissue [53]. Significant decreases in MAO-A and MAO-B in the brains of smokers relative to nonsmokers or to former smokers have been demonstrated using in vivo PET imaging with radiotracers specific for MAO-A and MAO-B [13,14]. Thus, present data showing lower binding of [3H]lazabemide to MAO-B in the amygdala further confirm the involvement the MAO-B in the pathology of tobacco dependency.
In summary, statistically significant changes in the amount of MAO-B in amygdala of subjects with major depression were not detected, suggesting that dopaminergic pathology of the amygdala in major depression may not involve altered metabolism by this oxidative pathway. An age-related increase in MAO-B and low MAO-B in cigarette smokers further validates findings of other laboratories.
Acknowledgments
The authors wish to thank Drs. Andrea M. Cesura and Edilio Borroni (F. Hoffman La Roche, Basel, Switherland) for the generous supply of the radioligand [3H]lazabemide. The authors gratefully acknowledge the contributions of Drs. Herbert Y. Meltzer and James C. Overholser for providing Axis I diagnoses of deceased subjects. The authors also kindly acknowledge helpful technical assistance provided by Wendy Hawkins and Hilary Flint. This work was supported by MH 46692, MH 63187, MH/AG 02031, and RR17701.
References
- 1.Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DD, Benca RM, Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. NeuroReport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- 2.Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, Seeburg PH, Shih JC. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. U. S. A. 1988;85:4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biegon A, Wolff M. Quantitative histochemistry of acetylcholinesterase in rat and human brain postmortem. J. Neurosci. Methods. 1986;16:39–45. doi: 10.1016/0165-0270(86)90006-3. [DOI] [PubMed] [Google Scholar]
- 4.Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol. Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 5.Carr LA, Basham JK. Effects of tobacco smoke constituents on MPTP-induced toxicity and monoamine oxidase activity in the mouse brain. Life Sci. 1991;48:1173–1177. doi: 10.1016/0024-3205(91)90455-k. [DOI] [PubMed] [Google Scholar]
- 6.Damier P, Kastner A, Agid Y, Hirsch EC. Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson’s disease? Neurology. 1996;46:1262–1269. doi: 10.1212/wnl.46.5.1262. [DOI] [PubMed] [Google Scholar]
- 7.Derry JM, Lan NC, Shih JC, Barnard EA, Barnard PJ. Localization of monoamine oxidase A and B genes on the mouse X chromosome. Nucleic Acids Res. 1989;17:8403. doi: 10.1093/nar/17.20.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann. N. Y. Acad. Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 9.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol. Biochem. Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 10.Ekblom J, Jossan SS, Bergstrom M, Oreland L, Walum E, Aquilonius SM. Monoamine oxidase-B in astrocytes. Glia. 1993;8:122–132. doi: 10.1002/glia.440080208. [DOI] [PubMed] [Google Scholar]
- 11.Emilsson L, Saetre P, Balciuniene J, Castensson A, Cairns N, Jazin EE. Increased monoamine oxidase messenger RNA expression levels in frontal cortex of Alzheimer’s disease patients. Neurosci. Lett. 2002;326:56–60. doi: 10.1016/s0304-3940(02)00307-5. [DOI] [PubMed] [Google Scholar]
- 12.First MB, Donovan S, Frances A. Nosology of chronic mood disorders. Psychiatry Clin. North Am. 1996;19:29–39. doi: 10.1016/s0193-953x(05)70271-9. [DOI] [PubMed] [Google Scholar]
- 13.Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan I, MacGregor RR, Alexoff D, Shea C, Schlyer DJ, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- 15.Fowler JS, Volkow ND, Wang GJ, Pappas N, Shea C, MacGregor RR, Logan J. Visualization of monoamine oxidase in human brain. Adv. Pharmacol. 1998;42:304–307. doi: 10.1016/s1054-3589(08)60750-4. [DOI] [PubMed] [Google Scholar]
- 16.Glover V, Sandler M, Owen F, Riley GJ. Dopamine is a monoamine oxidase B substrate in man. Nature. 1977;265:80–81. doi: 10.1038/265080a0. [DOI] [PubMed] [Google Scholar]
- 17.Gottfries CF, Oreland L, Wiberg A, Winblad B. Letter: brain-levels of monoamine oxidase in depression. Lancet. 1974;2:360–361. doi: 10.1016/s0140-6736(74)91752-8. [DOI] [PubMed] [Google Scholar]
- 18.Jahng JW, Houpt TA, Wessel TC, Chen K, Shih JC, Joh TH. Localization of monoamine oxidase A and B mRNA in the rat brain by in situ hybridization. Synapse. 1997;25:30–36. doi: 10.1002/(SICI)1098-2396(199701)25:1<30::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Jossan SS, Gillberg PG, d’Argy R, Aquilonius SM, Langstrom B, Halldin C, Oreland L. Quantitative localization of human brain monoamine oxidase B by large section autoradiography using L-[3H]deprenyl. Brain Res. 1991;547:69–76. doi: 10.1016/0006-8993(91)90575-g. [DOI] [PubMed] [Google Scholar]
- 20.Kasa K, Otsuki S, Yamamoto M, Sato M, Kuroda H, Ogawa H. Cerebrospinal fluid gamma-aminobutyric acid and homovanillic acid in depressive disorders. Biol. Psychiatry. 1982;17:877–883. [PubMed] [Google Scholar]
- 21.Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol. Psychiatry. 2002;52:740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- 22.Klimek V, Roberson G, Stockmeier CA, Ordway GA. Serotonin transporter and MAO-B levels in monoamine nuclei of the human brainstem are normal in major depression. J. Psychiatr. Res. 2003;37:387–397. doi: 10.1016/s0022-3956(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 23.Konradi C, Svoma E, Jellinger K, Riederer P, Denney R, Thibault J. Topograp hic immunocytochemical mapping of monoamine oxidase-A, monoamine oxidase-B and tyrosine hydroxylase in human post mortem brain stem. Neuroscience. 1988;26:791–802. doi: 10.1016/0306-4522(88)90099-1. [DOI] [PubMed] [Google Scholar]
- 24.Konradi C, Kornhuber J, Froelich L, Fritze J, Heinsen H, Beckmann H, Schulz E, Riederer P. Demonstration of monoamine oxidase-A and -B in the human brainstem by a histochemical technique. Neuroscience. 1989;33:383–400. doi: 10.1016/0306-4522(89)90218-2. [DOI] [PubMed] [Google Scholar]
- 25.Kornhuber J, Konradi C, Mack-Burkhardt F, Riederer P, Heinsen H, Beckmann H. Ontogenesis of monoamine oxidase-A and -B in the human brain frontal cortex. Brain Res. 1989;499:81–86. doi: 10.1016/0006-8993(89)91136-0. [DOI] [PubMed] [Google Scholar]
- 26.Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann JJ, Frances A, Kaplan RD, Kocsis J, Peselow ED, Gershon S. The relative efficacy of l-deprenyl, a selective monoamine oxidase type B inhibitor, in endogenous and nonendogenous depression. J. Clin. Psychopharmacol. 1982;2:54–57. doi: 10.1097/00004714-198202000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Mann JJ, Stanley M. Postmortem monoamine oxidase enzyme kinetics in the frontal cortex of suicide victims and controls. Acta Psychiatr. Scand. 1984;69:135–139. doi: 10.1111/j.1600-0447.1984.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 29.Mann JJ, Aarons SF, Wilner PJ, Keilp JG, Sweeney JA, Pearlstein T, Frances AJ, Kocsis JH, Brown RP. A controlled study of the antidepressant efficacy and side effects of (−)-deprenyl. A selective monoamine oxidase inhibitor. Arch. Gen. Psychiatry. 1989;46:45–50. doi: 10.1001/archpsyc.1989.01810010047007. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura S, Kawamata T, Akiguchi I, Kameyama M, Nakamura N, Kimura H. Expression of monoamine oxidase B activity in astrocytes of senile plaques. Acta Neuropathol. (Berl.) 1990;80:419–425. doi: 10.1007/BF00307697. [DOI] [PubMed] [Google Scholar]
- 31.Ordway GA, Farley JT, Dilley GE, Overholser JC, Meltzer HY, Balraj EK, Stockmeier CA, Klimek V. Quantitative distribution of monoamine oxidase A in brainstem monoamine nuclei is normal in major depression. Brain Res. 1999;847:71–79. doi: 10.1016/s0006-8993(99)02043-0. [DOI] [PubMed] [Google Scholar]
- 32.Quintana J. Platelet MAO deamination of serotonin in depressed patients. Changes after imipramine treatment and clinical correlations. Biol. Psychiatry. 1988;23:44–52. doi: 10.1016/0006-3223(88)90105-9. [DOI] [PubMed] [Google Scholar]
- 33.Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Rao BS. CFS amine metabolites in depression. Biol. Psychiatry. 1992;31:112–118. doi: 10.1016/0006-3223(92)90198-9. [DOI] [PubMed] [Google Scholar]
- 34.Richards JG, Saura J, Ulrich J, Da Prada M. Molecular neuroanatomy of monoamine oxidases in human brainstem. Psychopharmacology (Berlin) 1992;106 Suppl.:S21–S23. doi: 10.1007/BF02246228. [DOI] [PubMed] [Google Scholar]
- 35.Robinson DS, Sourkes TL, Nies A, Harris LS, Spector S, Bartlett DL, Kaye IS. Monoamine metabolism in human brain. Arch. Gen. Psychiatry. 1977;34:89–92. doi: 10.1001/archpsyc.1977.01770130091009. [DOI] [PubMed] [Google Scholar]
- 36.Roy A, De Jong J, Linnoila M. Cerebrospinal fluid monoamine metabolites and suicidal behavior in depressed patients. A 5-year follow-up study. Arch. Gen. Psychiatry. 1989;46:609–612. doi: 10.1001/archpsyc.1989.01810070035005. [DOI] [PubMed] [Google Scholar]
- 37.Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41–1049 and 3H-Ro 19–6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J. Neurosci. 1992;12:1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saura J, Bleuel Z, Ulrich J, Mendelowitsch A, Chen K, Shih JC, Malherbe P, Da Prada M, Richards JG. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience. 1996;70:755–774. doi: 10.1016/s0306-4522(96)83013-2. [DOI] [PubMed] [Google Scholar]
- 39.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. NeuroReport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 40.Shih JC. Molecular basis of human MAO A and B. Neuropsychopharmacology. 1991;4:1–7. [PubMed] [Google Scholar]
- 41.Sims KS, Williams RS. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;36:449–472. doi: 10.1016/0306-4522(90)90440-f. [DOI] [PubMed] [Google Scholar]
- 42.Slotkin TA, Seidler FJ, Ritchie JC. Regional differences in brain monoamine oxidase subtypes in an animal model of geriatric depression: effects of olfactory bulbectomy in young versus aged rats. Brain Res. 2000;882:149–154. doi: 10.1016/s0006-8993(00)02859-6. [DOI] [PubMed] [Google Scholar]
- 43.Sparks DL, Woeltz VM, Markesbery WR. Alterations in brain monoamine oxidase activity in aging, Alzheimer’s disease, and Pick’s disease. Arch. Neurol. 1991;48:718–721. doi: 10.1001/archneur.1991.00530190064017. [DOI] [PubMed] [Google Scholar]
- 44.Squires RF. Multiple forms of monoamine oxidase in intact mitochondria as characterized by selective inhibitors and thermal stability: a comparison of eight mammalian species. Adv. Biochem. Psychopharmacol. 1972;5:355–370. [PubMed] [Google Scholar]
- 45.Stenstrom A, Hardy J, Oreland L. Intra- and extra-dopamine-synaptosomal localization of monoamine oxidase in striatal homogenates from four species. Biochem. Pharmacol. 1987;36:2931–2935. doi: 10.1016/0006-2952(87)90205-x. [DOI] [PubMed] [Google Scholar]
- 46.Strolin BM, Dostert P. Monoamine oxidase, brain ageing and degenerative diseases. Biochem. Pharmacol. 1989;38:555–561. doi: 10.1016/0006-2952(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 47.Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann. Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- 48.Traskman L, Asberg M, Bertilsson L, Sjostrand L. Monoamine metabolites in CSF and suicidal behavior. Arch. Gen. Psychiatry. 1981;38:631–636. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]
- 49.Venero JL, de la RC, Machado A, Cano J. Age-related changes on monoamine turnover in hippocampus of rats. Brain Res. 1993;631:89–96. doi: 10.1016/0006-8993(93)91191-t. [DOI] [PubMed] [Google Scholar]
- 50.Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abel CW. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985;230:181–183. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- 51.Westlund KN, Denney RM, Rose RM, Abell CW. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience. 1988;25:439–456. doi: 10.1016/0306-4522(88)90250-3. [DOI] [PubMed] [Google Scholar]
- 52.Willner P. Dopamine and depression: a review of recent evidence: II. Theoretical approaches. Brain Res. 1983;287:225–236. doi: 10.1016/0165-0173(83)90006-1. [DOI] [PubMed] [Google Scholar]
- 53.Yu PH, Boulton AA. Irreversible inhibition of monoamine oxidase by some components of cigarette smoke. Life Sci. 1987;41:675–682. doi: 10.1016/0024-3205(87)90446-2. [DOI] [PubMed] [Google Scholar]
- 54.Zhu QS, Grimsby J, Chen K, Shih JC. Promoter organization and activity of human monoamine oxidase (MAO) A and B genes. J. Neurosci. 1992;12:4437–4446. doi: 10.1523/JNEUROSCI.12-11-04437.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]