Abstract
This prospective study examined object exploration behavior in 66 12-month-old infants, of whom nine were subsequently diagnosed with an autism spectrum disorder. Previous investigations differ on when the repetitive behaviors characteristic of autism are first present in early development. A task was developed that afforded specific opportunities for a range of repetitive uses of objects and was coded blind to outcome status. The autism/ASD outcome group displayed significantly more spinning, rotating, and unusual visual exploration of objects than two comparison groups. The average unusual visual exploration score of the autism/ASD group was over four standard deviations above the mean of the group with no concerns at outcome. Repetitive behaviors at 12 months were significantly related to cognitive and symptomatic status at 36 month outcome. These results suggest that repetitive or stereotyped behaviors may be present earlier than initially thought in very young children developing the autism phenotype.
Keywords: autism, diagnosis, early identification, repetitive behavior
Introduction
Repetitive behaviors constitute one of the three core domains of symptoms required for autism spectrum diagnoses. Multiple studies have found that differences in social-communicative behaviors like responding to name, monitoring faces, social smiling, and eye contact reliably distinguish children under 2 who will be diagnosed with autism or autism spectrum disorders (ASDs) from children with typical development or developmental delays (Lord, 1995; Nadig et al., 2007; Osterling and Dawson, 1994; Stone et al., 1999; Zwaigenbaum et al., 2005). There is lower consensus about how early repetitive and stereotyped behaviors are present. The goal of the present study was to examine whether the manner in which infants explore objects at 12 months of age differs in children later diagnosed with autism or ASD.
The literature is unclear on how early repetitive behaviors (RBs), including both motor stereotypies and repetitive use of objects, are present. While RBs are a reliable diagnostic feature in children older than 2 years of age (e.g. Richler et al., 2007; Turner, 1999) and are included in all major diagnostic systems, they have been documented inconsistently in studies of children younger than 3. One longitudinal study found no group differences in parent-reported repetitive behaviors at 20 months, but significantly higher rates by 42 months, concluding that ‘at least in some children, abnormalities on this third dimension only begin to emerge after infancy, later than the social and communication deficits are apparent’ (Cox et al., 1999, p. 730). Home video studies have found few significant differences in repetitive behaviors in the first and second years of life (Baranek, 1999; Werner and Dawson, 2005). Repetitive behaviors are less frequently endorsed by clinicians (Stone et al., 1999) or rated as clearly abnormal on the Autism Diagnostic Observation Schedule (Chawarska et al., 2007) than social or communication symptoms in the second and third years of life. A recent prospective study of infant siblings of children with autism found few differences in motor stereotypies or postures between toddlers subsequently diagnosed with ASD and unaffected siblings or controls at 12 or 18 months (Loh et al., 2007).
In contrast, a few studies have found differences in the frequency or severity of RBs in toddlers with autism under age 3. Wetherby et al. (2004) found that repetitive actions with objects and repetitive movements of the body/arms/hands were two of the nine ‘red flag’ behaviors that differentiated autism from both typical and delayed development in the second year of life. In another study, parents of children with autism retrospectively reported more repetitive motor movements (e.g. rocking, banging, standing on toes) than children with typical development by 10–12 months of age and significantly more than children with non-spectrum developmental delays (DDs) by 16–18 months (Werner et al., 2005).
Three recent studies have focused specifically on one type of RB –atypical use of objects – employing direct assessment rather than retrospective methods (home movies or parent report). Mottron et al. (2007) 458 demonstrated that lateral glances toward objects (defined as ‘fixating on a target with the pupils turned toward an extreme corner of the eye socket’, p. 27) were significantly more common in a group of children with autism (mean age 44 months) than children with typical development. Bruckner and Yoder (2007) found that children with autism (mean age 33 months) who spent a large proportion of time in restricted object use (defined as ‘the proportion of touched toys on which the child performed at least two differentiated play actions’, p. 166) showed poorer joint attention, imitation, and social engagement abilities. Finally, Wetherby and colleagues (2004) reported group differences in repetitive movements with objects (defined as ‘at least 3 consecutive movements with objects, such as taps, spins, bangs, lines up, rubs, twirls, rolls, collects’, p. 493) between toddlers with ASD and comparison toddlers with either typical or delayed development. The rate of RBs in the second year of life was significantly correlated with 36 month developmental outcomes in this study.
During the course of typical development, infants demonstrate rhythmic, repetitive movements, such as kicking, waving, bouncing, and banging (Thelen, 1979), which are similar in some ways to the stereotyped movements of older children with autism. Repetitive behaviors of this type are frequent in the first year of life, decrease after 12 months, manifest in older toddlers as ‘just right’ compulsive or sameness behaviors (Evans et al., 1997), and then reduce markedly in school age. This progression during typical development may account for the lack of group differences in some studies. Richler et al. (2007) used the ADI–R to examine parent-reported RBs in children with autism and those with non-spectrum DDs or typical development in the second year of life. Significant differences were found only on the repetitive-sensorimotor factor, which included behaviors that should be on the wane in typically developing children, such as repetitive use of objects and complex mannerisms. There were no group differences on an insistence-on-sameness factor, suggesting that only some aspects of RB are more common and severe in autism at very young ages. Previous studies that collapsed multiple types of RB into a single variable may have obscured group differences in certain components of RB, potentially accounting for the null results found in these studies.
The current investigation focused on one particular RB that is often seen in children with autism older than 2 years of age: atypical object use. Only two studies have examined this behavior in toddlers with autism using direct assessment methods (Mottron et al., 2007; Wetherby et al., 2004) and none as early as infancy. We assessed 12-month-olds participating in a prospective longitudinal study of infants at risk for autism, using a task that provided specific presses for both typical and atypical uses of objects. All participants tested at 12 months who also had diagnostic outcome data at 24 or 36 months were classified into three groups: autism/ASD, other delays, and no concerns. The study explored: (1) group differences in both typical and atypical uses of objects at 12 months; and (2) the relationship between atypical object use at 12 months and cognitive and symptomatic status at 36 months.
Method
Participants
Infants were recruited from families who had either an older child diagnosed with autism or an older child developing typically. Sixty-six infants were administered the Object Exploration task when they were 12 months old (35 in the autism sibling group, 31 in the typical sibling group) and had diagnostic outcome data at either 24 months (n = 29 total, of whom n = 16 were from the autism sibling group) or 36 months of age (n = 37 total, of whom n = 19 were from the autism sibling group). To better approximate the skewed gender ratio of autism, males were over-enrolled in both groups, with 62 percent of the total sample being male. The recruitment groups were well matched on demographic variables and there were no differences in gender ratio, chronological age at administration of the object task, family income, ethnicity, or racial background (all p > 0.30). Since the primary hypotheses focused on relationships between atypical object use and outcome status, no further analysis of differences as a function of recruitment group were conducted.
Participants were classified into one of three outcome groups at 24 and 36 months of age, using the following definitions:
Autism/ASD group: scored over the ASD cutoff on the Autism Diagnostic Observation Schedule and on the Social Communication Questionnaire, and the DSM-IV clinical best estimate judgment of an expert clinician was consistent with these scores. Outcomes of autism and ASD were grouped together because of the small size of the subgroups and because their distinction is tentative so early in development.
Other delays group: scored more than 1.5 standard deviations below the mean on one or more scales of the Mullen, or the clinical best estimate judgment of an expert clinician was of a behavior problem or developmental delay and did not meet autism/ASD criteria. Children in this group had clinical diagnoses of general developmental delay, speech/language delay, marked hyperactivity, or marked anxiety.
No concerns group: did not meet criteria for the other two outcome classifications.
At outcome assessment, nine children met criteria for the autism/ASD outcome group (eight of these diagnoses confirmed at 36 months), 10 met criteria for the other delays outcome group (six of these classifications confirmed at 36 months), and the remaining 47 fell in the no concerns outcome group (23 of these classifications confirmed at 36 months). For the 37 children with both 24 and 36 month outcome data, stability was relatively high. Overall, 31 of 37 classifications (83.8%) were the same at both ages. Of the six children who changed outcome groups between 24 and 36 months, one went from the autism/ASD to the other delays group, two went from the other delays to the autism/ASD group, and three went from the no concerns to the other delays group. In all cases, when outcome data were available at both 24 and 36 months of age, the later outcome classification was used in analyses.
The rates of adverse developmental outcomes were marginally higher in the autism sibling group (χ2 = 5.31, p = 0.07), with eight of nine children with autism/ASD classifications and six of 10 children with other delays having older siblings with autism. The only other significant difference between the three outcome groups on demographic variables was in gender, with the autism/ASD outcome group significantly more likely to be male. See Table 1 for characteristics of the sample.
Table 1.
Demographic characteristics of the sample by outcome groups
| Autism/ASD | Other delays | No concerns | Sig. | |
|---|---|---|---|---|
| Chronological age at task administration in months (mean, SD) | 12.0 (0.5) | 12.2 (0.3) | 12.1 (0.4) | n.s. |
| Gestational age in weeks (mean, SD) | 39.2 (1.9) | 39.2 (1.9) | 39.3 (1.5) | n.s. |
| Gender (% male) | 100.0 | 70.0 | 53.2 | p < 0.05 |
| Outcome assessment (% at 36 months) | 88.9 | 60.0 | 48.9 | n.s. |
| Ethnicity/race (% non-white, non-Hispanic) | 44.4 | 20.0 | 29.8 | n.s. |
| Income levela (mean, SD) | 3.1 (1.6) | 3.9 (1.5) | 3.9 (1.5) | n.s. |
3.0 = $50,000–$75,000.
Measures
Object Exploration task
Four objects (a round metal lid, a round plastic ring, a rattle, and a plastic baby bottle) were presented to the infant, one at a time, for 30 seconds each. Behavior was recorded on DVD and coded later by raters unaware of group membership, using Noldus Observer software. Eight uses of the objects were coded as either frequency or duration:1 four behaviors hypothesized to be typical, age-appropriate explorations of the objects (shaking, banging, mouthing, throwing), and four hypothesized to be atypical uses of the objects (spinning, rolling, rotating, unusual visual exploration).2 Coders were initially trained to 90 percent agreement on all codes. An additional 15 percent of the files were double-coded to examine ongoing reliability. Intraclass correlation co-efficients (ICCs) ranged from 0.73 for banging to 0.98 for mouthing (mean ICC across the eight codes was 0.91). See Table 2 for description of the codes.
Table 2.
Behaviors coded
| Code | Description |
|---|---|
| Typical uses of object | |
| Shakes/waves | Infant shakes, waves, or twiddles the object while holding it with one or both hands. Coded as duration |
| Bangs/taps | Infant uses one or both hands to hit, bang, or pound on an object, or uses an object to hit, bang, or pound another object, such as the table. Coded as duration |
| Mouths | Infant licks, sucks, or chews on an object. Instances where the child was sucking on the nipple of the bottle were not scored. Coded as duration |
| Throws/pushes | Infant throws the object, drops it off the table, or pushes it toward the examiner, ending their interaction with that object. Coded as frequency |
| Atypical uses of object | |
| Spins | Infant drops, tosses, or manipulates an object in order to make it spin or wobble. Coded as frequency |
| Rolls | Infant pushes a round object along a surface so that it rolls. Coded as frequency |
| Rotates | Infant turns, flips, or rotates object at least twice. Coded as duration |
| Unusual visual | Infant engages in prolonged visual inspection (>10 seconds), examines object from odd angles or peripheral vision, or squints or blinks repeatedly while examining object. Coded as duration |
Autism Diagnostic Observation Schedule (ADOS: Lord et al., 2000)
The ADOS is a semi-structured standardized interaction and observation that measures symptoms of autism. It has two empirically derived cutoffs, one for ASD and one for autistic disorder. It was administered at 24 and 36 months of age.
Social Communication Questionnaire (SCQ: Berument et al., 1999)
This parent-report questionnaire is composed of 40 yes/no questions, designed to evaluate communication skills and social functioning. It was originally developed for use with children over 4 years of age, but later studies have supported its use in younger children as well (sensitivity of 0.68 and specificity of 0.74 in discriminating ASD from non-spectrum cases: Corsello et al., 2007). The SCQ was administered at the 24 and 36 month visits.
Mullen Scales of Early Learning (MSEL: Mullen, 1995)
The MSEL is a standardized developmental test for children ages birth to 68 months. Four subscales were administered: fine motor, visual reception, expressive language, and receptive language.
Procedure
This study was conducted under the approval of the Institutional Research Review Board at the University of California, Davis. The study was explained to parents orally and in writing, all their questions were answered, and consent was obtained before conducting assessments. The Object Exploration task was administered at the 12 month visit as part of a larger experimental battery administered at that age. It was given in the last half-hour of a 2 hour session that included multiple breaks.
Results
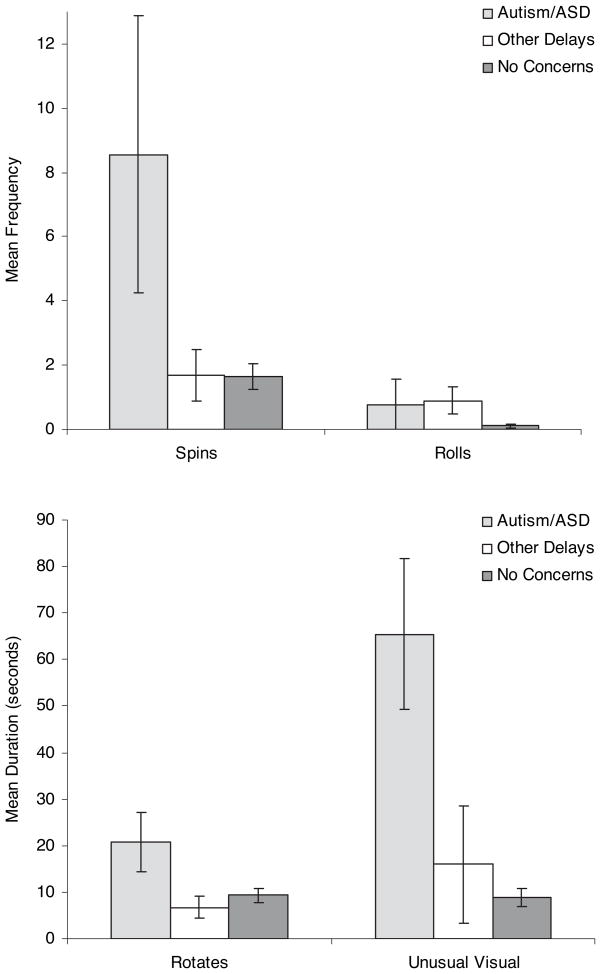
There were no group differences in three of four age-appropriate uses of the objects (banging, shaking, and mouthing; all p > 0.10). A significant group effect was observed for throwing (F = 6.25, p < 0.01), with the other delays group (mean = 8.20, SD = 5.12) significantly more likely to throw objects than the no concerns group (mean = 3.29, SD = 3.26; t = 3.52, d.f. = 56, p < 0.001). In contrast, as shown in Table 3, a significant group effect was found for each of the atypical uses of objects. Simple comparisons revealed that the autism/ASD group displayed significantly more rotating, spinning, and unusual visual exploration of objects than both the other delays and the no concerns groups (see Table 3 and Figure 1). For rolling, the other delays group rolled objects significantly more than the no concerns group (t = 2.21, d.f. = 56, p < 0.05). The comparison between the autism/ASD group and the no concerns group approached significance.
Table 3.
Means and standard deviations for atypical object behaviors
| Autism/ASD | Other delays | No concerns | F | Sig. | |
|---|---|---|---|---|---|
| Spins (frequency) | 8.56 (12.91) | 1.70b (2.54) | 1.63b (2.68) | 6.78 | p < 0.01 |
| Rolls (frequency) | 0.78 (2.33) | 0.90 (1.37) | 0.11c (0.37) | 3.48 | p < 0.05 |
| Rotates (duration, s) | 20.79 (18.85) | 6.75b (7.50) | 9.36b (10.48) | 4.33 | p < 0.05 |
| Unusual visual (duration, s) | 65.43 (48.67) | 15.95a (39.98) | 8.93a (13.38) | 18.30 | p < 0.001 |
Simple comparisons reveal differences from autism/ASD group at p < 0.001.
Simple comparisons reveal differences from autism/ASD group at p < 0.01.
Simple comparisons reveal differences from autism/ASD group at p < 0.10.
Figure 1.
Group differences in atypical uses of objects
To examine the utility of atypical object exploration in predicting outcome, the atypical object use variables were transformed into z-scores using the mean and standard deviation of the no concerns group. Seven of the nine infants (77.8%) with autism/ASD outcomes displayed at least one atypical object exploration that was 2 standard deviations or more above the no concerns group mean, compared to 50 percent of the other delays and 23.4 percent of the no concerns groups. This difference in the level of atypical object use exhibited by the three groups was highly statistically significant (χ2 = 8.46, p < 0.001). The most common atypical object use in the autism/ASD outcome group was unusual visual exploration, which was displayed by seven of the nine infants. The mean z-score of the autism/ASD group for unusual visual exploration, relative to the no concerns group, was 4.22.
It was hypothesized that (1) there would be continuity between atypical object use at 12 months and later repetitive behavior; (2) children with more atypical object use at 12 months would display more signs of autism at later ages; and (3) children with more atypical object use at 12 months would be functioning lower developmentally at later ages. To examine these predicted relationships, we correlated the rates of spinning, rotating, and unusual visual exploration at 12 months of age with the ADOS restricted and repetitive behavior score, the ADOS overall (communication + social) algorithm score, and the four Mullen scaled scores (visual reception, fine motor, receptive language, expressive language) in all participants with 36 month outcome data (n = 38).
Table 4 contains the results of the correlational analyses. Frequency of spinning objects at 12 months significantly predicted ADOS repetitive behavior and communication + social algorithm scores at 36 months. Duration of rotating objects at 12 months was marginally positively related to ADOS communication + social algorithm score and marginally negatively related to expressive language at 36 months. Finally, duration of unusual visual exploration at 12 months was significantly related to all 36 month variables: it was positively correlated with ADOS repetitive behavior and communication + social algorithm scores and negatively related to all four Mullen scores.
Table 4.
Intercorrelations among 12 month object exploration variables and 36 month outcome variables across all groups (n = 38)
| Spinning (frequency) | Rotating (duration) | Unusual visual (duration) | |
|---|---|---|---|
| ADOS RRB score | 0.34** | 0.22 | 0.39** |
| ADOS CST score | 0.48*** | 0.30* | 0.61*** |
| Mullen visual reception | −0.19 | −0.13 | −0.48*** |
| Mullen fine motor | −0.18 | −0.19 | −0.49*** |
| Mullen receptive language | −0.13 | −0.25 | −0.54*** |
| Mullen expressive language | −0.14 | −0.29* | −0.55*** |
ADOS = Autism Diagnostic Observation Schedule; RRB = restricted and repetitive behavior; CST = communication + social total.
p < 0.10;
p < 0.05;
p < 0.01.
To examine the influence of the infants with ASD outcomes on these results, correlations were recalculated for the n = 29 participants with 36 month outcome data who did not develop autism/ASD. This strengthened the relationship between rotating objects and the ADOS communication + social score, which had been marginally significant in the whole sample, but was statistically significant (r = 0.37, p < 0.05) in the subsample who did not develop autism/ASD. The significant negative relationships between unusual visual exploration and Mullen scores were also maintained (visual reception, r = −0.29, p < 0.10; fine motor, r = −0.44, p < 0.05; receptive language, r = −0.42, p < 0.05; expressive language, r = −0.46, p < 0.05) in 36-month-olds who did not develop autism/ASD. Further breakdown of the sample into siblings of children with autism (n = 12) and siblings of children with typical development (n = 17) revealed few differences in the magnitude of correlation coefficients in the two subgroups.3 Thus, the relationships between atypical object use and later developmental function were not driven exclusively by the subset of the sample who developed full-blown autism spectrum disorders, or by the subset who had older siblings with autism.
Discussion
In this prospective study we developed a task that provided specific presses for both typical and atypical uses of objects and administered it to 66 12-month-old infants, of whom nine were later diagnosed with autism or ASD. The study examined: (1) group differences in object use at 12 months; and (2) predictive relationships between atypical object use at 12 months and cognitive and symptomatic status at 36 months. The findings include significantly more atypical uses of the objects in the infants later diagnosed with autism/ASD, high rates particularly of one specific behavior – unusual visual exploration of objects – and strong relationships between early atypical object use and later social-communicative and cognitive functioning. These results suggest that emerging autism may be marked, contrary to earlier findings (Cox et al., 1999; Lord, 1995), by the presence of repetitive behaviors as early as the first birthday.
This study found that unusual visual exploration may be a particularly distinctive feature of the early autism phenotype, with seven of nine subjects displaying very high rates of this behavior. Similarly, Zwaigenbaum and colleagues (2005) reported that 12-month-old infants in a high-risk prospective sample who were later diagnosed with ASD spent longer periods of time looking at objects, by parent report, than low-risk infants and siblings who did not develop ASD. The results of the present study are also consistent with a recent report by Mottron et al. (2007), who found frequent lateral glances toward moving stimuli in a study of preschool-aged children with autism. Since lateral vision filters high spatial frequency (detail) information, they proposed that the high rate of lateral glances might be a compensatory attempt to regulate overwhelming amounts of detailed visual information in natural settings.
Another possible explanation for atypical object-based visual exploration is that attentional systems are disrupted in infants developing ASD, just as they are in older individuals with ASD. Multiple studies have shown deficits in various kinds of spatial orienting (e.g. Haist et al., 2005; Townsend et al., 2001) in children and adults with autism, suggesting impairments in frontal-parietal-cerebellar networks. It is unknown whether these attentional impairments are present in infancy, but if so, there could be implications for the manner in which attention is organized over time. If infants with ASD tend to look longer at objects once they can grasp and manipulate them, and experience even slight differences in orienting to other stimuli in the environment, this could result in a developmental cascade in which attention to objects grows over time. By the second year of life, this focus on objects and toys rather than people becomes clear (Chawarska and Volkmar, 2005; Swettenham et al., 1998).
Alternatively, the social-motivational hypothesis (Dawson et al., 2002; Schultz, 2005) posits that social stimuli (faces, voices) hold diminished reward value for infants with ASD, leading to reduced motivation to engage socially with people. If infants with ASD do not fully participate in interactions that are a source of rich sensory experiences, perhaps they seek out other avenues for sensory stimulation. In this case, the atypical visual interests in ASD might result from primary impairments in the social reward system in the first year of life. A recent study by Bruckner and Yoder (2007) reported that restricted object use was significantly correlated with joint attention and imitation scores 6 months later. These authors suggested that restricted object play reduced the amount of attention paid to people, which further constrained the number and quality of learning opportunities, perhaps resulting in secondary neurological effects (Mundy and Neal, 2001). We found similar predictive associations between early object use and later social, communication, and cognitive functioning. The current study was not, however, designed to distinguish among the various theoretical explanations for early atypical object use. Object exploration data were collected at only one time point and standardized measures of social behavior, such as the ADOS, were not administered concurrently. Future studies of infants at risk for ASD are needed that examine the development of both social and non-social attention in a more systematic manner in order to draw conclusions regarding underlying mechanisms and directionality of effects.
There are several explanations for why our results differ from previous studies that have failed to find early group differences in children later diagnosed with autism. Most of these explanations revolve around methodological issues. One of the strengths of the present study was that it was a prospective investigation, in which measures of object use were collected long before diagnoses were made and then behavior was analyzed by coders unaware of the outcome status of the infants. Many previous studies employed retrospective methods that may be less sensitive to subtle developmental differences such as atypical object use. Earlier investigations that used home movie methods may not have captured the behaviors observed in the present study because parents filming their child may filter abnormal behavior (Palomo et al., 2006) or do not tend to videotape children involved in object play. Parent report measures used in other studies typically do not ask about object use specifically (either omitting it altogether or collapsing it with other repetitive behaviors, such as motor stereotypies and compulsive behaviors). The objects we used were chosen to afford opportunities for specific repetitive behaviors, such as spinning and unusual looking, that were seen in pilot work during task development and have been described in other recent studies (Wetherby et al., 2004).
With the recent strong recommendations of the American Academy of Pediatrics (Johnson et al., 2007) that all children be screened for autism at least twice before the second birthday, there are implicit mandates for the identification of reliable early markers of autism and their incorporation into screening procedures. Several parent-report measures have been developed for use in the first and second years (Dietz et al., 2006; Reznick et al., 2007; Wetherby et al., 2002). The present study suggests that direct assessment and observation may also be helpful in early identification of developmental abnormalities. To be feasibly implemented in primary care settings, simple behavioral probes that can be administered quickly and interpreted efficiently are needed. The CHAT, a screening tool developed for use by pediatricians at the 18 month well-child visit (Baron-Cohen et al., 1992), included such direct probes, although object use was not among them. Earlier work from our lab suggests that an important probe may be observation of the infant’s response to name (Nadig et al., 2007). The work reported in this article suggests that another potential early marker of developing autism is atypical use of simple objects, particularly involving unusual or prolonged visual exploration and spinning or rotating of objects while examining them. Based on our own pilot work and studies of others (e.g. Wetherby et al., 2004), round flat objects, which can be spun or wobbled, and long cylindrical objects that can be shaken, twiddled or rolled, may be particularly successful in eliciting unusual behaviors in certain infants. It will be critical for future studies to examine whether ratings of atypical object use can improve sensitivity and specificity of early identification and the development of new screening instruments.
Acknowledgments
The work in this article was supported by grant R01 MH068398 from the National Institute of Mental Health. We are very grateful to Grace Baranek and Geraldine Dawson, who shared their repetitive behavior coding systems with us. Thank you to Diane Larzelere for preparation of the manuscript. Finally, we are greatly indebted to the children and families who participated in this longitudinal study.
Footnotes
Behaviors that occurred quickly, without explicit onset and offset times, such as throwing, rolling, and spinning an object, were coded as frequency (event) variables. All other object use behaviors, which were of more prolonged length and had onset and offset times that could be reliably determined, were coded as duration variables, in seconds.
Object uses were classified as atypical based on previous descriptions of repetitive behaviors with objects (Mottron et al., 2007; Wetherby et al., 2004) and on pilot work that examined how typically developing infants used the four objects included in this task. All four atypical behaviors occurred very infrequently or were never observed in typically developing infants in this pilot work.
The mean absolute value of the correlation coefficients reported in Table 4 was 0.29 for the autism siblings (n = 12) and 0.25 for the typical siblings (n = 17). Few correlations within the autism or typical sibling subgroups were statistically significant, however, due to the small sample sizes.
Contributor Information
SALLY OZONOFF, MIND Institute, Sacramento, USA.
SUZANNE MACARI, Yale University, USA.
GREGORY S. YOUNG, MIND Institute, Sacramento, USA
STACY GOLDRING, MIND Institute, Sacramento, USA.
MEAGAN THOMPSON, MIND Institute, Sacramento, USA.
SALLY J. ROGERS, MIND Institute, Sacramento, USA
References
- BARANEK G. Autism during Infancy: A Retrospective Video Analysis of Sensory-Motor and Social Behaviors at 9–12 Months of Age. Journal of Autism and Developmental Disorders. 1999;29:213–24. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- BARON-COHEN S, ALLEN J, GILLBERG C. Can Autism be Detected at 18 Months? The Needle, the Haystack, and the CHAT. The British Journal of Psychiatry. 1992;161 (6):839–43. doi: 10.1192/bjp.161.6.839. [DOI] [PubMed] [Google Scholar]
- BERUMENT SK, RUTTER M, LORD C, PICKLES A, BAILEY A. Autism Screening Questionnaire: Diagnostic Validity. The British Journal of Psychiatry. 1999;175:444–51. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- BRUCKNER CT, YODER P. Restricted Object Use in Young Children with Autism. Autism. 2007;11(2):161–71. doi: 10.1177/1362361307075709. [DOI] [PubMed] [Google Scholar]
- CHAWARSKA K, VOLKMAR F. Autism in Infants and Toddlers. In: VOLKMAR F, KLIN A, PAUL R, editors. Handbook of Autism and Pervasive Developmental Disorders. New York: Wiley; 2005. pp. 223–46. [Google Scholar]
- CHAWARSKA K, KLIN A, PAUL R, VOLKMAR F. Autism Spectrum Disorder in the Second Year: Stability and Change in Syndrome Expression. Journal of Child Psychology and Psychiatry. 2007;48(2):128–38. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- CORSELLO C, HUS V, PICKLES A, RISI S, COOK EH, LEVENTHAL BL, LORD C. Between a ROC and a Hard Place: Decision Making and Making Decisions About Using the SCQ. Journal of Child Psychology and Psychiatry. 2007;48:932–40. doi: 10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- COX A, KLEIN K, CHARMAN T, BAIRD G, BARON-COHEN S, SWETTENHAM J, DREW A, WHEELWRIGHT S. Autism Spectrum Disorders at 20 and 42 Months of Age: Stability of Clinical and ADI–R Diagnosis. Journal of Child Psychology and Psychiatry. 1999;40(5):719–32. [PubMed] [Google Scholar]
- DAWSON G, MUNSON J, ESTES A, OSTERLING J, MCPRTLAND J, TOTH K, CARVER L, ABBOTT R. Neurocognitive Function and Joint Attention Ability in Young Children with Autism Spectrum Disorder versus Developmental Delay. Child Development. 2002;73:345–58. doi: 10.1111/1467-8624.00411. [DOI] [PubMed] [Google Scholar]
- DIETZ C, SWINKELS S, VANDAALEN E, VAN ENGELAND H, BUITELAAR JK. Screening for Autistic Spectrum Disorder in Children Aged 14–15 Months. II: Population Screening with the Early Screening of Autistic Traits Questionnaire (ESAT), Design and General Findings. Journal of Autism and Developmental Disorders. 2006;36 (6):713–22. doi: 10.1007/s10803-006-0114-1. [DOI] [PubMed] [Google Scholar]
- EVANS DW, LECKMAN JF, CARTER A, REZNICK JS, HENSHAW D, KING RA, PAULS D. Ritual, Habit, and Perfectionism: The Prevalence and Development of Compulsive-Like Behavior in Normal Young Children. Child Development. 1997;68(1):58–68. [PubMed] [Google Scholar]
- HAIST F, ADAMO M, WESTERFIELD M, COURCHESNE E, TOWNSEND J. The Functional Neuroanatomy of Spatial Attention in Autism Spectrum Disorder. Developmental Neuropsychology. 2005;27:425–58. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- JOHNSON CP, MYERS SM THE COUNCIL ON CHILDREN WITH DISABILITIES. Identification and Evaluation of Children with Autistic Spectrum Disorders. Pediatrics. 2007;120:1183–215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- LOH A, SOMAN T, BRIAN J, BRYSON SE, ROBERTS W, SZATMARI P, SMITH IM, ZWAIGENBAUM L. Stereotyped Motor Behaviors Associated with Autism in High-Risk Infants: A Pilot Videotape Analysis of a Sibling Sample. Journal of Autism and Developmental Disorders. 2007;37(1):25–36. doi: 10.1007/s10803-006-0333-5. [DOI] [PubMed] [Google Scholar]
- LORD C. Follow-Up of Two-Year-Olds Referred for Possible Autism. Journal of Child Psychology and Psychiatry. 1995;36(8):1365–82. doi: 10.1111/j.1469-7610.1995.tb01669.x. [DOI] [PubMed] [Google Scholar]
- LORD C, RISI S, LAMBRECHT L, COOK EH, LEVENTHAL BL, DILAVORE PC, PICKLES A, RUTTER M. The Autism Diagnostic Observation Schedule–Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders. 2000;30:205–23. [PubMed] [Google Scholar]
- MOTTRON L, MINEAU S, MARTEL S, BERNIER CS, BERTHIAUME C, DAWSON M, LEMAY M, PALARDY S, CHARMAN T, FAUBERT J. Lateral Glances Toward Moving Stimuli among Young Children with Autism: Early Regulation of Locally Oriented Perception? Development and Psychopathology. 2007;19:23–36. doi: 10.1017/S0954579407070022. [DOI] [PubMed] [Google Scholar]
- MULLEN EM. Mullen Scales of Early Learning. Circle Pines, MN: AGS; 1995. [Google Scholar]
- MUNDY P, NEAL R. Neural Plasticity, Joint Attention and Autistic Developmental Pathology. In: GLIDDEN L, editor. International Review of Mental Retardation Research. Amsterdam: American Press; 2001. pp. 139–68. [Google Scholar]
- NADIG AS, OZONOFF S, YOUNG GS, ROZGA A, SIGMAN M, ROGERS SJ. A Prospective Study of Response to Name in Infants at Risk for Autism. Archives of Pediatric and Adolescent Medicine. 2007;161(4):378–83. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- OSTERLING J, DAWSON G. Early Recognition of Children with Autism: A Study of First Birthday Home Videotapes. Journal of Autism and Developmental Disorders. 1994;24(3):247–57. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- PALOMO R, BELINCHON M, OZONOFF S. Autism and Family Home Movies: A Comprehensive Review. Journal of Developmental & Behavioral Pediatrics. 2006;27:S59–S68. doi: 10.1097/00004703-200604002-00003. [DOI] [PubMed] [Google Scholar]
- REZNICK JS, BARANEK GT, REAVIS S, WATSON LR, CRAIS ER. A Parent-Report Instrument for Identifying One-Year-Olds at Risk for an Eventual Diagnosis of Autism: The First Year Inventory. Journal of Autism and Developmental Disorders. 2007;37(9):1691–710. doi: 10.1007/s10803-006-0303-y. [DOI] [PubMed] [Google Scholar]
- RICHLER J, BISHOP SL, KLEINKE JR, LORD C. Restricted and Repetitive Behaviors in Young Children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2007;37:73–85. doi: 10.1007/s10803-006-0332-6. [DOI] [PubMed] [Google Scholar]
- SCHULTZ R. Developmental Deficits in Social Perception in Autism: The Role of the Amygdala and Fusiform Face Area. International Journal of Developmental Neuroscience. 2005;23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- STONE WL, LEE EB, ASHFORD L, BRISSIE J, HEPBURN SL, COONROD EE, WEISS B. Can Autism Be Diagnosed Accurately in Children under 3 Years? Journal of Child Psychology and Psychiatry. 1999;40:219–26. [PubMed] [Google Scholar]
- SWETTENHAM J, BARON-COHEN S, CHARMAN T, COX A, BAIRD G, DREW A, REES L, WHEELWRIGHT S. The Frequency and Distribution of Spontaneous Attention Shifts between Social and Nonsocial Stimuli in Autistic, Typically Developing, and Nonautistic Developmentally Delayed Infants. Journal of Child Psychology and Psychiatry. 1998;39:747–53. [PubMed] [Google Scholar]
- THELEN E. Rhythmical Stereotypies in Normal Human Infants. Animal Behavior. 1979;27(3):699–715. doi: 10.1016/0003-3472(79)90006-x. [DOI] [PubMed] [Google Scholar]
- TOWNSEND J, WESTERFIELD M, LEAVER E, MAKEIG S, TZYY-PING J, PIERCE K, COURCHESNE E. Event-Related Brain Response Abnormalities in Autism: Evidence for Impaired Cerebello-Frontal Spatial Attention Networks. Cognitive Brain Research. 2001;11:127–45. doi: 10.1016/s0926-6410(00)00072-0. [DOI] [PubMed] [Google Scholar]
- TURNER M. Annotation: Repetitive Behavior in Autism: A Review of Psychological Research. Journal of Child Psychology and Psychiatry. 1999;40(6):839–49. [PubMed] [Google Scholar]
- WERNER E, DAWSON G. Validation of the Phenomenon of Autistic Regression Using Home Videotapes. Archives of General Psychiatry. 2005;62(8):889–95. doi: 10.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- WERNER E, DAWSON G, MUNSON J, OSTERLING J. Variation in Early Developmental Course in Autism and Its Relation with Behavioral Outcome at 3–4 Years of Age. Journal of Autism and Developmental Disorders. 2005;35(3):337–50. doi: 10.1007/s10803-005-3301-6. [DOI] [PubMed] [Google Scholar]
- WETHERBY AM, ALLEN L, CLEARY J, KUBLIN K, GOLDSTEIN H. Validity and Reliability of the Communication and Symbolic Behavior Scales Developmental Profile with Very Young Children. Journal of Speech and Language Hearing Research. 2002;45(6):1202–18. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- WETHERBY AM, WOODS J, ALLEN L, CLEARY J, DICKINSON H, LORD C. Early Indicators of Autism Spectrum Disorders in the Second Year of Life. Journal of Autism and Developmental Disorders. 2004;34(5):473–93. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- ZWAIGENBAUM L, BRYSON S, ROGERS T, ROBERTS W, BRIAN J, SZATMARI P. Behavioral Manifestations of Autism in the First Year of Life. International Journal of Developmental Neuroscience. 2005;23:143–52. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]