Abstract
Rationale
Long chain fatty acids (LCFA) are the preferred substrate for energy provision in hearts. However, the contribution of endogenous triacylglyceride (TAG) turnover to LCFA oxidation and the overall dependence of mitochondrial oxidation on endogenous lipid is largely unstudied.
Objective
We sought to determine the role of TAG turnover in supporting LCFA oxidation and the influence of the lipid-activated nuclear receptor, PPARα, on this balance.
Methods and Results
Palmitoyl turnover within TAG and palmitate oxidation rates were quantified in isolated hearts, from normal mice (non-transgenic, NTG) and mice with cardiac-specific overexpression of PPARα (MHC-PPARα). Turnover of palmitoyl units within TAG, and thus palmitoyl-CoA recycling, in NTG (4.5± 2.3 μmoles/min/gdw) was 3.75-fold faster than palmitate oxidation (1.2 ±0.4). This high rate of palmitoyl unit turnover indicates preferential oxidation of palmitoyl units derived from TAG in normal hearts. PPARα overexpression augmented TAG turnover 3-fold over NTG hearts, despite similar fractions of acetyl-CoA synthesis from palmitate and oxygen use at the same workload. Palmitoyl turnover within TAG of MHC-PPARα hearts (16.2 ± 2.9, P<0.05) was 12.5-fold faster than oxidation (1.3 ± 0.2). Elevated TAG turnover in MHC-PPARα correlated with increased mRNA for enzymes involved in both TAG synthesis, Gpam, Dgat1, and Agpat3, and lipolysis, Pnliprp1.
Conclusions
The role of endogenous TAG in supporting β-oxidation in the normal heart is much more dynamic than previously thought, and lipolysis provides the bulk of LCFA for oxidation. Accelerated palmitoyl turnover in TAG, due to chronic PPARα activation, results in near requisite oxidation of LCFA from TAG.
Keywords: PPARα, triacylglyceride, fatty acid oxidation
Introduction
Long chain fatty acids (LCFA) are well recognized as the preferred substrate for oxidative ATP production by cardiac mitochondria (1–6). To date, the exogenous, blood-borne LCFA have generally been considered as the primary source for fueling oxidative metabolism, with endogenous triacylglyceride (TAG) serving as a biochemically inert lipid store (7–9). The actual involvement of endogenous TAG in supplying LCFA for oxidation by the heart has largely gone unstudied, particularly in non-destructive studies of intact hearts. In light of the recent findings by Haemmerle, et al (10) of increased myocardial TAG in mouse hearts deficient of adipose triglyceride lipase (ATGL+/−), it is enticing to speculate that myocardial TAG is an important contributor to cardiac fatty acid oxidation. This work examines the contributions of steady state TAG to LCFA oxidation, through direct comparison of the rates of TAG turnover and LCFA oxidation in the intact, beating heart.
To probe the link between TAG dynamics and fatty acid oxidation (FAO) rates, we studied hearts of normal mice and a mouse model of a low level of cardiac overexpression of the peroxisome proliferator-activated receptor alpha (PPARα) following either normal diet or high fat diet (HFD). This model, which recapitulates many metabolic abnormalities of the diabetic heart, has been previously shown to have elevated TAG content and augmented expression of fatty acid oxidation enzymes that are exacerbated by HFD (5–6, 11–12). While PPARα has been clearly linked to altered expression of enzymes for fatty acid oxidation in diseased hearts, the potential role of, and mechanisms by which PPARα regulates fatty acid storage as TAG and the turnover of this endogenous lipid pool remains largely unknown. Through a comprehensive analysis of cardiac TAG dynamics and LCFA oxidation rates, the findings elucidate a preferential utilization of LCFA that cycles LCFA through the highly dynamic TAG pool as a primary source of fatty acid oxidation.
Methods
Animal model
Male mice with cardiac specific overexpression of PPARα, driven by the alpha myosin heavy chain promoter, (MHC-PPARα) and non-transgenic littermates (NTG), weighing ~25g at 12 weeks of age, were used for this study (12). We chose to study the previously described, low-level overexpressing MHC-PPARα transgenic mouse line (404-4), 404-4, that does not display cardiac dysfunction at this age, in contrast to mice with higher levels of transgene overexpression (12). Mice were backcrossed to C57Bl/6J six times. Mice were supplied either a regular chow diet (RCD) or high fat diet (HFD) (Teklad #97268) for two weeks prior to experimentation, and fed ad libitum in light and temperature controlled housing. All procedures were approved by the University of Illinois at Chicago Animal Care and Use Committee.
Isolated heart protocols
Mice were heparinized (50 U/10 g, i.p.) and anesthetized (ketamine, 80 mg/kg, plus xylazine,12 mg/kg, i.p.). Hearts were excised and perfused in retrograde fashion with modified Krebs-Henseleit buffer (118.5 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 1.2 mM MgSO4 and 1.2 mM KH2PO4) maintained at 37 °C, equilibrated with 95% O2/5% CO2 and containing 0.4 mM unlabeled palmitate/complexed to albumin (3:1 molar ratio) and 10 mM glucose. Left ventricular developed pressure (LVDP) and heart rate (HR) were continuously recorded from a water-filled baloon in the left ventricle with a pressure transducer for digital recording (Powerlab, AD Instruments, Colorado Springs, CO). At the end of each experiment, hearts were frozen in liquid N2 cooled tongs.
For TAG dynamics, isolated hearts from both NTG and MHC-PPARα mice were perfused in a 14.1 T NMR magnet at baseline workload (NTG N=7; MHC-PPARα N= 12) or with adrenergic challenge (0.1 μmole isoproterenol) (NTG N=5; MHC-PPARα N= 6). After collection of 13C-NMR background signals of naturally abundant 13C (1.1%), hearts were switched to buffer containing 0.4 mM [2,4,6,8,10,12,14,16 −13C8] palmitate (Isotec, Inc., Miamisburg, OH) plus 10 mM unlabeled glucose.
Perfusion with 13C-enriched media continued for 20 minutes at baseline workload for mice fed on RCD (MHC-PPARα, n=10; NTG n=12) and mice on HFD (MHC-PPARα, n=7; NTG, n=4), and for 10 minutes during adrenergic challenge (0.1 μM isoproterenol) (MHC-PPARα, n=5; NTG n=8). Additional hearts were perfused for 120 minutes to ensure stability of TAG turnover and content over time (n=4).
For palmitate oxidation rates, hearts from MHC-PPARα and NTG mice on either RCD (MHC-PPARα, n = 6; NTG, n = 4) or HFD (MHC-PPARα, n = 7; NTG, n = 5) were perfused for 30 minutes with 0.4 mM or 1.2 mM [4,6,8,10,12,14,16,−13C7] palmitate and 10 mM glucose.
NMR spectroscopy and tissue chemistry
NMR measurements of TAG turnover were performed on perfused hearts with sequential, proton-decoupled carbon-13 (13C) NMR spectra (2 min each) with 13C natural abundance correction, as previously reported (14–15).
13C enrichment of TAG in the heart was monitored from the NMR signal at 30.5 ppm from the TAG methylene groups. TAG turnover was calculated from total TAG content and enrichment over time (15–18). Kinetic analysis of dynamic 13C-spectra from hearts was performed as previously reported (14–15, 17–18).
Metabolic flux was determined during 13C palmitate oxidation in the intact mouse heart using a previously described method for kinetic analysis of the progressive 13C enrichment of glutamate, as detected via NMR (14, 20, 22–24).
For kinetic analysis of oxidative rates, glutamate, aspartate, citrate malate and α-ketoglutarate contents in frozen myocardial samples were assayed spectrophotometrically and fluorometrically (19–20). In vitro 13C NMR was performed on acid extracts of myocardium to determine fractional enrichment of [2-13C] acetyl CoA (21–22).
Lipid extracts were obtained from tissue and TAG quantified by colorimetric assay (Wako Pure Chemical Industries.) (15). The fractional 13C enrichment of TAG was assessed by liquid chromatography/mass-spectrometry (LC/MS) analysis. LCFA content in TAG, of carbon lengths 12–18, was determined by LCMS as a percentage of total LCFA. Total TAG turnover (nmoles TAG/min/mg protein) was quantified from 13C enrichment rates and the endpoint 13C enrichment (15–18).
Rates of palmitate unit turnover within the TAG pool were determined from TAG turnover rates and the percentage of acyl units represented by palmitate ([12C + 13C] palmitate) present in the TAG pool. LCMS analysis enabled determination of the percentage of each LCFA present in the TAG pool. From the stoichiometry of 3 fatty acyl groups per TAG molecule and the percentage of palmitate present in the TAG pool, TAG turnover rates were converted to rates of palmitate unit turnover within the TAG pool.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from hearts from MHC-PPARα and NTG mice on a regular chow diet (RCD: MHC-PPARα: N=6; NTG: N=7) or high fat diet (HFD: MHC-PPARα: N=5; NTG: N=7) with TRIzol reagent (Invitrogen) and reverse transcribed with Superscript III Reverse Transcriptase (Invitrogen). Equal amounts of cDNA were subjected to real-time PCR using SYBR Green as a probe as described previously (25). Data were corrected by expressing them relative to the levels of the invariant transcript Peptidylprolyl isomerase (Ppia, a.k.a. cyclophilin) and normalized to wild-type controls on standard chow, which were arbitrarily assigned as 1.0.
Results
Isolated Mouse Heart Hemodynamics and Oxygen Use
Baseline rate-pressure-products (RPP) were similar between hearts of transgenic (MHC-PPARα) and nontransgenic (NTG) mice: MHC-PPARα: 35,000 ± 3,000 beats*mmHg/min; NTG: 35,000 ± 3,000 beats*mmHg/min. Baseline oxygen consumption (MVO2) were also similar between MHC-PPARα and NTG mice, MHC-PPARα: 42.2 ± 9.1 μmoles/min/g; NTG: 38.0 ± 3.8 μmoles/min/g. With isoproterenol stimulation, RPP increased by 44% ± 4.9% and 42% ± 8.3% in the NTG and MHC-PPARα mice, respectively (P<0.05) and oxygen use increased to MHC-PPARα: 74.4 ± 18.1 μmoles/min/g; NTG: 65.9 ± 8.0 μmoles/min/g. Both NTG and MHC-PPARα hearts showed similar RPP under each condition. Thus, the metabolic demand, which is dominated by work performance, was also similar between groups, despite the significant differences in lipid dynamics. A 2 week HFD did not affect MVO2 (MHC-PPARα: 36.4 ± 2.2 μmoles/min/g; NTG: 38.0 ± 6.9) nor RPP (MHC-PPARα: 34,000 ± 4,000 beats*mmHg/min; NTG: 36,000 ± 2,000 beats*mmHg/min).
TAG content and dynamics
MHC-PPARα hearts displayed elevated TAG content (Figure 1) (11). TAG stores remained constant over the duration of each protocol, confirming steady state conditions for turnover measurements (Figure 2) (26). TAG contents at both 20 minutes and 2 hours of perfusion were similar (NTG: 15 ± 1 nmole/mg protein at 20 minutes of perfusion vs. 14 ± 1 at 120 minutes; MHC-PPARα: 20 ± 2 nmole/mg protein at 20 minutes vs. 26 ± 2 at 120 minutes). LC/MS analysis of purified TAG samples revealed that at baseline 13C palmitate comprised a significantly larger percentage of fatty acyl groups within TAG in MHC-PPARα hearts than NTG (Table 1). This finding is consistent with the augmented long-chain fatty acid storage as TAG in MHC-PPARα, which results in higher TAG content.
Figure 1.
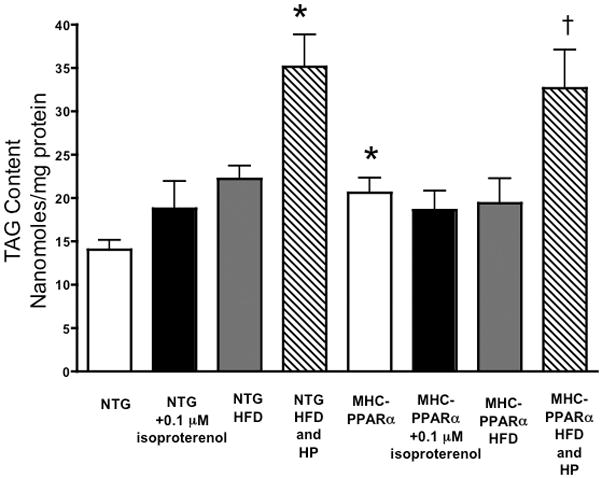
Triacylglyceride content in hearts of MHC-PPARα low over-expressing mice and nontransgenic littermates on a regular chow diet or high fat diet (HFD) perfused with 0.4 mM palmitate or 1.2 mM palmtiate (high palmitate, HP). TAG content reported in nanomoles/mg protein. * P < 0.05, vs. NTG baseline; † P < 0.05, vs NTG HFD and MHC-PPARα HFD.
Figure 2.
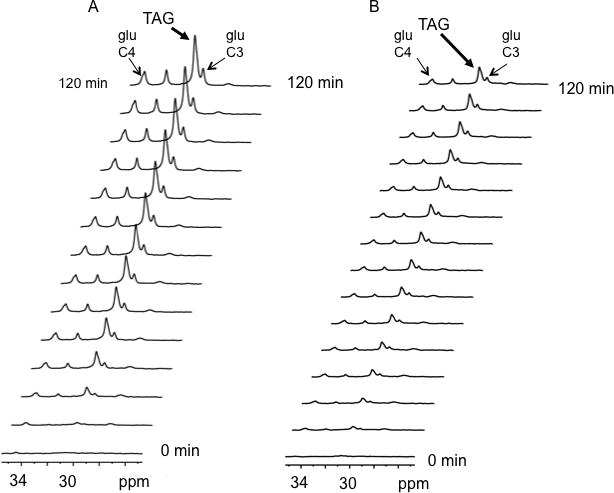
Representative 13C spectra from isolated mouse hearts displaying progressive 13C enrichment of TAG and glutamate over 120 minutes from A) MHC-PPARα and B) NTG hearts perfused with 0.4 mM [2,4,6,8,10,12,14,16 13C8] palmitate + 10 mM unlabeled glucose. The spectra signals from 13C enriched methylene carbons of TAG, at 30.5 ppm, due to 13C palmitate storage and the 4- and 3-carbons of glutamate produced by oxidation of 13C palmitate, at 34 and 28 ppm respectively (glu C-4 and glu C-3).
Table 1.
Total palmitate (endogenous 12C-palmitate + exogenous 13C-palmitate) as a percentage of total fatty acyl units in triacylglyceride (TAG). Rate of palmitate turnover in TAG (μmoles/min/gdw).
| NTG | MHC-PPARα | |||
|---|---|---|---|---|
| Baseline | 0.1 μM Isoproterenol | Baseline | 0.1 μM Isoproterenol | |
| Total Palmitate (% Units of Total Fatty Acyl Units of Triacylglyceride) | 23.4 ± 1.9 | 14.6 ± 4.1 | 32.7 ± 2.5 | 35.6 ± 3.5 * |
| Palmitate Turnover in TAG (μmoles/min/g dw) | 4.5 ± 0.4 | 4.0 ± 2.3 | 16.2 ± 2.9 † | 36.7 ± 7.7 ‡ |
Significantly different versus NTG baseline conditions, P<0.05.
P<0.005, vs. NTG baseline.
P<0.005, vs baseline MHC-PPARα.
β-adrenergic challenge did not alter myocardial TAG in either group. β-adrenergic stimulation did not affect the 13C palmitate incorporation into TAG, despite a potentiated increase in TAG turnover (Table 1; Figure 3). TAG content was maintained in both groups, despite the higher energetic demands of increased workload.
Figure 3.
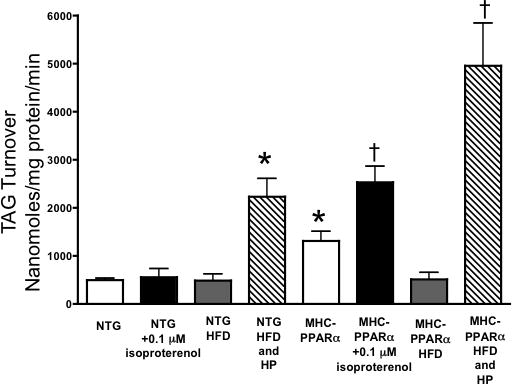
Triacylglyceride turnover in MHC-PPARα low over-expressing mice and NTG littermates. * P < 0.05, vs. NTG baseline TAG. † P < 0.05, vs. NTG + isoproterenol and PPARα baseline.
HFD did not affect TAG content in hearts perfused with 0.4 mM palmitate. Elevated concentrations of palmitate in the buffer (1.2 mM) did increase TAG content in hearts of mice fed a HFD (Figure 1). This different response between 0.4 and 1.2 mM palmitate could be due to net use of TAG stores with lower exogenous FA concentration, partially depleting the otherwise elevated TAG content. However, TAG turnover significantly increased in both NTG and MHC-PPARα hearts perfused with 1.2 mM palmitate. HFD did not alter expression of genes that regulate TAG turnover, thus the increases in TAG turnover appear related to the increase in exogenous FA supply.
Overall, TAG dynamics were significantly elevated in the hearts of MHC-PPARα mice, yet the 13C enrichment of acetyl CoA from palmitate was similar in both groups, irrespective of diet (Figure 4). The combined results of TAG turnover and palmitate oxidation indicate that both groups rely to a great extent on LCFA that initially esterified to TAG prior to oxidation.
Figure 4.

A. Acetyl CoA enrichment from 13C palmitate in MHC-PPARα low over-expressing mice (PPARα) and NTG littermates. B. Rates of palmitate oxidation and MHC-PPARα low over-expressing mice (PPARα) and NTG littermates (μmoles/min/gdw). C. Palmitate turnover of TAG units in NTG and MHC-PPARα low over-expressing mice (MHC-PPARα) (μmoles/min/gdw). D. Acetyl CoA enrichment from 13C palmitate in MHC-PPARα low over-expressing mice (PPARα) and NTG littermates fed a high fat diet (HFD) for 2 weeks and perfused with 1.2 mM Palmitate. E. Palmitate oxidation rates from MHC-PPARα low over-expressing mice (PPARα) and NTG littermates (μmoles/min/gdw) fed a HFD and perfused with 1.2 mM Palmitate. F. Palmitate turnover of TAG units in NTG and MHC-PPARα low over-expressing mice (MHC-PPARα) fed a HFD and perfused with 1.2 mM Palmitate (μmoles/min/gdw). * P<0.05 vs. NTG.
Palmitoyl unit turnover within TAG and oxidation rates
Table 1 shows palmitate content (12C and 13C) in TAG, as a percentage of total fatty acyl groups, and palmitate turnover rates within the TAG pool for each group. Palmitate turnover within the TAG pool of MHC-PPARα hearts was four-fold higher than that of NTG hearts. Unlike NTG, MHC-PPARα hearts responded to isoproterenol challenge by doubling the turnover rate of palmitate within TAG.
Palmitate oxidation rates (Figure 4B), were 1.2 ± 0.4 and 1.3 ± 0.2 μmoles/min/gdw in NTG and MHC-PPARα hearts, respectively (Figure 4B), consistent with previous reports (5, 11, 27–30). Palmitate oxidation rates at baseline work from mice on a RCD or a HFD were similar between the MHC-PPARα and NTG groups. In contrast, TAG turnover was much greater in the MHC- PPARα hearts than NTG. Comparison of these palmitate unit turnover rates within TAG at baseline (RCD: NTG = 4.5 ±0.4 to PPARα = 16.2 ±2.9 μmoles/min/gdw; HFD: NTG = 22.9 ±0.5 to PPARα = 63.12 ±26 μmoles/min/gdw) to the respective rates of palmitate oxidation (RCD: NTG = 1.2 ± 0.4 and PPARα = 1.3 ± 0.2 μmoles/min/gdw; HFD: NTG = 0.9 ±0.23 and PPARα = 1.1 ± 0.1 μmoles/min/gdw) indicates a much more rapid rate of palmitate turnover within TAG compared to that of palmitate oxidation (Figure 4D).
From the measured palmitate turnover within the TAG pool and previously published acyl-CoA pool size data, the time for complete turnover of the available acyl-CoA pool is 16 and 4 seconds in NTG and MHC-PPARα hearts, respectively (11). Thus, the turnover of the palmitoyl pool that is available for oxidation is 4 times faster in MHC-PPARα hearts versus NTG hearts. Accounting for palmitate alone, complete exchange of the cytosolic palmitoyl-CoA pool with palmitoyl units from lipolysis of TAG would require 0.33 seconds and 0.09 seconds, for NTG and MHC-PPARα hearts, respectively.
These relative rates indicate a large contribution from total TAG lipolysis to palmitate oxidation in the intact heart. Specifically, the difference between the rates of palmitate turnover in TAG and palmitate oxidation in MHC-PPARα hearts, which is an order of magnitude, indicates a high rate of mixing between exogenous palmitate and TAG-derived palmitate within the pool of fatty acids available for oxidative metabolism. These data provide evidence that the preferential oxidation of TAG-derived fatty acid units is augmented by PPARα activation.
Gene expression
Transcription levels for enzymes involved in TAG trafficking and turnover was determined (Figure 5). In all MHC-PPARα hearts tested, PPARα expression was induced 9–13 fold (not shown). mRNA’s for Gpam, Agpat3 & 5, and Dgat1, all key enzymes involved in TAG synthesis, were higher in MHC-PPARα mice than NTG (Figure 5). Increased Agpat3 is consistent with findings of PPARα regulation of Agpat3 (31). The results for Gpam and Dgat confirm previous findings in MHC-PPARα hearts by Finck et al (32). Pnliprp1 was also increased in MHC-PPARα hearts and is consistent with increased lipolysis (Figure 5). These changes suggest that PPARα activation determines TAG turnover by regulating the expression of genes involved in both TAG synthesis and lipolysis.
Figure 5.
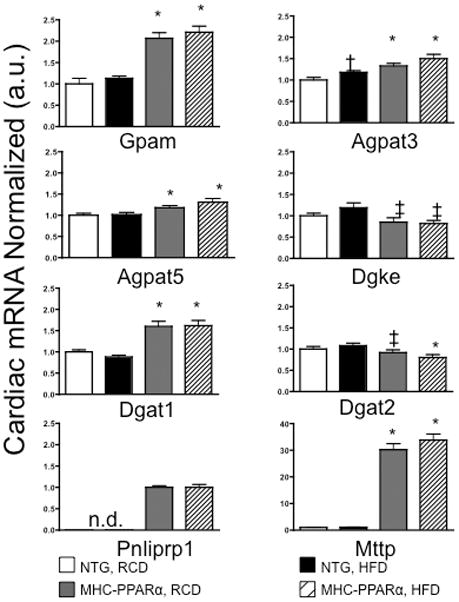
mRNA levels of genes encoding enzymes that regulate TAG synthesis and lipolysis in heart tissue from NTG and MHC-PPARα mice fed a regular chow diet (RCD) or a high fat diet (HFD). * P < 0.03, vs NTG. † Significantly different compared to NTG—RCD. ‡ P < 0.05, vs NTG—HFD. n.d., not detectable.
Discussion
The current study provides evidence that, in normal hearts, fatty acyl units from a comparatively dynamic triacylglyceride pool are preferentially oxidized due to the relative rates of TAG turnover and palmitate oxidation. In otherwise normal, NTG mouse hearts, the high turnover rate of palmitoyl units within TAG, relative to the slower palmitate oxidation rate, indicates a large contribution from TAG to βoxidation. Chronic activation of PPARα, in MHC-PPARα mouse hearts drives oxidation of TAG-derived LCFA to near complete levels, through greatly accelerated TAG turnover rates. While total palmitate oxidation in both the NTG and the MHC-PPARα hearts were relatively similar, the rate of palmitate turnover of TAG units was 4 times greater in the transgenic than NTG hearts at baseline. The augmented TAG dynamics in MHC-PPARα hearts, and thus increased contribution of TAG to β-oxidation, correlates with elevated transcript levels of enzymes involved in both TAG synthesis and degradation. Thus, this mechanism of preferential oxidation of TAG versus exogenous free fatty acids is driven, at least in part, by PPARα via regulation of the expression of enzymes that determine the rates of TAG synthesis and lipolysis.
Both exogenous palmitate and palmitoyl units derived from lipolysis of the intracellular steady state TAG pool contribute to palmitoyl-CoA in the cytosol, as a “commonly accessible” pool for either reincorporation into TAG or oxidation within the mitochondria (Figure 6). From previously reported measures of the acyl pool sizes, the total fatty acyl pool is 140 nmoles/g and 100 nmoles/g in NTG and MHC-PPARα hearts, respectively (11). The palmitoyl-CoA pool sizes were reported at 25 nmoles/g for both NTG and MHC-PPARα (11). Because the rate of turnover of palmitate TAG units is significantly greater than the palmitate oxidation rate, there is significant cycling of palmitoyl units through the TAG pool prior to beta-oxidation. Hearts of PPARα transgenic mice undergo significantly higher rates of TAG turnover compared to NTG, indicating an elevated recruitment of the TAG pool, which is consistent with the upregulation of known lipid metabolism and LCFA oxidative enzymes in this model (Figure 7). In light of work showing rescue of metabolism when hearts with high PPARα overexpression are crossed with mice deficient in the LCFA transporter, CD36, the observed group differences in TAG turnover relative to oxidation suggest a mechanism whereby hearts respond to imbalances between LCFA uptake and oxidation (6).
Figure 6.
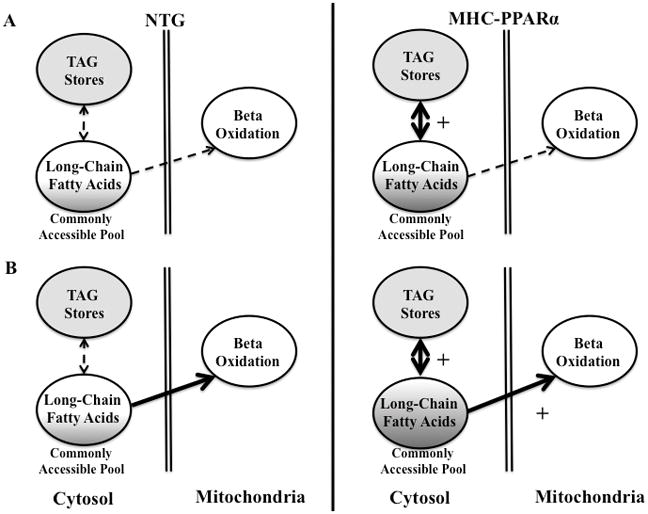
A. Fate of LCFA in the myocardium at baseline. Both MHC-PPARα and NTG hearts exhibit cytosolic mixing of endogenous LCFA (shown in the solid grey) with exogenous LCFA. B. Fate of LCFA during β-adrenergic stimulation. MHC-PPARα hearts exhibit increased mixing to meet energy demands, shown in darker shade. Thickness of arrow indicates pathway preference. Shaded grey areas represent mixing of exogenous and endogenous LCFA. Both figures show bidirectional pathway of LCFA entering and exiting the TAG pool.
Figure 7.
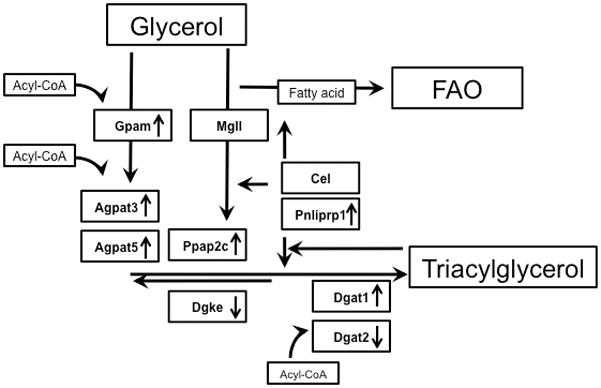
Schematic of enzymatic pathways for the synthesis of triacylglycerol. Up arrow indicates an increased expression in the transgenic model. Down arrow indicates a decreased enzyme expression.
Differences in the time required for complete turnover of the palmitoyl-content in the TAG pool, mean that 26% of the cytosolic palmitoyl-CoA pool is oxidized in NTG hearts, whereas 8% of palmitoyl-CoA in this pool is oxidized in MHC-PPARα hearts. Nearly 4 times more of the cytosolic palmitoyl-CoA pool in NTG hearts than MHC-PPARα is oxidized during the time required for complete exchange of the palmitoyl-CoA pool with TAG. Thus, faster TAG turnover, and palmitoyl-CoA exchange with TAG, corresponds to a lower percentage of the palmitoyl-CoA pool being oxidized in the same time period. The distinction between the influence of relative rates of palmitate turnover within TAG and palmitate oxidation and the source of palmitoyl-CoA, indicates that palmitate preferentially enters the TAG pool prior to lipolysis and ultimately, β-oxidation (Figure 6A) in the MHC-PPARα heart. With such rapid turnover, LCFA enter the cell, are stored, and then following lipolysis are finally oxidized. These findings are also consistent with the hypothesis that preference for TAG-derived LCFA drives elevated TAG turnover in PPARα hearts.
During adrenergic challenge, myocardial TAG content remained constant, but TAG turnover in MHC-PPARα hearts was significantly faster than in NTG. Surprisingly, adrenergic stress potentiated the elevated TAG dynamics in the MHC-PPARα hearts, indicating homeostatic mechanisms exist that maintain this enlarged endogenous lipid pool. Consequently, β-adrenergic stimulation accelerates exchange of palmitate through the TAG pool prior to oxidation. The greater rate of palmitate turnover in TAG, during β-adrenergic stimulation of the MHC-PPARα hearts, indicates a further increase in the contribution of stored fatty acyl units (i.e. palmitate) from TAG to the common acyl-CoA pool that supplies β-oxidation (Figure 6B). Rather than primarily shifting toward glucose oxidation to meet immediate energy demands, there is a remarkable increase in the shuttling of LCFA through the TAG pool in the MHC-PPARα. Importantly, not only are LCFA being recruited from the TAG pool to meet energy demands, but they are also being cycled through it, which in itself is an energy consuming process. The constant TAG pool size during elevated turnover reflects the impact of increased activity of the enzymes that regulate catabolic and anabolic TAG turnover pathways, in response to stress in MHC-PPARα hearts. This increase in TAG synthesis and lipolysis supports β-oxidation during β-adrenergic challenge (Figure 6B).
Our findings are consistent with, and further supported by longstanding findings that exogenous TAG-derived acyl esters contribute to β-oxidation as opposed to free fatty acids (33–34). We now identify a similar role for FA derived from endogenous TAG, and underscore that FA-acyl CoA that enter the heart after hydrolysis of exogenous TAG are largely recycled through an endogenous TAG pool. While TAG stores are known to contribute to LCFA oxidation, the extent of that contribution has not previously been comprehensively analyzed, in part due to a lack of steady state TAG content, known isotopic enrichment levels, and/or serial measurements of actual TAG dynamics in the same hearts (9).
The current protocol enabled quantitative analysis at steady state without perturbing TAG dynamics and content. These steady-state measures allowed direct measurement of the dynamics of stored and oxidized LCFA. Importantly, the highly regulated processes of lipid storage and oxidation preclude any conclusive predictions from observing increased protein levels alone. Indeed, the observed rates of turnover in the TAG pool of the intact, beating heart could not otherwise be predicted (Figure 7). Therefore, the near requisite oxidation of LCFA from TAG in MHC-PPARα could only have been discerned from the relative rates of palmitoyl turnover within TAG and palmitate oxidation by mitochondria in the intact heart.
Changes in substrate oxidation and lipid handling have previously been implicated in various cardiomyopathies (9,13,17). Increased PPARα expression leads to an increase in myocardial LCFA uptake with augmented FA oxidation, which mimics the metabolic phenotype of the diabetic heart (9). Conversely, PPARα expression is reduced in the hypertrophic heart where FAO is limited and turnover of TAG is slowed (13,17). While the previous studies revealed FAO defects that contributed to cardiomyopathy and LCFA storage as TAG, this current study provides a direct link between PPARα expression and TAG turnover, where an increase in PPARα expression resulted in an increase in TAG turnover dynamics. Thus, MHC-PPARα hearts exhibit a more dynamic TAG pool than do NTG. Active recruitment of LCFA from TAG indicates that the TAG pool is integral in maintaining elevated energy demands in the PPARα over-expressing heart. Because lipid dysregulation is at the center of metabolic disorders such as diabetes, the ability to efficiently access the TAG pool to maintain energy demands and changes in the TAG pool dynamics may be a key limitation of metabolic syndromes.
While transgenic mouse models are powerful tools, caution should be taken to not over interpret the findings for human disease states. However, rodent models can mimic the physiology of human cardiomyopathies (35), and the mechanisms elucidated in this study contribute to a new understanding of the dynamic balance of lipid utilization/storage in the heart.
In summary, this study reveals a mechanism for handling LCFA in cardiomyocytes within intact beating hearts, whereby the TAG pool is an integral, dynamic source of LCFA for oxidation. The kinetic data indicate continuous mixing and rapid cycling of extracellular and TAG-derived LCFA in the cytosol, by which the TAG pool is a preferred source of LCFA oxidation. Increased TAG turnover and increased transcription of genes encoding enzymes for both TAG synthesis and degradation, induced by chronic PPARα activation, augmented the contribution of lipolysis to LCFA oxidation in MHC-PPARα transgenic mouse hearts. In addition to elucidating the dynamic nature of myocardial TAG stores relative to LCFA oxidation rates, the current findings demonstrate the action of PPARα on the relative contribution of lipid stores to oxidative energy metabolism.
Novelty and Significance.
What Is Known?
The heart relies on long chain fatty acids (LCFA) as the primary fuel for energy metabolism, oxidizing both circulating lipid and free fatty acids, as well as stored fats from the endogenous triacylglyceride (TAG) pool.
Altered lipid dynamics in the heart are linked to the development of cardiomyopathy.
The lipid-activated nuclear receptor, peroxisome proliferator-activated receptor α (PPARα), induces expression of enzymes for fatty acid metabolism.
What New Information Does This Article Contribute?
The application of 13C NMR for serial measures of TAG turnover in intact mouse hearts demonstrates that the TAG pool is more dynamic than previously thought.
Rates of TAG turnover and LCFA oxidation indicate that LCFA from lipolysis of endogenous TAG is preferentially oxidized over exogenous LCFA for energy metabolism.
PPARα activation induces the expression of enzymes involved in the turnover of the TAG pool, augmenting the contribution of TAG to β-oxidation.
Human and animal studies link altered lipid storage to cardiomyopathy. The current study used 13C NMR for comparing the storage kinetics and oxidation rates of 13C enriched palmitate from sequential measurements in individual mouse hearts. We found that the turnover of palmitoyl units within TAG was almost 4-fold faster than palmitate oxidation. This rate difference indicates preferential oxidation of LCFA from the lipolysis of TAG versus oxidation of exogenous LCFA. Hearts of transgenic mice (TG), overexpressing the nuclear receptor, PPARα, showed palmitoyl turnover in TAG to be 13-fold faster than oxidation, indicating near requisite oxidation of LCFA from TAG. Increased TAG turnover in PPARα– TG hearts correlated to elevated transcription levels for enzymes involved in TAG synthesis and lipolysis. While PPARα is known to induce expression of enzymes for LCFA metabolism, this is the first demonstration that PPARα drives the dynamics of TAG synthesis/lipolysis. The findings elucidate the role of intramyocardial TAG in providing the majority of LCFA to fuel β-oxidation and the influence of PPARα on this process. Thus, altered cardiac lipid storage dynamics determine the contribution of stored lipids to oxidative energy metabolism, which may be a factor in linking imbalances in lipid metabolism to cardiomyopathy.
Supplementary Material
Acknowledgments
Sources of Funding: This work is supported by grants from the N.I.H., R37HL49244, R01HL62702, and T32HL07692.
Abbreviations
- Agpat3
1-acylglycerol-3-phosphate O-acyltransferase 3
- Agpat5
1-acylglycerolphosphate acyltransferase epsilon
- Cel
carboxyl ester lipase
- Dgat1
diacylglycerol acetyltransferase 1
- Dgat2
diacylglycerol acetyltransferase 2
- Dgke
diacylglycerol kinase, epsilon
- FAO
fatty acid oxidation
- Gpam
glycerol-3-phosphate acyltransferase, mitochondrial
- gdw
gram dry weight
- HFD
high fat diet
- LCFA
long-chain fatty acid
- LC/MS
liquid chromatography/mass spectrometry
- LVDP
left ventricular developed pressure
- MHC-PPARα
myosin heavy chain-peroxisome proliferator-activated receptor alpha transgene
- Mttp
mitochondrial triglyceride transfer protein
- MgII
monoglyceride lipase
- NTG
nontransgenic
- Pnliprp1
Pancreatic lipase related protein 1
- PPARα
peroxisome proliferator-activated receptor alpha
- RCD
regular chow diet
- RPP
rate pressure product
- TAG
triacylglyceride
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–59. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 2.Lopaschuk GD, Collins-Nakai RL, Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res. 1997;26:1172–80. doi: 10.1093/cvr/26.12.1172. [DOI] [PubMed] [Google Scholar]
- 3.Russell LK, Finck BN, Kelly DP. Mouse models of mitochondrial dysfunction and heart failure. J Mol Cell Cardiol. 2005;38:81–91. doi: 10.1016/j.yjmcc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Finck BN. The role of the peroxisome proliferators-activated receptor alpha pathway in pathological remodeling of the diabetic heart. Curr Opin Clin Nutr Metab Care. 2004;7:391–6. doi: 10.1097/01.mco.0000134371.70815.32. [DOI] [PubMed] [Google Scholar]
- 5.Sambandam N, Morabito D, Wagg C, Finck BN, Kelly DP, Lopaschuk GD. Chronic activation of PPARαis detrimental to cardiac recovery of ischemia. Am J Physiol Heart Circ Physiol. 2006;290:H87–95. doi: 10.1152/ajpheart.00285.2005. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–17. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- 7.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:3199–36. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 8.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Phyiol Heart Circ Physiol. 2006;291:H1489–1506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 9.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem. 1991;266:8162–70. [PubMed] [Google Scholar]
- 10.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–7. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, Cho YR, Finck BN, Kim HJ, Higashimori T, Hong EG, Lee MK, Danton C, Deshmukh S, Cline GW, Wu JJ, Bennett AM, Rothermel B, Kalinowski A, Russell KS, Kim YB, Kelly DP, Kim JK. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-αcauses insulin resistance in heart and liver. Diabetes. 2005;54:2514–24. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 12.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. PNAS. 2003;100:1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced CPT1 activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115(15):2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell JM, Alpert NM, White LT, Lewandowski ED. Coupling of mitochondrial fatty acid uptake to oxidative flux in the intact heart. Biophysical J. 2002;82:11–18. doi: 10.1016/S0006-3495(02)75369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Amer J Physiol Endocrinol Metab. 2006;290:E448–E455. doi: 10.1152/ajpendo.00139.2005. [DOI] [PubMed] [Google Scholar]
- 16.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–717. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell JM, Fields AD, Sorokina N, Lewandowski ED. Absence of endogenous lipid oxidation in heart failure exposes limitations for triacylglycerol storage and turnover. J Mol Cell Cardiol. 2008;44:315–22. doi: 10.1016/j.yjmcc.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, Kelly DP. The transcriptional coactivator PGC-1αis essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol. 2008;295:H185–96. doi: 10.1152/ajpheart.00081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson JR, Corkey BE. Assays of intermediates of the citric acid cycle and related compounds by fluorometric enzyme methods. In: Colowick SP, Kaplan NO, editors. Methods in Enzymology. New York, NY: editors. Academic; 1969. pp. 439–513. [Google Scholar]
- 20.Yu X, White LT, Doumen C, Daminco LA, LaNoue KF, Alpert NM, Lewandowski ED. Kinetic analysis of dynamic 13C NMR spectra: metabolic flux, regulation, and compartmentation in hearts. Biophys J. 1995;69:2090–102. doi: 10.1016/S0006-3495(95)80080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malloy CR, Sherry AD, Jeffrey FMH. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J Biol Chem. 1988;265:6964–6971. [PubMed] [Google Scholar]
- 22.Lewandowski ED, Doumen C, White LT, LaNoue KF, Damico LA, Yu X. Multiplet structure of 13C NMR signal from glutamate and direct detection of TCA cycle intermediates. Magn Reson Med. 1996;35:149–154. doi: 10.1002/mrm.1910350203. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell JM, Doumen C, La Noue KF, White LT, Xin Y, Alpert NM, Lewandowski ED. Dehydrogenase regulation of metabolite oxidation and efflux from mitochondria in intact hearts. Am J Physiol Heart Circ. 1998;274:H467–76. doi: 10.1152/ajpheart.1998.274.2.H467. [DOI] [PubMed] [Google Scholar]
- 24.Yu X, Alpert NM, Lewandowki ED. Modeling enrichment kinetics from dynamic 13C-NMR spectra: theoretical analysis and practical considerations. Am J Physiol Cell Physiol. 1997;272:C2037–48. doi: 10.1152/ajpcell.1997.272.6.C2037. [DOI] [PubMed] [Google Scholar]
- 25.Sena S, Rasmussen IR, Wende AR, McQueen AP, Theobald HA, Wilde N, Pereira RO, Litwin SE, Berger JP, Abel ED. Cardiac hypertrophy caused by peroxisome proliferator-activated receptor-g agonist treatment occurs independently of changes in myocardial insulin signaling. Endocrinology. 2007;148:6047–6053. doi: 10.1210/en.2006-1559. [DOI] [PubMed] [Google Scholar]
- 26.Stowe KA, Burgess SC, Merritt M, Sherry AD, Malloy CR. Storage and oxidation of long-chain fatty acids in C57/BL6 mouse heart as measured by NMR spectroscopy. FEBS Lett. 2006;580:4282–8. doi: 10.1016/j.febslet.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 27.Belke D, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–13. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 28.Belke D, Larsen TS, Lopaschuk GD, Severson DL. Glucose and fatty acid metabolism in the isolated working mouse heart. Am J Physiol. 1999;277:R1210–17. doi: 10.1152/ajpregu.1999.277.4.R1210. [DOI] [PubMed] [Google Scholar]
- 29.Belke D, Larsen TS, Gibbs EM, Severson DL. Glucose metabolism in perfused mouse hearts overexpressing human GLUT-4 glucose transporter. Am J Physiol Endocrinol Metab. 2001;280:E420–7. doi: 10.1152/ajpendo.2001.280.3.E420. [DOI] [PubMed] [Google Scholar]
- 30.Cambell FM, Kozak R, Wagner A, Altarejos JY, Dyck JRB, Belke DD, Severson DL, Kelly DP, Lopaschuk GD. A role for peroxisome proliferators-activated receptor α(PPARα) in the control of cardiac malonyl-CoA levels. J Biol Chem. 2002;277:4098–10330. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- 31.Lu B, Jian YJ, Zhou Y, Xu FY, Hatch GM, Choy PC. Cloning and characterization of murine 1-acyl-sn-glycerol-3phosphate acyltransferases and their regulation by PPARαin murine heart. Bichem J. 2005;385:469–477. doi: 10.1042/BJ20041348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARαoverexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballard FB, Danforth WH, Naegle S, Bing R. Myocardial metabolism of fatty acid. J Clin Invest. 1960;39:717–23. doi: 10.1172/JCI104088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danforth WH, Ballard FB, Kako K, Choudhury JD, Bing RJ. Metabolism of the Heart in Failure. Circulation. 1960;21:112–23. doi: 10.1161/01.cir.21.1.112. [DOI] [PubMed] [Google Scholar]
- 35.Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Disease Models and Mechanisms. 2009;2:454–66. doi: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


