Summary
Hypertension is a multifactorial disease associated with significant morbidity. Increased sympathetic nervous system activity has been noted as an important etiologic factor and is, in part, regulated by afferent input arising from arterial and cardiopulmonary baroreceptors, activation of which causes inhibition of sympathetic output. It was thought for many years that baroreceptors control only short-term blood pressure changes, a conclusion stemming from observations in sinoaortic denervation (SAD) animal models and the phenomenon of rapid baroreceptor resetting, also seen in animal models. Newer observations, however, indicate that SAD is rather imperfect and resetting is rarely complete. Recent studies reveal that baroreceptors control sympathetic output on a more long-term basis and participate in fluid volume regulation by the kidney, and thus have the potential to adjust blood pressure chronically. Importantly, these findings are consistent with studies and observations in humans. Meanwhile, a model of electrical stimulation of the carotid sinus has been developed and successfully tested in animals. Following these encouraging results human trials to evaluate the clinical application of electrical carotid sinus manipulation in the treatment of systemic hypertension have commenced, and results so far indicate that this represents an exciting potential tool in the clinician’s armament against chronic arterial hypertension.
Keywords: Baroreceptor, barereflex, hypertension, sinoaortic denervation
BACKGROUND
Hypertension is a common contributor to morbidity and mortality from cardiovascular disease and has a complex and multifactorial etiology, which includes both genetic and environmental influences. Hypertension usually runs an indolent course and can be clinically silent until complications in the form of end organ damage to the heart, brain, kidneys, or large arteries develop [1]. Even though the number of patients who are treated with antihypertensives has risen significantly over the last several decades, almost three quarters of patients who receive treatment fail to achieve adequate blood pressure control, which has been attributed to problems with patient compliance, financial barriers and, to some extent, physician-related lack of aggressiveness in medical management [2–4].
Several reports have identified that increased sympathetic nerve activity (SNA) is a major actor in the development of essential hypertension, a condition in which increased cardiac and renal norepinephrine production and muscle SNA have been demonstrated via microneurography [5–9]. A variety of causes can precipitate sympathetic nervous system overactivity. One of the most well known is inhibition of arterial baroreceptor function. These baroreceptors, which are involved in the regulation of blood pressure in both animals and humans, provide a common pathway through which seemingly unrelated events ultimately regulate systemic blood pressure. The clinical implication is that any manipulation to alter the activity of this pathway might lead to improved blood pressure control.
The idea of carotid sinus stimulation as a means of lowering systemic blood pressure is not new [4]. However, after disappointing results from initial studies, as well as the emergence of the long-held belief that the arterial baroreceptors account for only short-term blood pressure regulation, skepticism emerged, and this idea was abandoned. However, since a series of recent animal experiments have suggested that baroreflex-mediated pressure changes persist in the long-term, the whole subject of baroreceptor significance in blood pressure control has been revisited. In this paper we review the basic anatomy, physiology and cardiovascular pathophysiology of arterial baroreceptors, their significance in long and short term systemic blood pressure control, and the first animal and human data that indicate electrical manipulation of baroreceptors may be of benefit in the long term control of systemic hypertension.
OVERVIEW OF ARTERIAL BARORECEPTORS
Anatomy and physiology
Baroreceptors are stretch-sensitive fibers located primarily in the aortic arch and each of the carotid sinuses near the area where the common carotid artery bifurcates [10]. Afferent fibers from these carotid sinus baroreceptors join their respective glossopharyngeal nerve and project to the nucleus tractus solitarii in the dorsal medulla, which is under cortical command. In turn, they project to efferent cardiovascular neurons in the medulla and spinal cord. Meanwhile, there are extra-carotid arterial baroreceptors found in the aortic arch and stretch-sensitive receptors in the heart and pulmonary vessels, which are called cardiopulmonary receptors. All of these extra-carotid baroreceptors transmit their afferent information via the vagi nerves to the same brainstem nuclei. The efferent limbs of the baroreflex loop consist of sympathetic and parasympathetic fibers to the heart, the smooth muscle of the peripheral blood vessels, and other organs such as the kidney.
Regardless of anatomical location, baroreceptors function to provide the afferent signals in a negative-feedback circuit in the medulla that maintains mean arterial pressure (MAP) at normal levels. In a simplified paradigm, increase in MAP leads to stimulation of baroreceptors, which ultimately leads to attenuation of the sympathetic outflow to the peripheral vessels and the heart. This, in turn, restores MAP to normal levels. Conversely, a decrease in MAP unloads the baroreceptors and leads to increased sympathetic outflow, vasoconstriction, and increased cardiac output; MAP is then normalized [11]. Arterial baroreceptors are actively engaged at resting blood pressures and are, therefore, loaded at rest. Thus, a normal resting and otherwise “stable” hemodynamic state reflects an ongoing, baseline level of baroreceptor reflex activity.
Aortic baroreceptors had long been thought to be the main participants in baroreflex control of heart rate, with the carotid baroreceptors contributing only about 30%. This was based on experiments where aortic baroreceptors were selectively unloaded; a stable carotid sinus transmural pressure was achieved by application of neck suction and/or pressure during infusion of vasoactive substances. Heart rate was measured [12]. Subsequent investigations similarly found that baroreflex control of heart rate is determined more by the distensibility of the aortic arch than that of the carotid sinus [13]. However, in a more recent study using a nonpharmacologic method to induce abrupt hypotension involving unilateral leg cuff occlusion, it was shown that the contributions of aortic and carotid baroreceptors to reflex control of heart rate are actually fairly equivalent [14].
The carotid sinus has also been extensively studied with regards to its role in maintaining cardiovascular homeostasis. Clinically, exaggeration of the carotid sinus reflex is widely known as carotid sinus syndrome (CSS). This can be elicited by simply applying manual pressure over the carotid sinus; syncope can occur from the resulting bradycardia and/or hypotension. Accumulated evidence indicates that disturbance of the carotid sinus or the more central portions of the baroreflex pathway accounts for the blood pressure and heart rate alterations that constitute the fundamental clinical hallmarks of this disease. Surgical treatment for CSS in the form of unilateral or bilateral carotid sinus denervation has largely been abandoned. Cardiac pacing in selected patients, alongside conventional medical treatment, is today the main treatment modality [15].
Finally, baroreceptors are distinct from chemoreceptors, which are distinct collections of highly specialized cells located in the carotid and aortic bodies. These units regulate ventilatory and arterial pressure changes during acute hypoxia and detect changes in arterial carbon dioxide tension levels and pH. Afferent fibers from the carotid and aortic bodies carry impulses via the glossopharyngeal and vagus nerves towards medullary centers, including the nucleus tractus solitarii. Central chemoreceptive areas located at the rostral ventrolateral medulla mediate the response to changing pH and regulate respiratory and circulatory responses during hypercapnia and chronic disturbances of acid–base balance [16].
Baroreceptor resetting
Baroreceptor resetting refers to a shift in the pressure threshold required to activate a receptor in the direction of the prevailing MAP [17, 18]. During resetting, the baroreceptor mechanism is adjusted to a higher operating pressure and therefore maintains rather than suppresses the hypertension. There appear to be two different forms of resetting, distinguished mainly by the underlying mechanism that causes the pressure threshold change. The first form is known as acute baroreceptor resetting. It is initiated by rapid changes in the pressure to which the receptors are exposed for a short period of time, typically 20 min or less [19]. This initial shift in pressure threshold is stable for at least an hour, occurs without a change in the sensitivity of the receptors, and is fully reversible [19]. The second type is known as chronic resetting, in which the baroreceptor sensitivity is reduced. The pressure threshold is again shifted in the direction of the pressure change but this time the changes are not readily reversible [20].
The first evidence that baroreceptors reset appeared in 1956 [21]. At that time, a study of sinus nerve and aortic nerve recordings in dogs with renal hypertension demonstrated an elevated pressure threshold to induce baroreceptor firing and an elevation of the pressure at which firing became continuous; this was significant when compared to normal controls. To confirm that the baroreflex pathway in these dogs remained functional, a separate set of experiments were conducted to document pressor responses by occluding the carotid artery before and during the development of renal hypertension, on a weekly basis. There was no dampening of the pressor response to carotid occlusion as the hypertension progressed, which indicates the dogs’ baroreflex pathway was intact in the presence of renal hypertension. Subsequent studies confirmed the presence of resetting in receptors from both the aortic wall and the carotid sinus [20].
The mechanisms that underlie resetting and the relative importance of acute and chronic baroreceptor adaptation are still poorly understood. Myelinated fibers are present in baroreceptor neural pathways and are related to rapid resetting, whereas unmyelinated fibers appear to reset in models of chronic hypertension [22, 23]. It has also been shown that overall, the magnitude of resetting does not appear to correlate with the degree of change in MAP [19]. Resetting in the context of chronic hypertension has been attributed to direct damage to the receptors, an alteration in the coupling between the receptors and the vascular walls, genetically determined properties of the receptors themselves, and decreased distensibility of the vascular walls in which the receptors are embedded [24–27]. The latter possibility is particularly intriguing, given the prevalence of coexistent atherosclerosis and hypertension. It appears that wall distensibility is one of the main determinants of sensitivity for baroreceptor activation, which is reduced as the distensibility of the blood vessel decreases [28]. The pressure threshold at which the baroreceptor becomes activated, however, depends on the arterial pressure as well as other factors that have not yet been defined [28].
Pathophysiology
Baroreceptor function changes with aging and atherosclerosis (see Thrasher [29] for review) and these trends are summarized as follows. Stiffening of the vascular wall occurs during the development of an atherosclerotic plaque; this reduced distensibility leads to decreased deformation of the receptors in response to pressure changes and, as a result, reduced efferent signaling and increased sympathetic outflow. To confirm this Crandall et al. experimentally reduced the compliance of animals’ common, internal, and external carotid arteries by applying specially designed plastic clamps around the vessels [30]. They noticed a change in the MAP from 126 to 167 mm Hg after clamp placement. A similar increase was noted when the compliance was decreased in a group of dogs that had undergone SAD. The compliance-induced hypertension was treated successfully with phenoxybenzamine, but it recurred after cessation of the treatment [30].
Several years later, in an effort to simulate the limited expansion of the atherosclerotic vascular wall, Burstyn et al. applied casts made of dental cement around the carotid sinus of animals [31]. They observed that the systolic pressure increased 38% above baseline and was sustained for 50 days after casting compared to an increase of only 9% in controls who had the cast placed on the internal carotid above the sinus. The integrity of the sinus nerves was verified at the end of this experiment by showing a normal pressor response to common carotid occlusion, which was comparable to that of controls. Bradycardia in response to acute increases in MAP was reduced in the group with sinus casts compared with the control animals, a finding also consistent with diminished baroreceptor stimulation.
Both of the aforementioned studies involved animal models in which only the carotid sinus baroreceptors were manipulated, while the aortic baroreceptors remained intact. These indicate that quantitative differences in afferent signaling are important for blood pressure control, and that complete destruction or stimulation of all groups of baroreceptors is not necessary in order to achieve blood pressure effects. Finally, it is worth mentioning that dietary manipulation in order to decrease arterial distensibility in animals using a diet rich in vitamin D or high fat and cholesterol results in diffuse plaque formation in the aorta and the carotid arteries, diminished arterial wall distensibility as assessed with pressure volume studies and, ultimately, hypertension [27, 32].
Carotid endarterectomy (CEA) is a frequently performed procedure for treating atherosclerotic disease of the carotid artery, which is usually located at the bifurcation. It is an open procedure that involves exposure of the bifurcation, arteriotomy, and removal of the plaque and diseased endothelium. CEA may be the ideal real life model to test the hypothesis that changing the distensibility of the vascular wall containing the carotid baroreceptors can alter MAP. Only few relevant studies have appeared in the literature, and they mainly focus on the short term effect of carotid endarterectomy on MAP. For instance, the closed loop gain of the carotid occlusion reflex almost doubles immediately after unilateral CEA, a benefit that disappears two months after the procedure [33, 34]. Baroreflex sensitivity and MAP improve a few days after unilateral CEA in hypertensive patients, and there seems to be a correlation between improvement in baroreceptor reflex and decrease in MAP [35]. In a more long-term study, however, baroreceptor sensitivity and MAP six months after CEA were the same as preoperatively [36]. In a subset of patients who were hypertensive preoperatively, a slight decrease in the MAP was noted but it was not significantly different from that of the normotensive group. Despite technical and methodological limitations, these studies underscore the notion that improvement of the carotid sinus distensibility after CEA has a short-term impact on blood pressure regulation favoring a decrease in MAP. Of note, the opposite result has also been reported; CEA has been shown to result in an early postoperative hypertensive response, presumably because of destruction of carotid sinus fibers [37]. This effect likely obscures the improvement in baroreflex function obtained from improved distensibility after endarterectomy.
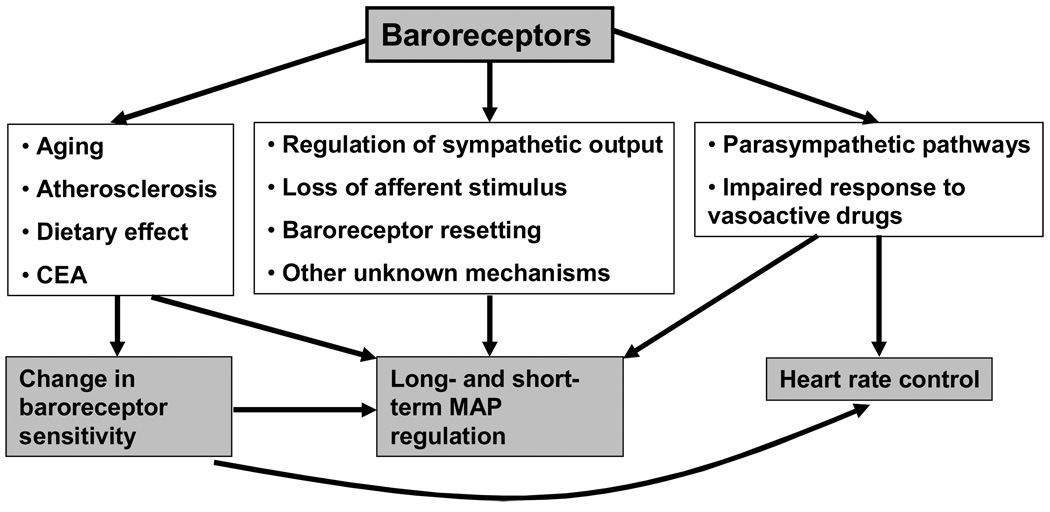
Aging is associated with a diminished ability to maintain blood pressure homeostasis, presumably due to stiffening of the arterial wall. Increases seen in MAP have been compared between different age groups in response to phenylephrine injection before and after ganglionic blockade with trimethaphan [38]. MAP rose more in the older patient group before blockade and less after ganglionic blockade compared to the younger patient group, suggesting a diminished ability of aged subjects to adjust to blood pressure increase. In addition to blood pressure, baroreceptors control changes in the heart rate that have also been shown to correlate with age. Aging leads to a decline in the baroreceptor-dependent heart rate control, an effect that is partially reversible with regular exercise [39]. Furthermore, the baroreceptor reflex control of HR in response to vasoactive agents has been shown to be altered independently by hypertension and by aging in both animal models and human subjects [40, 41]. As part of this impaired response one would expect a proportional decrease in SNA with advancing age, however, conclusive evidence about this is lacking; some studies indicate that the sensitivity of the baroreflex control on muscle SNA decreases with age, but others report no difference in baroreflex control of muscle SNA in age-matched hypertensive and normotensive elderly patient groups [42, 43]. Thus, there are the complex function and multiple interactions among arterial baroreceptors, cardiovascular system and neural pathways (Figure 1).
Figure 1.
A schematic representation of the complex function and multiple interactions among arterial baroreceptors, cardiovascular system and neural pathways. The main baroreceptor function consists of blood pressure regulation through a variety of mechanisms that include modification of the sympathetic output, loss of afferent baroreceptor stimulus in cases of baroreceptor denervation, baroreceptor resetting and possibly other mechanisms unknown at present. Changes of the vascular physiology due to aging, atherosclerosis, diet, or after carotid endarterectomy alter baroreceptor sensitivity that subsequently impacts blood pressure and heart rate control. Finally, heart rate changes due to fluctuations in the parasympathetic activity or altered response to vasoactive medications and overall baroreceptor sensitivity have an impact on the short- and long-term blood pressure regulation.
ARTERIAL BARORECEPTORS AND SHORT-TERM REGULATION OF SYSTEMIC BLOOD PRESSURE
Arterial baroreceptors control the sympathetic drive to the heart and the peripheral blood vessels. They constantly adjust the sympathetic activity in relation to systemic blood pressure changes in order to maintain homeostasis. Based on this mechanism, it was thought that severing baroreceptor afferents would result in chronically increased sympathetic activity, thereby causing sustained hypertension, a hypothesis that was validated by a number of early experiments [44]. Subsequent work, however, did not confirm this. In animal studies using dogs, SAD resulted in a very labile MAP in response to physical movement and environmental stimuli, but only slight MAP increase compared to controls [45]. It also substantially decreased the ability to maintain a stable blood pressure after hemorrhage [46]. Similar effects of SAD, namely temporary increases in MAP without significant persistent hypertension, were reported in rats, rabbits, cats, and monkeys [47–50]. It was then realized that over time, both hypertension and SNS activity subside. It was based on these observations that many researchers concluded that baroreceptors account for short-term blood pressure control only. As mentioned before, this discouraged the aggressive investigation of the baroreflex pathway as a means of blood pressure control.
Several mechanisms were proposed to explain the absence of long-term MAP regulation by baroreceptors. One of the most popular theories involved the phenomenon of baroreceptor resetting that was more extensively discussed in the previous section. Another explanation was that in addition to carotid sinus and aortic baroreceptors, cardiopulmonary receptors actively participate in the short-term blood pressure control, as evidenced by the fact that combined SAD and cardiopulmonary receptor denervation causes an increase in MAP in dogs [10]. A third explanation is related to evidence that the gain from the baroreceptor control system was not sufficiently strong to explain the long-term consistency of blood pressure. Studies in anesthetized dogs and rabbits, for instance, showed that arterial baroreceptors provided only 65–75% compensation for a given change in arterial pressure [51]. A last potential mechanism was thought to be the loss of afferent baroreceptor activation after SAD. Loss of this continuous stimulus leads to modification of the neural processing within the nucleus of the solitary tract (NTS) or other nuclei of the baroreflex pathway, as evidenced by experiments in SAD animal models [52, 53].
ARTERIAL BARORECEPTORS AND LONG-TERM REGULATION OF SYSTEMIC BLOOD PRESSURE
The notion that baroreceptors participate only in short term blood pressure regulation remained dominant for several years. More recent studies, however, cast doubt on the accuracy of the SAD model, and a more critical evaluation of the original experimental models has demonstrated flaws that could have led to misinterpretation of the early results [54]. In the following section, we summarize the evidence that indicates a more complex and long-term role of the baroreflex in hypertension.
Animal observations
Kidney-mediated regulation of intravascular and total body fluid volume is an important component of the long-term adjustment to the hypertensive state. Renal sympatho-inhibition has been suggested to represent a long-term compensatory mechanism in cases of volume excess [55]. The interplay between the sympathetic nervous system, intravascular volume status, control of sodium excretion, and baroreceptor activity has been well established in animal models of blood pressure regulation, and points towards the long-term effect of baroreceptors on blood pressure control. For example, King et al studied mechanisms of circulatory adaptation in different scenarios of catecholamine excess [56]. They noted that in dogs that were infused continuously with the potent vasopressor norepinephrine (NE), the MAP remained stable, but heart rate and cardiac output were reduced throughout the period of infusion; however, ganglionic blockade established after atropine infusion increased the heart rate and the MAP. This indicates a continuous buffering of the pressor activity via autonomic nervous system reflex mechanisms. In a separate series of experiments, salt loading in baroreceptor-intact rats and rats with SAD caused minimal changes in MAP in the first group and significant increase in the latter, implying that the baroreceptors were buffering the effects of large increases in dietary sodium chloride intake on MAP [57].
Lohmeier studied responses to salt loading in dogs using a split-bladder preparation and unilateral renal denervation [55]. This model allowed for differential assessment of sodium excretion from denervated and innervated kidneys, which in turn provided an indirect measure of the level of renal SNA. In response to angiotensin II infusion, sodium excretion in the innervated kidney increased, demonstrating that diminished renal SNA may be an important buffering mechanism in a chronic hypertension model. The authors repeated the experiment after cardiopulmonary and arterial baroreceptor denervation. In the denervated group, there was no change noted in sodium excretion, indicating that the decrease in the renal SNA disappeared after abolishment of the afferent baroreceptor pathway [58]. A later study further investigated the significance of baroreceptor pathways in a 5 day model of chronic angiotensin II-induced hypertension. Activation of neural baroreceptor pathways was assessed by determining levels of Fos-like protein in neurons of the nucleus tractus solitarius, caudal ventrolateral medulla and rostral ventrolateral medulla. Angiotensin II-induced hypertension was associated with increased baroreceptor pathway activity and, subsequently, suppressed sympathetic output [59].
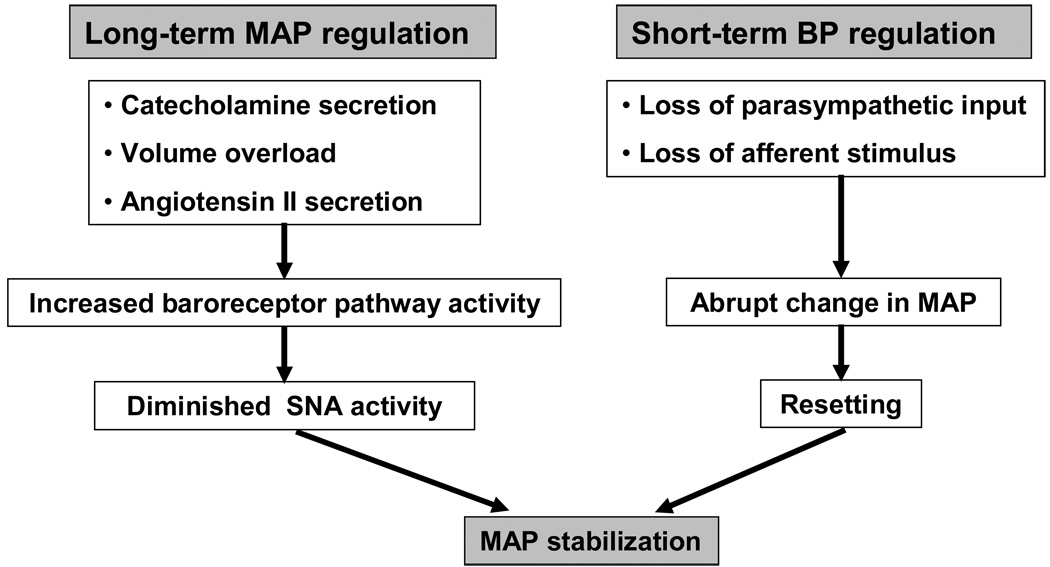
A chronic baroreceptor unloading model was developed in dogs by denervating the aortic arch, brachiocephalic and subclavian trunks and one carotid sinus, leaving the other carotid sinus intact. Then, the common carotid artery proximal to the only innervated carotid sinus was ligated and measurements of MAP were made [29]. An increase of approximately 20–30 mm Hg was noted in the animals in the first week postoperatively; this declined to about 10 mm Hg above controls after two to three weeks and was maintained at this level. The increase was significant statistically when compared to control dogs, suggesting that chronic baroreceptor unloading results in long-term MAP changes [29]. Evidence of increased SNA was also present in these animals; plasma renin activity was increased despite the fact that both systemic and renal perfusion pressures remained elevated. Furthermore, the pressure natriuresis mechanism in this animal model appeared to have been reset as well to a higher level, a phenomenon that is also mediated via sympathetic pathways (Figure 2).
Figure 2.
Long- and short-term blood pressure regulation by arterial baroreceptors. Loss of parasympathetic input as well as loss of afferent stimulus in cases of baroreceptor denervation results in an abrupt increase in the MAP. Baroreceptors then rapidly reset and the blood pressure returns to pre-denervation levels. However, baroreceptors participate in long-term blood regulation. Experimental evidence indicates that in cases of catecholamine secretion, volume overload or increased angiotensin II secretion, increased baroreceptor pathway activity is followed by diminished sympathetic output and pressure normalization.
Human observations
Purposeful experimenting with SAD or manipulation of the renal SNA with angiotensin II infusion in humans is a difficult undertaking. Therefore, information on baroreceptor denervation comes from observations, mainly on patients who underwent surgical removal of carotid sinus or aortic baroreceptors for a variety of reasons. There is one report of apparent SAD in a patient who received large doses of radiation in the upper chest and neck for the treatment of cancer, who several years later had a bypass graft in his carotid arteries, essentially destroying his baroreceptors. Soon after, he developed increased and labile MAP, an elevated resting heart rate that remained unaffected by positional changes, as well as a high level of resting muscle sympathetic nervous system activity, with evidence of loss of afferent baroreceptor input, all consequences similar to those observed in SAD animals[60].
In 1965, bilateral carotid sinus denervation was performed on two patients with normal baseline blood pressure as a treatment for chonic lung disease. This led to a marked blood pressure increase that persisted for approximately 12 weeks after the operation. At the same time, the heart rate response to positional changes was abolished for several weeks [61]. In another series, four patients underwent bilateral carotid body tumor resection. Heart rate and blood pressure responses to positional changes were substantially impaired in these subjects, whereas continuous ambulatory MAP measurement demonstrated elevated daytime MAP in three out of four patients, with normal night time MAP in all four. Abnormalities in MAP variability persisted for up to two years. This study seemed to show a correlation between the increase in ambulatory blood pressure and the degree of impairment of baroreflex dysfunction [62]. Similar results have been seen in a study of patients who had neck irradiation and developed evidence of baroreceptor malfunction. In this group, baroreflex responses were absent and the MAP was persistently elevated with markedly increased variability, whereas the sympathetic and parasympathetic efferent function was found to be intact. Ultrasound examination demonstrated intimal thickening in the carotid arteries, which may be associated with diminished distensibility and impairment of the baroreflex pathway [63].
Taken together, these data indicate that destruction of arterial baroreceptors in humans has a more chronic effect than that seen in animals. Complete denervation of carotid baroreceptors produces a sustained increase in MAP and pressure lability in humans, even when aortic baroreceptors are functioning normally, whereas less than complete sinus denervation decreases baroreflex sensitivity and increases MAP variability but does not result in an elevated MAP [61–64]. Animal data do not show this chronic effect of carotid sinus denervation on either the level or the variability of MAP, which is likely because aortic baroreceptors can compensate for the lost afferent input from the destroyed carotid sinus pathways in animals. It is not clear why the aortic baroreceptors in humans, as opposed to animals, do not provide adequate blood pressure control. One possibility is that aortic baroreceptor pathways are iatrogenically damaged during the procedure that led to carotid sinus denervation, for instance radiation or removal of carotid body tumors. Alternatively, aging-related loss of baroreceptor sensitivity to buffer blood pressure changes is possible [29]. Finally, it has been shown that human subjects are more active and alert for longer periods of time during the day compared with animals under normal housing conditions, a behavioral pattern that must be factored into the changes observed in MAP after baroreceptor denervation [62].
SUSTAINED BAROREFLEX-INDUCED REGULATION OF SYSTEMIC BLOOD PRESSURE
In a landmark study, Lohmeier et al established a model of continuous baroreceptor activation as a means of lowering the systemic blood pressure and studied properties of neuron-regulated renin secretion [18]. Electrodes connected to a pulse generator were implanted around the carotid sinus and were used to electrically activate the carotid baroreflex of conscious dogs. The MAP was reduced quickly on the first day of the study compared to control values obtained from the same animals prior to the electrode activation, and more importantly, remained suppressed throughout the seven-day study period. MAP on the seventh day of study was found to be 72±5 mm Hg, as opposed to the pre-treatment control value of 93±3 mm Hg. The heart rate also decreased from 64±4 bpm to 51±3 bpm at the end of the study period. These hemodynamic effects were reversible upon discontinuation of the electrical stimulation of the carotid sinus, contradicting older findings on quick baroreceptor resetting. The authors attributed this response to the fact that during electrical stimulation of the afferent pathway, the blood pressure sensing step that takes place at the baroreceptor level was bypassed, eliminating a component of chronic adaptation that might take place in the baroreceptors themselves via the resetting processes. A thirty-five percent reduction in plasma norepinephrine concentration was noted, which was reversible. Plasma renin levels did not change during the hypotensive period, indicating a baroreceptor-induced inhibition of the renal sympathetic activity that is reflected in the circulating renin plasma levels.
This idea was tested in eleven patients who underwent routine carotid endarterectomy [65]. Prior to the operation, electrodes were placed around the carotid sinus and dose response curves were generated for different levels of electrode activation. A reduction in the average blood pressure was noted from 144±9 mmHg to 131±9 mm Hg when a stimulus of up to 3 V was applied to the electrode. Greater reduction was evident for a subgroup of patients that had the procedure performed under local anesthesia, presumably because of the diminished cardiovascular reflex sensitivity during general anesthesia, which has previously been described [66].
As a result of these encouraging data, the first human clinical trials were undertaken. An early case report of a patient who suffered from hypertensive and diabetic end organ damage and had to undergo renal transplantation was promising [67]. Prior to intervention, the patient was persistently hypertensive, despite treatment with five antihypertensive medications. A programmable pulse generator was placed underneath the pectoralis major muscle and connected to electrodes applied around both carotid sinuses to apply continuous baroreflex activation. A reduction in the systolic and diastolic blood pressure by 38 and 8 mm Hg respectively was described upon initial testing. The blood pressure remained well controlled during the one month follow up.
Since then, a multicenter phase II trial has been undertaken to study the use of an implantable carotid sinus stimulator in drug-resistant patients. In this study, a device called the CVRx Rheos Baroreflex Hypertension Therapy System was implanted in patients who were on three or more antihypertensives and had systolic blood pressure (SBP) of 160 mmHg or higher. This programmable pulse generator is capable of delivering between 1 and 7.5 V in a temporally variable pattern, via two electrodes that are placed around the carotid bulb bilaterally, requiring open surgical exposure and intraoperative mapping for the site of maximal hemodynamic effect. An implantable pulse generator is then tunneled to a pocket below the clavicle, much like a conventional cardiac pacemaker. Due to potential blunting of the baroreflex by anesthetic agents, meticulous anesthesia was performed, with minimal use of baroreceptor-reflex blunting agents. Intraoperative findings after the generators were turned on were very encouraging: the mean SBP decreased from 170 to 133 mmHg at 6 V of electrical stimulation, the mean diastolic blood pressure (DBP) decreased from 88 to 64 mm Hg, and the pulse pressure decreased from 81 to 68 mm Hg. Heart rate also decreased from 71 bpm to 63 bpm. Following surgery and before discharge home, patients underwent dose-response testing, which revealed a linear relationship between voltage dose and hemodynamic response, with the maximal response seen at around 4.8 +/− 1.5 V. During the follow up period, one patient developed infection that necessitated removal of the generator [68].
More recently, the Device Based Therapy in Hypertension (DEBuT-HT) Trial was implemented, as a subset of the above study, to assess the safety and efficacy of chronic use of the electric carotid sinus baroreceptor stimulation Rheos device. The authors were especially interested in the role of sympathetic and parasympathetic nervous activity in the hemodynamic alterations seen with stimulation of the carotid baroreceptor (SCB). Twenty-one patients were included in the study, and 24-hour ECG was implemented to study heart rate variability (HRV) and heart rate turbulence (HRT), which was measured as turbulence onset (or acceleration after a premature beat) and turbulence stop (subsequent deceleration), all of which are thought to be mediated by parasympathetic and sympathetic activity. Office blood pressure was also measured and again found to be significantly decreased in these patients when the stimulator was on versus when it was off. The authors concluded that alterations in HRV and HRT were consistent with an overall increase in vagal tone and decreased sympathetic activity; thus, the chronic lowering of blood pressure seen with SCB in these patients was likely mediated through “sympathovagal modulation” [69].
CONCLUSIONS
There is currently a shift in the paradigm of baroreceptor-mediated blood pressure regulation. The long-held belief that baroreflex only accounts for the short-term blood pressure fluctuation has now been seriously questioned and essentially disproven. Recent data, including investigations in humans, indicate that arterial baroreceptors are indeed implicated in long-term blood pressure control. A model of electrical stimulation of the carotid sinus has been established and clinical studies are under way to investigate the potential of carotid sinus manipulation in the treatment of refractory hypertension. If successful, baroreflex control using electrical stimulation has the potential to revolutionize the way we view and treat hypertension and usher in a new era in the management of carotid sinus related pathology.
Acknowledgments
Source of support: This study was partially supported by the Michael E. DeBakey Department of Surgery at the Baylor College of Medicine, Houston, TX, USA and a grant (T32 HL083774) from the National Institutes of Health, USA.
REFERENCES
- 1.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia JS, McNagny SE, Rask KJ. Correlates of controlled hypertension in indigent, inner-city hypertensive patients. J Gen Intern Med. 1997;12(1):7–14. doi: 10.1046/j.1525-1497.1997.12107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shea S, Misra D, Ehrlich MH, et al. Predisposing factors for severe, uncontrolled hypertension in an inner-city minority population. N Engl J Med. 1992;327(11):776–781. doi: 10.1056/NEJM199209103271107. [DOI] [PubMed] [Google Scholar]
- 4.Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339(27):1957–1963. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 5.Julius S, Esler M. Autonomic nervous cardiovascular regulation in borderline hypertension. Am J Cardiol. 1975;36(5):685–696. doi: 10.1016/0002-9149(75)90170-8. [DOI] [PubMed] [Google Scholar]
- 6.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62(2):347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 7.Esler M. Sympathetic nervous system: contribution to human hypertension and related cardiovascular diseases. J Cardiovasc Pharmacol. 1995;26 Suppl 2:S24–S28. [PubMed] [Google Scholar]
- 8.Yamada Y, Miyajima E, Tochikubo O, et al. Age-related changes in muscle sympathetic nerve activity in essential hypertension. Hypertension. 1989;13(6 Pt 2):870–877. doi: 10.1161/01.hyp.13.6.870. [DOI] [PubMed] [Google Scholar]
- 9.Anderson EA, Sinkey CA, Lawton WJ, et al. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14(2):177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 10.Persson P, Ehmke H, Kirchheim H, et al. Effect of sino-aortic denervation in comparison to cardiopulmonary deafferentiation on long-term blood pressure in conscious dogs. Pflugers Arch. 1988;411(2):160–166. doi: 10.1007/BF00582309. [DOI] [PubMed] [Google Scholar]
- 11.Thrasher TN. Unloading arterial baroreceptors causes neurogenic hypertension. Am J Physiol Regul Integr Comp Physiol. 2002;282(4):R1044–R1053. doi: 10.1152/ajpregu.00431.2001. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson DW, Abboud FM, Mark AL. Relative contribution of aortic and carotid baroreflexes to heart rate control in man during steady state and dynamic increases in arterial pressure. J Clin Invest. 1985;76(6):2265–2274. doi: 10.1172/JCI112236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenard Z, Studinger P, Kovats Z, et al. Comparison of aortic arch and carotid sinus distensibility in humans--relation to baroreflex sensitivity. Auton Neurosci. 2001;92(1–2):92–99. doi: 10.1016/S1566-0702(01)00309-5. [DOI] [PubMed] [Google Scholar]
- 14.Fadel PJ, Stromstad M, Wray DW, et al. New insights into differential baroreflex control of heart rate in humans. Am J Physiol Heart Circ Physiol. 2003;284(2):H735–H743. doi: 10.1152/ajpheart.00246.2002. [DOI] [PubMed] [Google Scholar]
- 15.Healey J, Connolly SJ, Morillo CA. The management of patients with carotid sinus syndrome: is pacing the answer? Clin Auton Res. 2004;14 Suppl 1:80–86. doi: 10.1007/s10286-004-1012-2. [DOI] [PubMed] [Google Scholar]
- 16.Timmers HJ, Wieling W, Karemaker JM, et al. Denervation of carotid baro- and chemoreceptors in humans. J Physiol. 2003;553(Pt 1):3–11. doi: 10.1113/jphysiol.2003.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger E. Neurogenic mechanisms in hypertension: resetting of the baroreceptors. Hypertension. 1986;8 Supplement I I7-114. [Google Scholar]
- 18.Lohmeier TE, Irwin ED, Rossing MA, et al. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43(2):306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 19.Munch PA, Andresen MC, Brown AM. Rapid resetting of aortic baroreceptors in vitro. Am J Physiol. 1983;244(5):H672–H680. doi: 10.1152/ajpheart.1983.244.5.H672. [DOI] [PubMed] [Google Scholar]
- 20.Sleight P, Robinson JL, Brooks DE, et al. Characteristics of single carotid sinus baroreceptor fibers and whole nerve activity in the normotensive and the renal hypertensive dog. Circ Res. 1977;41(6):750–758. doi: 10.1161/01.res.41.6.750. [DOI] [PubMed] [Google Scholar]
- 21.McCubbin JW, Green JH, Page IH. Baroceptor function in chronic renal hypertension. Circ Res. 1956;4(2):205–210. doi: 10.1161/01.res.4.2.205. [DOI] [PubMed] [Google Scholar]
- 22.Seagard JL, Gallenberg LA, Hopp FA, et al. Acute resetting in two functionally different types of carotid baroreceptors. Circ Res. 1992;70(3):559–565. doi: 10.1161/01.res.70.3.559. [DOI] [PubMed] [Google Scholar]
- 23.Jones JV, Thoren PN. Characteristics of aortic baroreceptors with non-medullated afferents arising from the aortic arch of rabbits with chronic renovascular hypertension. Acta Physiol Scand. 1977;101(3):286–293. doi: 10.1111/j.1748-1716.1977.tb06010.x. [DOI] [PubMed] [Google Scholar]
- 24.Aars H. Aortic baroreceptor activity in normal and hypertensive rabbits. Acta Physiol Scand. 1968;72(3):298–309. doi: 10.1111/j.1748-1716.1968.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown AM, Saum WR, Tuley FH. A comparison of aortic baroreceptor discharge in normotensive and spontaneously hypertensive rats. Circ Res. 1976;39(4):488–496. doi: 10.1161/01.res.39.4.488. [DOI] [PubMed] [Google Scholar]
- 26.Andresen MC, Kuraoka S, Brown AM. Baroreceptor function and changes in strain sensitivity in normotensive and spontaneously hypertensive rats. Circ Res. 1980;47(6):821–828. doi: 10.1161/01.res.47.6.821. [DOI] [PubMed] [Google Scholar]
- 27.Angell-James JE. Arterial baroreceptor activity in rabbits with experimental atherosclerosis. Circ Res. 1974;40(4):27–39. doi: 10.1161/01.res.40.4.27. [DOI] [PubMed] [Google Scholar]
- 28.Andresen MC. Short- and long-term determinants of baroreceptor function in aged normotensive and spontaneously hypertensive rats. Circ Res. 1984;54(6):750–759. doi: 10.1161/01.res.54.6.750. [DOI] [PubMed] [Google Scholar]
- 29.Thrasher TN. Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R819–R827. doi: 10.1152/ajpregu.00813.2004. [DOI] [PubMed] [Google Scholar]
- 30.Crandall EE, Mc CH, Sukowski EJ, et al. Pathogenesis of experimental hypertension produced by carotid sinus area constriction in dogs. Circ Res. 1957;5(6):683–692. doi: 10.1161/01.res.5.6.683. [DOI] [PubMed] [Google Scholar]
- 31.Burstyn PG, Horrobin DF, Lloyd IJ. Chronic hypertension in rabbits induced by bilateral placement of rigid casts around the carotid sinus regions. Cardiovasc Res. 1972;6(1):54–56. doi: 10.1093/cvr/6.1.54. [DOI] [PubMed] [Google Scholar]
- 32.Angell-James JE. Pathophysiology of aortic baroreceptors in rabbits with vitamin D sclerosis and hypertension. Circ Res. 1974;34(3):327–338. doi: 10.1161/01.res.34.3.327. [DOI] [PubMed] [Google Scholar]
- 33.Tyden G, Samnegard H, Thulin L, et al. Effect of carotid endarterectomy on baroreflex sensitivity in man. Intraoperative studies. Acta Chir Scand Suppl. 1980;500:67–69. [PubMed] [Google Scholar]
- 34.Tyden G, Samnegard H, Melcher A, et al. Effect of carotid endarterectomy on the antihypertensive properties of the carotid sinus reflex. Acta Chir Scand. 1981;147(1):15–17. [PubMed] [Google Scholar]
- 35.Hirschl M, Hirschl MM, Magometschnigg D, et al. Arterial baroreflex sensitivity and blood pressure variabilities before and after carotid surgery. Klin Wochenschr. 1991;69(16):763–768. doi: 10.1007/BF01797615. [DOI] [PubMed] [Google Scholar]
- 36.Dehn TC, Angell-James JE. Long-term effect of carotid endarterectomy on carotid sinus baroreceptor function and blood pressure control. Br J Surg. 1987;74(11):997–1000. doi: 10.1002/bjs.1800741113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nouraei SA, Al-Rawi PG, Sigaudo-Roussel D, et al. Carotid endarterectomy impairs blood pressure homeostasis by reducing the physiologic baroreflex reserve. J Vasc Surg. 2005;41(4):631–637. doi: 10.1016/j.jvs.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Jones PP, Christou DD, Jordan J, et al. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107(13):1770–1774. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- 39.Monahan KD, Dinenno FA, Seals DR, et al. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001;281(1):H284–H289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 40.Hajduczok G, Chapleau MW, Johnson SL, et al. Increase in sympathetic activity with age. I. Role of impairment of arterial baroreflexes. Am J Physiol. 1991;260(4 Pt 2):H1113–H1120. doi: 10.1152/ajpheart.1991.260.4.H1113. [DOI] [PubMed] [Google Scholar]
- 41.Gribbin B, Pickering TG, Sleight P, et al. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29(4):424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 42.Davy KP, Tanaka H, Andros EA, et al. Influence of age on arterial baroreflex inhibition of sympathetic nerve activity in healthy adult humans. Am J Physiol. 1998;275(5 Pt 2):H1768–H1772. doi: 10.1152/ajpheart.1998.275.5.H1768. [DOI] [PubMed] [Google Scholar]
- 43.Grassi G, Seravalle G, Bertinieri G, et al. Sympathetic and reflex alterations in systo-diastolic and systolic hypertension of the elderly. J Hypertens. 2000;18(5):587–593. doi: 10.1097/00004872-200018050-00012. [DOI] [PubMed] [Google Scholar]
- 44.Ferrario CM, McCubbin JW, Page IH. Hemodynamic characteristics of chronic experimental neurogenic hypertension in unanesthetized dogs. Circ Res. 1969;24(6):911–922. doi: 10.1161/01.res.24.6.911. [DOI] [PubMed] [Google Scholar]
- 45.Cowley AW, Jr, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32(5):564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- 46.Thrasher TN, Keil LC. Arterial baroreceptors control blood pressure and vasopressin responses to hemorrhage in conscious dogs. Am J Physiol. 1998;275(6 Pt 2):R1843–R1857. doi: 10.1152/ajpregu.1998.275.6.R1843. [DOI] [PubMed] [Google Scholar]
- 47.Osborn JW, England SK. Normalization of arterial pressure after barodenervation: role of pressure natriuresis. Am J Physiol. 1990;259(6 Pt 2):R1172–R1180. doi: 10.1152/ajpregu.1990.259.6.R1172. [DOI] [PubMed] [Google Scholar]
- 48.Saito M, Terui N, Numao Y, et al. Absence of sustained hypertension in sinoaortic-denervated rabbits. Am J Physiol. 1986;251(4 Pt 2):H742–H747. doi: 10.1152/ajpheart.1986.251.4.H742. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez AJ, Bertinieri G, Belli L, et al. Reflex control of blood pressure and heart rate by arterial baroreceptors and by cardiopulmonary receptors in the unanaesthetized cat. J Hypertens. 1985;3(4):327–335. doi: 10.1097/00004872-198508000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Cornish KG, Gilmore JP. Sino-aortic denervation in the monkey. J Physiol. 1985;360:423–432. doi: 10.1113/jphysiol.1985.sp015625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev. 1992;72(1):231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- 52.Schreihofer AM, Sved AF. Nucleus tractus solitarius and control of blood pressure in chronic sinoaortic denervated rats. Am J Physiol. 1992;263(2 Pt 2):R258–R266. doi: 10.1152/ajpregu.1992.263.2.R258. [DOI] [PubMed] [Google Scholar]
- 53.Ito S, Sved AF. Influence of GABA in the nucleus of the solitary tract on blood pressure in baroreceptor-denervated rats. Am J Physiol. 1997;273(5 Pt 2):R1657–R1662. doi: 10.1152/ajpregu.1997.273.5.R1657. [DOI] [PubMed] [Google Scholar]
- 54.Sleight P. Arterial baroreflexes can determine long-term blood pressure. Baroreceptors and hypertension: time for a re-think? Exp Physiol. 2004;89(4):337–341. doi: 10.1111/j.1469-445x.2004.t01-1-00053.x. [DOI] [PubMed] [Google Scholar]
- 55.Lohmeier TE, Hildebrandt DA, Hood WA. Renal nerves promote sodium excretion during long-term increases in salt intake. Hypertension. 1999;33(1 Pt 2):487–492. doi: 10.1161/01.hyp.33.1.487. [DOI] [PubMed] [Google Scholar]
- 56.King BD, Sack D, Kichuk MR, et al. Absence of hypertension despite chronic marked elevations in plasma norepinephrine in conscious dogs. Hypertension. 1987;9(6):582–590. doi: 10.1161/01.hyp.9.6.582. [DOI] [PubMed] [Google Scholar]
- 57.Osborn JW, Hornfeldt BJ. Arterial baroreceptor denervation impairs long-term regulation of arterial pressure during dietary salt loading. Am J Physiol. 1998;275(5 Pt 2):H1558–H1566. doi: 10.1152/ajpheart.1998.275.5.H1558. [DOI] [PubMed] [Google Scholar]
- 58.Lohmeier TE, Lohmeier JR, Haque A, et al. Baroreflexes prevent neurally induced sodium retention in angiotensin hypertension. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1437–R1448. doi: 10.1152/ajpregu.2000.279.4.R1437. [DOI] [PubMed] [Google Scholar]
- 59.Lohmeier TE, Lohmeier JR, Warren S, et al. Sustained activation of the central baroreceptor pathway in angiotensin hypertension. Hypertension. 2002;39(2 Pt 2):550–556. doi: 10.1161/hy0202.103003. [DOI] [PubMed] [Google Scholar]
- 60.Aksamit TR, Floras JS, Victor RG, et al. Paroxysmal hypertension due to sinoaortic baroreceptor denervation in humans. Hypertension. 1987;9(3):309–314. doi: 10.1161/01.hyp.9.3.309. [DOI] [PubMed] [Google Scholar]
- 61.Holton P, Wood JB. The effects of bilateral removal of the carotid bodies and denervation of the carotid sinuses in two human subjects. J Physiol. 1965;181(2):365–378. doi: 10.1113/jphysiol.1965.sp007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smit AA, Timmers HJ, Wieling W, et al. Long-term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation. 2002;105(11):1329–1335. doi: 10.1161/hc1102.105744. [DOI] [PubMed] [Google Scholar]
- 63.Sharabi Y, Dendi R, Holmes C, et al. Baroreflex failure as a late sequela of neck irradiation. Hypertension. 2003;42(1):110–116. doi: 10.1161/01.HYP.0000077441.45309.08. [DOI] [PubMed] [Google Scholar]
- 64.Timmers HJ, Karemaker JM, Wieling W, et al. Baroreflex control of muscle sympathetic nerve activity after carotid body tumor resection. Hypertension. 2003;42(2):143–149. doi: 10.1161/01.HYP.0000080495.07301.31. [DOI] [PubMed] [Google Scholar]
- 65.Schmidli JS, Irwin E, Peters T, Cain C, Martin R, Kieval R, Carrel T. Electrical activation of the baroreflex in man: A step towards a novel treatment for hypertension? Vascular. 2004;12:S95. [abstract] [Google Scholar]
- 66.Godet G, Bernard JM, Bertrand M, et al. Baroreflex activity in carotid endarterectomy during general anesthesia. Ann Fr Anesth Reanim. 1989;8(2):93–97. doi: 10.1016/s0750-7658(89)80159-5. [DOI] [PubMed] [Google Scholar]
- 67.Mohaupt MS, Cain C, Frey FJ, et al. Chronic Electrical Activation of the Carotid Sinus Baroreflex by Implanted Electrodes for Blood Pressure Reduction in Man: First Experience in a Hypertensive Patient. Kidney Blood Press Res. 2004;27:299. [Google Scholar]
- 68.Illig KA, Levy M, Sanchez L, et al. An implantable carotid sinus stimulator for drug-resistant hypertension: surgical technique and short-term outcome from the multicenter phase II Rheos feasibility trial. J Vasc Surg. 2006;44(6):1213–1218. doi: 10.1016/j.jvs.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 69.Wustmann K, Kucera JP, Scheffers I, et al. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54(3):530–536. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]