Abstract
Neurogenesis of dentate gyrus granule cells is generally considered to be negatively regulated by glucocorticoids. We tested the hypothesis that exposure to low plasma corticosteroid levels starting in the early postnatal period enhances granule cell proliferation rate during adulthood. Rat pups were adrenalectomized (ADX) on postnatal day 10 and were then “clamped” throughout life at low corticosterone levels via oral supplementation. Neurogenesis was determined using BrdU immunochemistry at 3 and 12 months in clamped rats as compared with age-matched, sham-operated controls. Rate of neurogenesis did not differ between the groups at either 3 or 12 months. It was significantly lower in 12-month-old compared with 3-month-old rats, despite the presence of an age-dependent increase of plasma corticosterone only in the sham-ADX rats. Granule cell layer volume, granule cell density, and granule cell degeneration (determined using apoptotic markers) were indistinguishable in the two groups, further supporting the comparable rate of neurogenesis under differing chronic glucocorticoid levels. In addition, whereas acute deprivation of plasma glucocorticoids (adrenalectomy) in adult rats evoked a burst of granule cell neurogenesis, complete elimination of these hormones (by stopping hormone supplementation) in adult, early-life ADX/clamped rats did not. These data do not support a simple inverse relationship between chronic plasma glucocorticoid levels and granule cell neurogenesis. Specifically, chronic modulation of glucocorticoid levels commencing early in life evokes additional, adaptive, and compensatory mechanisms that contribute to the regulation of granule cell proliferation.
Keywords: BrdU, granule cells, mineralocorticoid receptor, dentate gyrus, adrenalectomy
INTRODUCTION
In mammalian brain, the dentate gyrus (DG) is among the few areas where production of significant numbers of new neurons continues throughout life (Altman and Das, 1965; Bayer et al., 1982; Kuhn et al., 1996; Eriksson et al., 1998; Kornack and Rakic, 1999). This ongoing neurogenesis provides a continuous supply of newly formed, undifferentiated granule cells (Cameron and McKay, 2001; Bender et al., 2001; van Praag et al., 2002; Kempermann et al., 2003), which may play a role in certain memory processes of the aging hippocampus (van Praag et al., 1999; Lemaire et al., 2000; Shors et al., 2001; Drapeau et al., 2003). In rat DG, neurogenesis reaches a peak during the second postnatal week (Schlessinger et al., 1975), then declines progressively during adulthood (Seki and Arai, 1995; Kuhn et al., 1996; Kempermann et al., 1998; Nichols et al., 2001; Bizon and Gallagher, 2003; Heine et al., 2004). The loss of neurogenic potential of the aged DG has been proposed as a contributor to age-related deficits in cognitive functions involving the hippocampus (Cameron and McKay, 1999; Drapeau et al., 2003; but see Bizon and Gallagher, 2003; Merrill et al., 2003; Heine et al., 2004). Therefore, understanding the mechanisms that are responsible for age-related loss of neurogenic potential in the DG may be helpful for eventual strategies for prevention of aging-associated cognitive decline.
Among factors that influence granule cell neurogenesis, the role of adrenal steroids has been extensively studied. Glucocorticoids inhibit (Gould et al., 1991; Cameron and Gould, 1994), and adrenalectomy during adulthood increases granule cell neurogenesis (Gould et al., 1992; Rodriguez et al., 1998; Cameron and McKay, 1999; Nichols et al., 2001). In general, the rate of granule cell proliferation in the rat is inversely related to basal glucocorticoid levels. For example, adrenal steroid levels in the neonatal rat are relatively low for the first 2 postnatal weeks (Levine, 1970; Walker et al., 1991; Yi and Baram, 1994; Gould and Tanapat, 1999), coinciding with the period of maximal granule cell proliferation (Schlessinger et al., 1975). Basal plasma corticosterone (CORT; the major glucocorticoid in the rat) levels rise gradually after this period (Walker et al., 1991), concurrent with a decline in granule cell neurogenesis (Kuhn et al., 1996; Nichols et al., 2001; Bizon and Gallagher, 2003; Heine et al., 2004). During aging (> 12 months), plasma CORT reaches its highest levels (e.g., Sapolsky, 1992; but see Heine et al., 2004), whereas granule cell production decelerates further (Kuhn et al., 1996; Nichols et al., 2001; Bizon and Gallagher, 2003; but see Heine et al., 2004). This intriguing correlation of plasma glucocorticoids and granule cell neurogenesis has led to the hypothesis that elevated glucocorticoid levels are responsible for decreased neurogenesis in the aging hippocampus (Cameron and McKay, 1999; Drapeau et al., 2003). Here we tested this hypothesis by performing adrenalectomy in early postnatal rats and then keeping these rats on chronically low CORT levels (clamping) throughout life. If indeed glucocorticoid levels determine the rate of neurogenesis in the aging hippocampus, then granule cell production should be significantly increased in chronically clamped rats when compared with age-matched controls.
MATERIALS AND METHODS
Experimental Design
To evaluate neurogenesis, rats were injected with 5-bromo-2′-deoxyuridine (BrdU; Roche, Indianapolis, IN; 150 mg/kg body weight i.p.) and sacrificed 24 h (Experiments 1–4) or 28 days (Experiment 5) later:
Experiment 1: Neurogenesis was evaluated at 12 months of age in rats that were adrenalectomized (ADX) on postnatal day (P) 10 and maintained on CORT supplement (ADX/clamped; n = 7), and compared with sham-ADX controls (n = 6).
Experiment 2: Neurogenesis was evaluated at 3 months of age in rats that were ADX on P10 and maintained on CORT supplement (n = 5) compared with sham-ADX controls (n = 5).
Experiment 3: To examine the neurogenic potential following the lifelong differing CORT levels, rats that had been ADX/clamped on P10 (n = 4) were deprived of their source of CORT 6 days before sacrifice (at 3 months of age), by stopping their CORT supplementation. Neurogenesis rate was compared with that of sham-ADX controls (n = 3).
Experiment 4: To compare the acute effects of steroid withdrawal on neurogenesis in our experiments to those published, rats that were ADX as adults (3 months of age; n = 5) were injected with BrdU 6 days after adrenalectomy (with no CORT supplement), and their neurogenesis rate was compared with that of sham-ADX controls (n = 4).
Experiment 5: To determine the fate of BrdU-labeled cells, 3-month-old rats (n = 3) were injected with BrdU and sacrificed 28 days after injection.
Experiment 6: To consider the potential role of adrenal steroid receptors in the adaptation to early- life onset chronic low plasma CORT levels, the expression of glucocorticoid and mineralocorticoid receptors (GR and MR, respectively) was studied. Rats were ADX and “clamped” on P10 and sacrificed on P19, P30, 3 or 12 months (n = 5 per group).
Animals
Sprague-Dawley-derived male rats (Zivic-Miller, Zelienople, PA) were born in our vivarium and maintained in this National Institutes of Health (NIH)-approved animal facility on a 12-h light/dark cycle with access to unlimited lab chow and water. Delivery was verified at 12-h intervals, and the date of birth was considered day 0. Litters were culled to 12 pups if needed and mixed among experimental groups: thus, effects of experimental manipulations were compared among littermates. For technical reasons, the experiments were performed in several batches, but each batch included both control and experimental groups. Following weaning, all rats were housed 2–3 per cage.
Surgical Procedures
Adrenalectomy or sham-adrenalectomy was performed under halothane anesthesia (~5 min/animal) on 10-day or 3-month-old rats via small bilateral dorsal incisions (Brunson et al., 2001a,b). The completeness of the adrenalectomy was verified by visual inspection at the time of sacrifice, as well as by assessment of plasma CORT. To permit normal mineralocorticoid function and based on pilot experiments, ADX animals were supplemented with aldosterone (subcutaneously, 2 µg/100 g body weight/day) during P10–21 (Walker et al., 1990; Brunson et al., 2001b). After weaning (P21), CORT (10 mg/L) was applied to the drinking solution (0.9% saline) (Walker et al., 1990; Akana and Dallman, 1997). This supplementation (clamping) leads to chronic “basal” glucocorticoid levels (Walker et al., 1990; Akana and Dallman, 1997), saturating MR but not GR (Reul and de Kloet, 1985; Herman, 1993). At the time of sacrifice, blood samples were collected via cardiac puncture and CORT levels were determined using a commercial radioimmunoassay (RIA) kit (ICN, Irvine, CA) as previously described (Eghbal-Ahmadi et al., 1999). Assay sensitivity was 0.5 µg/dl and intra-assay variability was approximately 5–7%.
All experiments were initiated at 8–10 a.m. to minimize diurnal variability of stress hormones (Watts et al., 2004). The experiments were also designed to minimize pain and discomfort, were carried out according to NIH guidelines and were approved by the Institutional Animal Care Committee.
Tissue Processing
Rats used for the neurogenesis study were sacrificed via injection of a lethal dose of pentobarbital and perfused transcardially with 0.9% saline followed by cold 4% paraformaldehyde/0.1 M phosphate buffer (pH 7.3). Before starting perfusion, blood samples were collected from the left ventricle. Perfused brains were removed from the skull, postfixed (4 h in 4% paraformaldehyde/0.1 M phosphate buffer), cryoprotected (48 h in 25% sucrose/0.1 M phosphate buffer) and frozen in −50°C cold isopentane. Serial coronal sections (50 µm) were cut throughout the entire hippocampus on a cryostat. Slides were either processed immediately (BrdU analysis, co-localization study) or mounted on glass slides and stored for future use. For in situ hybridization analyses, animals were sacrificed by rapid decapitation, and brains were immediately frozen on powdered dry ice.
BrdU Detection and Analysis
Every sixth section of a hippocampal series was processed for BrdU detection, as described previously (Bender et al., 2001). Free-floating sections were transferred to 2× SSC, then immersed in 50% formamide/2× SSC (2 h, 65°C), followed by 2 M hydrochloric acid (30 min at 37°C) to denature DNA. After neutralization with 0.1 M sodium borate (pH 8.5) and pre-incubation with phosphate-buffered saline (PBS) containing 0.3% Triton-X and 3% normal goat serum (NGS), sections were incubated with anti-BrdU (rat monoclonal IgG, Accurate Chemicals, Westbury, NY; 1:1,000) at room temperature (RT) for 24 h, followed by a biotinylated secondary antibody (anti-rat IgG, 1:600; Chemicon, Temecula, CA) and the avidin-biotin-peroxidase reaction components (Vector Laboratories, Burlingame, CA). BrdU-labeled nuclei were visualized by incubating sections in 0.04% 3, 3′ diaminobenzidine-solution containing 0.002% H2O2, 0.01% NiCl2, and 0.01% CoCl2. Eight anatomically matched sections were chosen from each series for BrdU analysis. In these sections, BrdU-immunoreactive nuclei were counted in the dorsal left and right hippocampus. Only nuclei that were located in granule cell layer or subgranular zone (defined as a two-cell body wide zone along the inner border of the granule cell layer) were included in the analysis. Data were correlated to granule cell layer surface area determined in adjacent cresyl violet-stained sections (see below) and presented as “BrdU-positive cells/mm2 granule cell layer.” Data presentation as cell densities was chosen over a stereological analysis calculating total numbers, because the latter is more likely to incur sampling errors that could obscure minor differences among experimental groups. In these, as in the following experiments, all counts were performed by an observer blinded to the experimental group status of the sections.
Immunohistochemistry
Co-localization of BrdU with mature granule cell (calbindin D28k), glial fibrillary acidic protein (GFAP), or epithelial (rat endothelial cell antigen, RECA1) markers was examined using immunofluorescence. Free-floating sections were pre-treated as described above for BrdU, then incubated with rat anti-BrdU together with one of the following primary antibodies: polyclonal rabbit anti-calbindin D28k (1:800; Chemicon), monoclonal mouse anti-GFAP (1:1,000; Chemicon) or monoclonal mouse anti-RECA1 (1:20; SeroTec, Raleigh, NC), for 48 h at 4°C. First antibodies were subsequently detected with Alexa Fluor 488-conjugated secondary antibodies (Calbindin D28k, GFAP, RECA1) and biotinylated anti-rat IgG antibody followed by streptavidin Alexa Fluor 568-conjugate (BrdU; all fluorescent products were purchased from Molecular Probes, Eugene, OR). For each marker, two sections per brain were analyzed using a Zeiss LSM510 Meta confocal microscope (Argon Laser 488 mm and He–Ne Laser 543 mm). The number of double-labeled cells was determined and correlated to the total number of BrdU-labeled cells in these sections. Data are presented as “percentage of BrdU-labeled cells.”
For GFAP-immunohistochemistry, slide-mounted sections were incubated in 10 mM Na-citrate, pH 9.0 (30 min at 80°C), for antigen retrieval (Jiao et al., 1999). After cooling, sections were quenched with 0.3% H2O2 and immersed in 0.01 M PBS containing 0.3% Triton X-100, 0.2% sodium dodecyl sulfate (SDS) 1% bovine serum albumin (BSA), and 2% NGS for 1 h to block unspecific binding sites. GFAP antiserum (see above) was added and sections were incubated for >48 h at 4°C. Antigen binding was visualized using the avidin-biotin-peroxidase technique as described above. GFAP-immunopositive glial cells located in granule cell layer or subgranular zone were counted in two sections per brain (from dorsomedial hippocampus). Only cells with clearly visible somata and associated radial processes were included in the analysis. Numbers were correlated to granule cell layer surface area and are presented as “GFAP-positive cells/mm2 granule cell layer.”
Analysis of Granule Cell Layer Volume and Granule Cell Density
Sections adjacent to those chosen for BrdU detection were stained with 1% cresyl violet acetate and analyzed for the following parameters. First, granule cell layer surface area was measured in each section using a counting grid; granule cell layer volume was then determined by multiplying the average value (total surface area/number of analyzed sections) with the distance from the septal to the temporal pole as calculated from the total number of sections in the series. Second, granule cell densities were determined by systematically counting granule cell nuclei included in a counting grid positioned above the midpoint of the granule cell suprapyramidal layer. Counting was performed in 10 units (1 U = 25 µm × 25 µm) of the grid by focusing through the entire vertical depth of the section at each position (revealing 3–4 layers of granule cells). Samples were taken from the eight sections (left and right hippocampus = 16 values per rat), which were adjacent to the eight sections used for BrdU analysis. Granule cell densities were computed as: N (neurons/mm3) = A[M/(L+M)]/V, where A is the number of counted nuclei, L is the average length of nuclei, M is the section thickness, and V is the volume of the sampling area (Bender et al., 2003). All measurements and cell counts were performed under 400× magnification.
Analysis of Granule Cell Degeneration
Two separate methods were used to determine granule cell degeneration. First, granule cells were considered to be undergoing apoptosis based on morphological characteristics, including pyknotic, shrunken nuclei and an eosinophilic cytoplasm (Gerth et al., 1998). Thus, cells exhibiting dense chromatin forming crescent or ring-like structures or discrete clumps were counted in the cresyl violet-stained sections (see above). Single pyknotic nuclear clumps were excluded. Cells were counted in the same counting grid (and at the same positions) used to determine granule cell densities, and data are presented as “pyknotic cells/mm2 granule cell layer.” Second, in addition, terminal-transferase-mediated dUTP nick-end-labeling (TUNEL) was performed to detect cells undergoing apoptosis (modified from Heine et al., 2004). Slide-mounted sections (a series adjacent to those chosen for BrdU detection) were pre-treated in 0.1 M sodium citrate buffer (pH 6.0) in a microwave oven set at full power for 2.5 min. After cooling the jars, sections were pre-incubated with Proteinase K buffer (10 mM Tris-HCl, 2.6 mM CaCl2, pH 7.6) for 10 min, followed by 20 µg/ml Proteinase K (Promega, Madison, WI) for 15 min at RT. After a brief rinse in double-distilled water and wash in PBS, sections were pre-incubated with terminal transferase (TdT) buffer (0.2 M sodium cacodylate/0.025 M Tris-HCl/0.25 mg/ml BSA, pH 6.6) for 10 min and incubated for 60 min at 37°C with 1 µl TdT and 1 µl biotin-16-UTP (Promega) per 100 µl reaction mixture. TdT reaction was stopped in 2× SSC (5 min), endogenous peroxidase activity was blocked with 0.3% H2O2 in PBS (15 min) and sections were transferred to streptavidin-peroxidase (Promega) for 1 h. Labeling was visualized by incubating sections in 3,3′ diaminobenzidine-solution containing H2O2, NiCl2, and CoCl2 (see above). TUNEL-positive cells were counted in the entire granule cell layer of at least four sections per brain (labeled cells in the two innermost layers were not included to avoid potential false-positives due to BrdU-induced DNA-breaks). Data are presented as “TUNEL-positive cells/mm2 granule cell layer.”
In situ hybridization histochemistry (ISH) for GR and MR mRNA: ISH and riboprobe labeling were performed as described previously (Eghbal-Ahmadi et al., 1999). Coronal sections (20 µm) of dorsal hippocampus were cut using a cryostat. Before hybridization, sections were brought to room temperature, air dried and fixed in fresh 4% buffered paraformaldehyde for 20 min, followed by dehydration and rehydration through graded ethanols. Sections were exposed to 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8) for 8 min and were dehydrated through graded ethanols. Prehybridization and hybridization steps were performed in a humidified chamber at 55°C in a solution of 50% formamide, 5× SET, 0.2% SDS, 5× Denhardt’s, 0.5 mg/ml salmon sperm DNA, 0.25 mg/ml yeast tRNA, 100 mM dithiothreitol and 10% dextran sulfate. After 1-h prehybridization, sections were hybridized overnight with antisense riboprobes radioactively labeled with 35S-CTP (GR probe, a kind gift from Dr. J.N. Masters) or 35S-UTP (MR probe, a kind gift from Dr. S. Rivest). The specificity of the probes has previously been established. After hybridization (with 1 × 106 cpm of riboprobe per section), sections were washed in 2× SSC for 5 min at room temperature and were exposed to RNase A (200 µg/ml) in 10 mM Tris-HCl (pH 8)/NaCl for 30 min at 37°C. Sections underwent serial washes of increasing stringency at 55°C, the most stringent being at 0.03 × SSC for 1 h. Hybridized and washed sections were apposed to film (Hyperfilm β-Max, Amersham, IL) for 10–14 days. Semiquantitative analysis of the ISH signal was performed on digitized films using the ImageTool software program (UTHSC, San Antonio, TX) as previously described. For analysis, three matched dorsal hippocampal sections/animal were sampled, using unbiased methods (Eghbal-Ahmadi et al., 1999).
Statistical Analysis
The results are depicted as means with standard errors (SEM). Statistical significance was set at P < 0.05. Since most analyses compared two experimental groups, differences were evaluated using unpaired Student’s t-test. For Figure 1, plasma corticosterone levels were analyzed with a two-way analysis of variance (ANOVA) with treatment (ADX) as a between subject variable and age as the within subject variable.
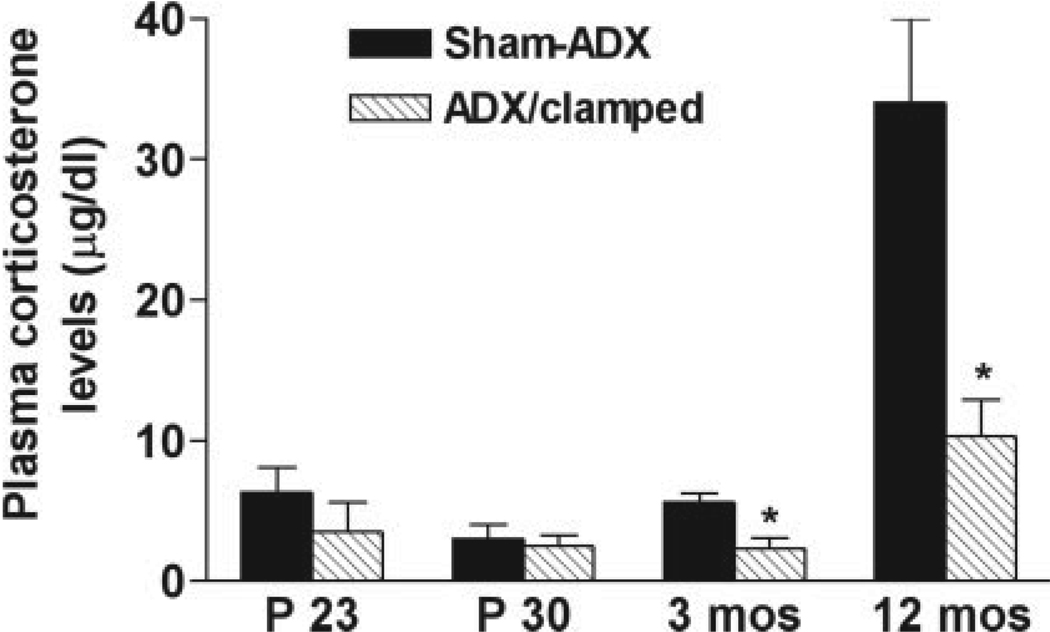
FIGURE 1.
Morning plasma corticosterone (CORT) levels of sham-adrenalectomized (Sham-ADX) or ADX/clamped rat groups. Starting after weaning (P23) plasma CORT levels of ADX/clamped animals averaged ~2.5 µg/dl, and remained at this range during adulthood. By middle age (12 months) increased plasma levels were found (see text), but these remained much lower than those in intact rats. In the latter group, an effect of age on plasma CORT was apparent (two-way ANOVA; F = 25.5, P < 0.0001).
RESULTS
Adrenalectomy/Clamping Paradigm Results in Lifelong Reduction of Plasma Glucocorticoids
Adrenalectomy on P10, with CORT supplementation, influenced CORT plasma levels for life (effect of treatment: F = 25.5, P < 0.0001; Fig. 1). As shown in Figure 1, the adrenalectomy followed by “clamping” of CORT led to relatively constant low plasma levels (2.3–2.5 µg/dl) that are typical of basal CORT throughout the majority of morning hours (Dallman et al., 1987; Watts et al., 2004). Statistical divergence from sham-ADX levels emerged at 3 months, and was maintained to 12 months. Note that controls were sacrificed immediately upon removal from the cage at 8–10 a.m. However, it is reasonable to consider that in animals with intact adrenals, stressors (e.g., cage changes, other human contact) evoked occasional bursts of stress-level plasma glucocorticoids, that saturate GRs (Reul and de Kloet, 1985; Spencer et al., 1990). Note also that in both groups plasma CORT was increased at 12 months.
Hippocampal Neurogenesis Rate Does Not Differ Between ADX/Clamped and Sham-ADX Controls
While plasma CORT levels clearly distinguished the ADX/clamped from the intact group at both 3 months and 12 months, the number of BrdU-positive cells were similar in these two groups. As shown in Figure 2A, the rate of cell proliferation in 12-month-old rats was significantly lower than that of 3-month-old animals (P < 0.0001) for both the intact and ADX/clamped groups. However, proliferation rates did not differ between these groups at either age (P = 0.73 and 0.84 at 3 months and 12 months, respectively).
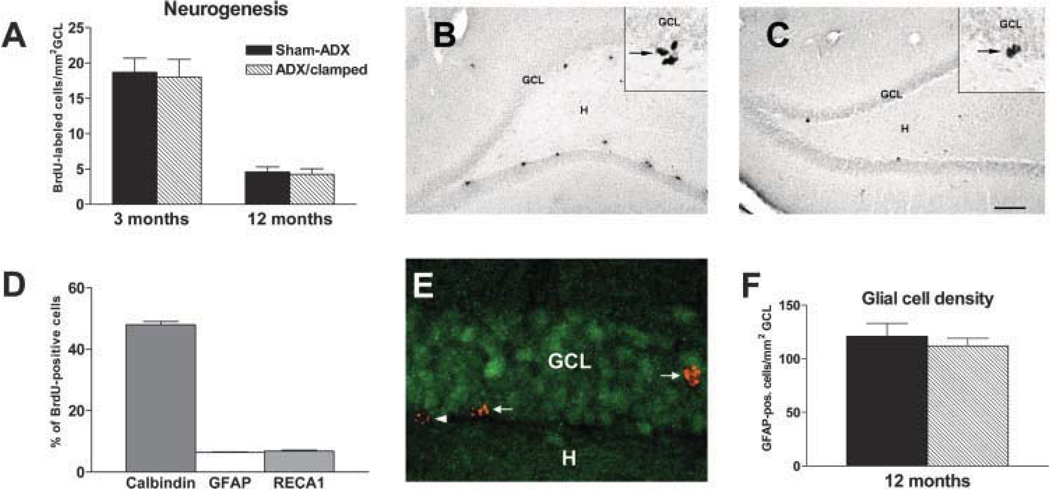
FIGURE 2.
Early postnatal adrenalectomy and persistent low-level plasma CORT do not influence neurogenesis. A: Rates of neurogenesis are similar in 3-month-old intact rats sham-adrenalectomized (Sham-ADX) compared with those reared at chronic low CORT levels (ADX/clamped). By 12 months, neurogenesis is diminished in both groups. B,C: Low magnification of the dentate gyrus in these two ages, highlighting the reduced abundance of BrdU-labeled cells at the hilar border of 12-month-old rats. An intact 3-month-old rat (B) and an intact 12-month-old (C) rat. Insets: Representative high-magnification photographs of BrdU-labeled nuclei (arrows) at the base of the granule cell layer (GCL). D: Twenty-eight days after BrdU injection (into 3-month-old rats), most BrdU-labeled cells (~48%) expressed calbindin D28k, indicating differentiation into mature granule cells. Minor populations were identified as glial (GFAP-expressing, ~6%) or epithelial (RECA1-expressing, ~7%) cells. E: Confocal image showing BrdU-labeled cells (red) that have migrated into GCL and express calbindin D28k (green; arrows). BrdU-labeled cells in subgranular zone, which do not express calbindin D28k (arrowhead), most likely represent glial, epithelial or slowly differentiating, immature granule cells. F: Density of glial cells in GCL (+subgranular zone) is not different in ADX/clamped compared with age-matched Sham-ADX control rats, indicating that lifelong exposure to low CORT does not alter glial cell production significantly. *P < 0.05, Student’s t-test). H, hilus. Scale bars = 200 µm in B,C; 20 µm in insets; 15 µm E. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 2 illustrates a typical DG after BrdU labeling in a 3-month (Fig. 2B) and a 12-month-old rat (Fig. 2C). Reduction in the number of BrdU-incorporating nuclei in the latter is apparent. This reduction was similar in magnitude in both groups of rats (75% in intact; 76% in ADX/clamped rats). In contrast, the plasma CORT level was higher in the intact group (34 ± 6 µg/dl) versus the ADX/clamped group (10 ± 3 µg/dl; Fig. 1).
Mitotically active cells in subgranular zone do not only produce granule cells but also include progenitors of other cell types, e.g., epithelial cells and glial cells (Palmer et al., 2000) (Fig. 2D). Glial cells, in particular, have previously been shown to be sensitive to alterations in CORT levels (Gould et al. 1992; Nichols et al., 2001). To estimate the relative contribution of glial cells to the population of BrdU-incorporating cells, we injected BrdU into 3-month-old rats and determined the phenotype of BrdU-labeled cells 28 days later. At that time point, the majority of labeled cells (48%) had migrated into the granule cell layer and co-localized Calbindin D28k, suggesting differentiation into mature granule cells (Fig. 2D,E). Only 6% expressed the glial cell marker GFAP, whereas 7% had an epithelial cell phenotype; 39% of BrdU-labeled cells, mainly those still residing in or close to the subgranular zone, did not co-localize any of the cell-type markers studied. Because these cells probably represent slowly maturing granule cells (Heine et al., 2004), it can be estimated that the majority (>50%) of the BrdU-labeled cells in the current study differentiates into granule cells.
While only 6% of BrdU positive cells co-expressed GFAP at a given time during adulthood, this fact cannot exclude a subtle shift in the granule cell/glial cell production ratio after chronically low CORT, leading to a dissociation of the number of BrdU-labeled cells from the true granule cell production rates in the clamped rats. This possibility should result in altered glial cell density in DG. Therefore, the density of GFAP-expressing glial cells was compared between 12-month-old rats that had been clamped on low CORT throughout life and controls. As shown in Figure 2F, glial cell densities did not differ in clamped and sham-ADX controls, rendering a significant effect of lifelong low CORT on glial cell production rate unlikely.
Granule Cell Number and Viability Do Not Differ in Early-Life ADX/Clamped Rats and Controls
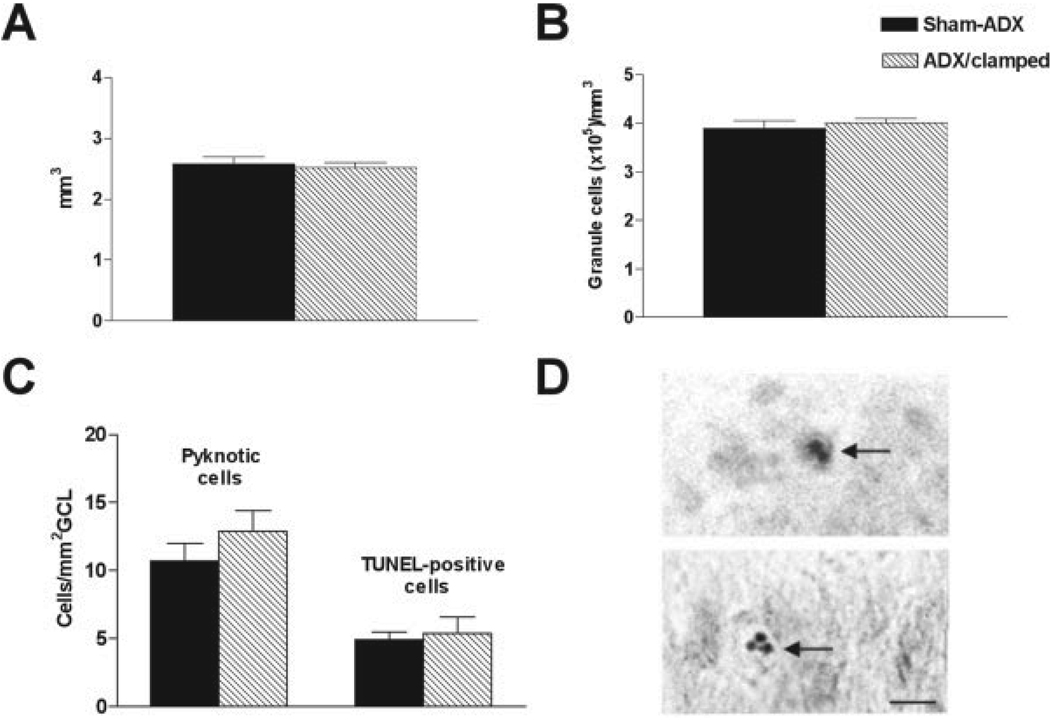
A modest but enduring increase of granule cell production in rats with constant low levels of CORT might be missed by the single sampling time-point using BrdU. However, such increased production (assuming equal cell death) should result in increased total numbers of granule cells. This can be measured as increased granule cell layer volume, higher granule cell packing density or both. As shown in Figure 3A,B, the volume and packing density of the granule cell layer were virtually identical in 12-month-old animals subjected to differing CORT levels throughout life. Similarly, no significant difference was found in 3-month-old rats (volume: 2.75 ± 0.12 mm3 in clamped vs. 2.93 ± 0.17 mm3 in control rats; density: 3.48 ± 0.1 × 105 cells/mm3 in clamped vs. 3.63 ± 0.2 × 105 cells/mm3 in control rats).
FIGURE 3.
Total numbers of granule cells are not higher in adrenalectomized (ADX)/clamped rats, and this fact is not due to increased cell death. Granule cell layer (GCL) volume (A), packing density (B), or apoptosis rate (C) were not different in 12-month-old ADX/clamped rats, when compared with age-matched sham-operated (Sham-ADX) rats. D: Examples of TUNEL-positive (upper panel) and pyknotic (lower panel) granule cell nuclei in GCL of a 12-month-old rat. Scale bar = 10 µm.
It could be argued that the lack of increased numbers of granule cells in the ADX/clamped group was attributable to an increased death rate, as found after adrenalectomy in adult rats (Sloviter et al., 1993). Therefore, we analyzed granule cell degeneration in 12-month-old intact and ADX/clamped animals (Fig. 3C) using two methods: (1) density of pyknotic nuclei; and (2) density of TUNEL-positive (apoptotic) nuclei. In both analyses, rates of granule cell degeneration were not significantly different between the groups (the lower number of TUNEL-positive compared with pyknotic nuclei is probably the result of a narrower time window for TUNEL detection of apoptotic nuclei). Taken together, these data support the notion that the rate of granule cell neurogenesis is not significantly affected by the chronically low plasma glucocorticoid levels.
Rate of Neurogenesis is Increased After Adrenalectomy of Adult Rats
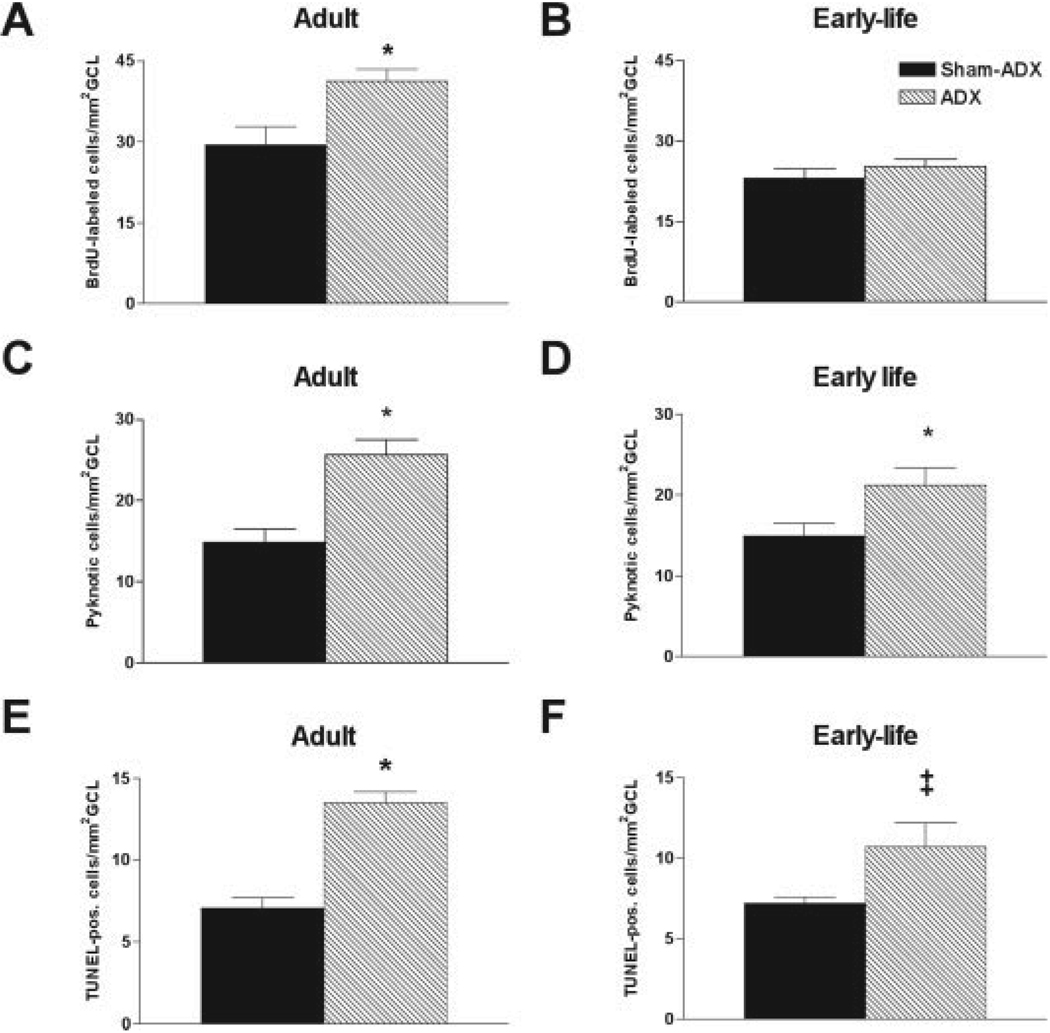
The failure to detect increased neurogenesis rates in rats exposed to enduring low levels of plasma glucocorticoids might derive from technical issues with the BrdU method (Cameron and McKay, 2001; Rakic, 2002; Hayes and Nowakowski, 2002). Therefore, granule cell production was examined in 3-month-old rats that were ADX as adults and kept without CORT supplementation for 6 days (serum CORT levels of these rats were undetectable, i.e., <0.5 µg/dl in four of five animals, and 1.1 µg/dl in one). As expected from previous reports (e.g., Cameron and Gould, 1994; Rodriguez et al., 1998; Cameron and McKay, 1999; Nichols et al., 2001), adrenalectomy in adult rats provoked an increase of granule cell proliferation in subgranular zone (Fig. 4A). The lack of enhanced granule cell production in the ADX/clamped group in response to chronic low plasma CORT is therefore not explained by a lack of sensitivity of the BrdU method in our hands and requires other explanations.
FIGURE 4.
Differential response of intact and adrenalectomized (ADX)/clamped rats to complete corticosterone (CORT) withdrawal. A: Adrenalectomy of 3-month-old rats resulted in increased granule cell production. B: Withdrawal of CORT from 3-month-old ADX/clamped rats did not enhance neurogenesis. However, apoptosis rate was significantly increased in both the adult-ADX (C,E) and the early-life ADX/clamped rats (D,F) after CORT withdrawal. (*P < 0.05; ‡P = 0.06; Student’s t-test). GCL, granule cell layer.
Granule Cells From Rats With “Lifelong” Low CORT Die Upon Corticoid Withdrawal, but Their Proliferative Response is Impaired
Adrenalectomy early in life, with clamping of plasma CORT at levels saturating MR but not GR (de Kloet et al., 1990), might lead to adaptation of the granule cells to these hormone levels. Therefore, we investigated the responsiveness of granule cell neurogenesis to total elimination of plasma CORT, by withdrawing the hormone from the drinking solution of 3-month-old rats that were ADX/clamped on P10. In comparison with total withdrawal of CORT from intact 3-month-old rats (via adrenalectomy at this age, see above), a 6-day withdrawal of CORT from rats raised with chronic low CORT levels did not provoke enhanced neurogenesis (cf. Fig. 4B with Fig. 4A).
However, the effective elimination of plasma CORT induced apoptosis in the early-life ADX/clamped group, as demonstrated with both pyknotic nuclei and TUNEL analysis (Fig. 4D,F). This effect, likely attributable to loss of MR activation (Reul and de Kloet, 1985; Hornsby et al., 1996; Gerth et al., 1998), was similar in magnitude to the apoptosis evoked by a 6 day adrenalectomy in previously intact 3-month-old rats (Fig. 4C,E). Taken together, these data suggest that early-life onset of chronically low CORT levels influenced differentially the proliferative and apoptotic responses of granule cells to corticosteroid deprivation.
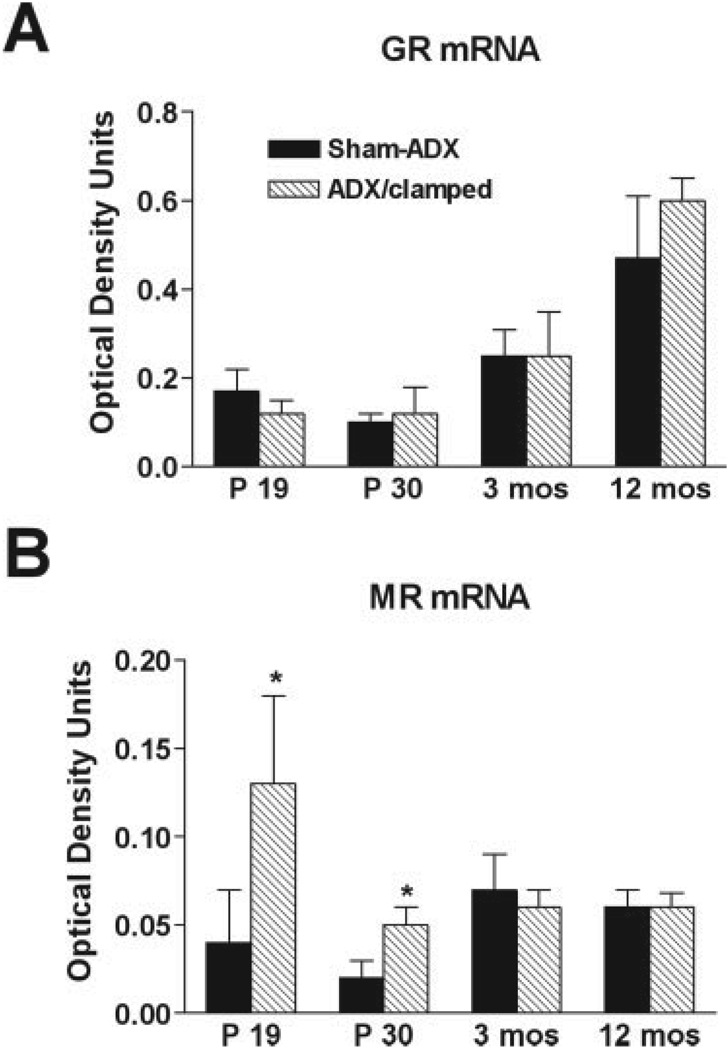
Transient Increase in Expression of Mineralocorticoid Receptor mRNA in Granule Cells of Chronically Clamped Rats
Granule cell neurogenesis and neurodegeneration are linked to the level of activation of the two corticosteroid receptors (Reul and de Kloet, 1985), the low-affinity GR (Woolley et al., 1991; Gass et al., 2000) and the high-affinity MR (Hornsby et al., 1996), respectively. The expression levels of these receptors vary inversely with CORT levels in both adult (Herman et al., 1989; Kalman and Spencer, 2002) and developing (Avishai-Eliner et al., 1999, 2001) rat DG. In turn, receptor levels help govern glucocorticoid secretion and plasma levels. Thus, higher expression of these receptors is associated with more efficient transduction of corticoid-evoked signals, including negative feedback of the hypothalamic-pituitary adrenal axis. Therefore, we studied the expression of MR and GR mRNAs in granule cells 9 days, 20 days, 3 months, and 12 months after ADX of neonatal (P10) rats, that were clamped with low CORT levels.
Unexpectedly, GR mRNA expression in granule cells of chronic low-CORT rats was not augmented at any time point when compared with sham-operated controls (Fig. 5A). This suggests that invariant low CORT levels during the critical period of granule cell proliferation lead to escape of GR expression levels from the effects of plasma glucocorticoids. For MR mRNA, its expression was strongly upregulated immediately after ADX (ADX: 0.13 ± 0.05 relative optical density [ROD] units vs. control: 0.04 ± 0.03 ROD units; P = 0.049, t-test), and remained significantly higher than the control level for at least 20 days (ADX: 0.05 ± 0.01 ROD units vs. control: 0.02 ± 0.01 ROD units; P = 0.043, t-test; Fig. 5B). This increased MR expression level could amplify the effects of a given level of circulating CORT on hippocampal granule cells.
FIGURE 5.
Chronic low glucocorticoid levels selectively alter expression of hippocampal corticosteroid receptors. A: No difference was detected in GR mRNA levels in dentate gyrus of ADX/clamped rats at any time point compared with sham-adrenalectomized (ADX) controls. B: Levels of MR mRNA were transiently enhanced in granule cells of ADX/clamped animals. Upregulation of MR mRNA was pronounced during the first weeks after adrenalectomy and returned to control levels by 3 months of age. *P < 0.05, Student’s t-test).
DISCUSSION
The major findings of these experiments are: (1) lifelong exposure to low plasma glucocorticoids, when commencing early in life, does not lead to chronic increase of granule cell neurogenesis; (2) hippocampal granule cell production correlates inversely with age in both intact and ADX/clamped animals, suggesting that aging-related reduction of neurogenesis is not governed exclusively by glucocorticoids; (3) the neurogenic potential (but not death-mechanisms) of granule cells in response to total deprivation of corticosteroids is lost when low plasma steroid levels are imposed starting early in life; and (4) increased MR levels and loss of GR regulation by plasma glucocorticoids may contribute to the adaptation of granule cell neurogenesis to lifelong low plasma levels of these hormones.
High levels of glucocorticoids inhibit production of new hippocampal granule cells (Gould et al., 1991, 1998; Cameron and Gould, 1994; Ambrogini et al., 2002). In addition, production of new granule cells declines with age (Seki and Arai, 1995; Kuhn et al., 1996; Kempermann et al., 1998; Nichols et al., 2001; Bizon and Gallagher, 2003; Heine et al., 2004), when glucocorticoid levels are higher (Sapolsky, 1992). Therefore, it has been hypothesized that chronic plasma glucocorticoid levels are a key regulator of the rate of granule cell neurogenesis (Gould et al., 1992; Cameron and McKay, 1999; Drapeau et al., 2003).
To consider the effects of lifelong low plasma corticosteroid on rates of granule cell neurogenesis, we adrenalectomized neonatal rats and clamped them at low CORT throughout life. This procedure eliminated daily circadian peaks and the stress-associated plasma glucocorticoid bursts. Surprisingly, granule cell production rate was similar in the two groups at 3 months of age. Further, although the procedure blunted the age-dependent increase of plasma CORT (the remaining increase is likely due to reduced clearance of CORT and/or contribution of ectopic adrenal hormone-secreting tissue in an occasional animal), the age-dependent decline of neurogenesis persisted in 12-month-old ADX/clamped rats.
Several paradigms of BrdU labeling have been used by a number of investigators to examine granule cell neurogenesis in adult rats (Cameron and McKay 1999, 2001; Hayes and Nowakowski, 2002; Heine et al., 2004), each providing advantages and disadvantages. We used a single, high dose pulse of BrdU followed by detection of BrdU incorporation after a short survival period (24 h). While this paradigm detects only a subset of the cells in S-phase, it provides an accurate estimate of the proliferative activity of hippocampal progenitor cells at a given time and under a given condition, whereas multiple injections of BrdU and/or longer survival incur the risk of disproportional representation of multiply-dividing cells in the analysis. In addition, with extended survival periods, cell death may occur, leading to an underestimate of the number of proliferating cells (Cameron and McKay, 2001). The disadvantage of this approach is a result of the relatively low number of cells in adult DG that are labeled, potentially affecting the sensitivity of the analysis. However, using this method, we detected enhanced granule cell proliferation after total CORT withdrawal in adult-ADX rats, but not in rats that had been clamped on low CORT throughout life (Fig. 4). These results suggest that the paradigm is sufficiently sensitive to detect real differences in proliferative capacity of granule cell progenitors. Thus, the similar proliferation rates in the experimental groups suggest that early-life onset reduction of plasma CORT results in adaptive mechanisms that overcome the inverse relationship of plasma CORT and neurogenesis—mechanisms that might contribute to altered neurogenesis during aging. This conclusion is further supported by the observation that other parameters (e.g., granule cell volume and density) were also not different in chronically clamped compared with age-matched control rats.
Logical candidates for mediating granule cell adaptation to chronic changes in circulating corticosteroids are compensatory or adaptive changes in the expression of their receptors (Herman and Spencer, 1998). Such changes of receptor expression would facilitate a stable corticosteroid environment, which is particularly important in the neonatal rat to ensure proper brain development (Bohn, 1980; de Kloet et al., 1988). Indeed, we found a transient but robust enhancement of MR mRNA expression that lasted throughout the developmental period, when MR expression is dynamically regulated (Vazquez et al., 1998). Thus, by upregulating MR expression, granule cells may attempt to compensate for abnormally low CORT levels and maintain MR signaling at a level necessary for both control of neurogenesis and protection against apoptosis (Woolley et al., 1991; Hornsby et al., 1996; Gerth et al., 1998; Montaron et al., 2003). Indeed, our data, together with previous studies, suggest that this adaptive mechanism might be successful: chronically ADX/clamped rats, when tested as adults, performed without impairment in hippocampus mediated learning and memory tasks, even at age 12 months (Brunson et al., 2001b). As found here, increased MR expression did not persist into adulthood. In addition, when deprived entirely from adrenal steroids, granule cells from early-life clamped animals underwent apoptosis to a degree similar to that of animals ADX as adults, suggesting that compensatory mechanisms to protect from MR- dependent cell death are limited.
The apoptosis after total steroid withdrawal for 6 days in early-life ADX/clamped animals also excluded the possibility that granule cell neurogenesis was not enhanced because of altered clearance of CORT and/or long-lasting storage of the hormone in their tissues. In addition, the apparent dissociation between steroid-withdrawal-induced apoptosis and neurogenesis in these animals supports previous notions that neurogenesis and degeneration are independently regulated processes (Montaron et al., 2003). Finally, the similar rates of cell death observed in early-life ADX/clamped and intact rats throughout their lives (see Fig. 3C) does not support the notion that persistent, rapid turnover of granule cells, i.e., rapid cycling of proliferation and death evoked by low CORT, led to depletion of the precursor pool of the ADX/clamped rats.
An additional potential adaptive mechanism to changes in plasma CORT levels involves modulation of GR expression. In intact aging rats, increased GR in the granule cell layer was found (Fig. 5A), despite increased circulating CORT. Interestingly, this increase in GR expression was found also in early-life clamped rats, where age-related increased CORT was modest. This finding has two implications. First, it suggests a lifelong escape of the regulation of GR expression from plasma glucocorticoid levels in the early-life ADX group. Second, for both groups of aging rats, increased levels of GR have been considered a successful, compensatory mechanism for facilitating more effective negative glucocorticoid feedback, shutting off further activation of CORT secretion during stress (Liu et al., 1997). Thus, enhanced GR expression in aging rats is generally considered neuroprotective (Sapolsky, 1992; Stein-Behrens and Sapolsky, 1992).
While altered regulation of GR and MR expression following lifelong exposure to low plasma CORT was found here, the reduced neurogenic rate with age persisted, as found in intact animals. Thus, granule cell production rates were significantly lower in aging rats regardless of whether their plasma CORT increased with age. These observations suggest that diminished granule cell production is largely regulated by additional, age-dependent factors that are not necessarily glucocorticoid-dependent. This conclusion is supported by the recent findings of Heine et al. (2004). These authors carried out a thorough analysis of lifetime granule cell production in Wistar rats, but found no correlation between basal corticosteroid levels and neurogenesis in middle-aged (12 months) and aged (24 months) rats.
Candidate mechanisms include growth factors (see Fabel et al., 2003, for a recent review). True, the production and release of some growth factors is influenced by glucocorticoids, probably via their binding to GR on cells neighboring the granule cell precursors (Cameron et al., 1993; Fabel et al., 2003). However, the findings here suggest that age-related effects might overcome the influence of glucocorticoid levels on release or action of neurogenesis-promoting factors. For example, the expression of insulin-like growth factor 1 (IGF1), a mediator of increased hippocampal neurogenesis in response to physical exercise (van Praag et al., 1999; Trejo et al., 2001), decreases upon aging (Anlar et al., 1999; Sonntag et al., 1999), potentially compromising neurogenesis (Åberg et al., 2000; Lichtenwalner et al., 2001). In addition, neuronal precursors in DG may be subject to replicative senescence, permitting only a defined number of proliferation cycles throughout life (Nichols et al., 2001; Olariu et al., 2003). Of note, the mechanisms for this effect may also involve glucocorticoids (e.g., via their actions on cellular oxidative stress, Roy and Sapolsky, 2003).
In summary, the regulation of granule cell neurogenesis throughout the life cycle is a complex and dynamic process. Both acute and chronic levels of plasma corticosteroids play a critical role in modulating granule cell production (and death). By manipulating glucocorticoid levels throughout life, the current studies illustrate an interesting divergence of neurogenesis and plasma corticosteroid levels, as well as adaptive mechanisms. In particular, these studies suggest that aging-related reduction of granule cell production rates is not a simple function of plasma glucocorticoids. Understanding the additional mechanisms involved should provide important information for prevention of aging-related cognitive dysfunction.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: NS 28912; Grant number: NS 39307.
The authors thank Dr. Yuncai Chen for his excellent contributions and Michele Hinojosa for editorial assistance.
REFERENCES
- Åberg MAI, Åberg DN, Hedbacker H, Oscarsson J, Hagberg H, Eriksson PS. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akana SF, Dallman MF. Chronic cold in adrenalectomized, corticosterone (B)-treated rats: facilitated corticotropin responses to acute restraint emerge as B increases. Endocrinology. 1997;138:3249–3258. doi: 10.1210/endo.138.8.5291. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GP. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;301:325–342. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Barbanti I, Cuppini R. Persistently high corticosterone levels but not normal circadian fluctuations of the hormone affect cell proliferation in the adult rat dentate gyrus. Neuroendocrinology. 2002;76:366–372. doi: 10.1159/000067581. [DOI] [PubMed] [Google Scholar]
- Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-1 and central nervous system development. Horm Metab Res. 1999;31:120–125. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Hatalski CG, Tabachnik E, Eghbal-Ahmadi M, Baram TZ. Differential regulation of glucocorticoid receptor messenger RNA (GR-mRNA) by maternal deprivation in immature rat hypothalamus and limbic regions. Dev Brain Res. 1999;114:265–268. doi: 10.1016/s0165-3806(99)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Bender RA, Lauterborn JC, Gall CM, Cariaga W, Baram TZ. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur J Neurosci. 2001;13:679–686. doi: 10.1046/j.1460-9568.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epilepsy. J Neurosci. 2003;23:6826–6836. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Bohn MC. Granule cell genesis in the hippocampus of rats treated neonatally with hydrocortisone. Neuroscience. 1980;5:2003–2012. doi: 10.1016/0306-4522(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ. Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression. Ann Neurol. 2001a;49:304–312. [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender RA, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001b;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;6:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variation on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Rosenfeld P, Van Eekelen JAM, Sutanto W, Levine S. Stress, glucocorticoids and development. Prog Brain Res. 1988;3:101–120. doi: 10.1016/S0079-6123(08)60500-2. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Reul JM, Sutanto WJ. Corticosteroids and the brain. J Steroid Biochem Mol Biol. 1990;37:387–394. doi: 10.1016/0960-0760(90)90489-8. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fabel K, Toda H, Fabel K, Palmer T. Copernican stem cells: regulatory constellations in adult hippocampal neurogenesis. J Cell Biochem. 2003;88:41–50. doi: 10.1002/jcb.10377. [DOI] [PubMed] [Google Scholar]
- Gass P, Kretz O, Wolfer DP, Berger S, Tronche F, Reichardt HM, Kellendonk C, Lipp HP, Schmid W, Schütz G. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep. 2000;1:447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth A, Hatalski CG, Avishai-Eliner S, Baram TZ. Corticotropin releasing hormone antagonist does not prevent adrenalectomy-induced apoptosis in the dentate gyrus of the rat hippocampus. Stress. 1998;2:159–169. doi: 10.3109/10253899809167280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus. II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joёls M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Herman JP. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol Neurobiol. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PubMed] [Google Scholar]
- Herman JP, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. J Neurosci. 1998;18:7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Patel AD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralcorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Hornsby CD, Grootendorst J, de Kloet ER. Dexamethasone does not prevent seven-day ADX-induced apoptosis in the dentate gyrus of the rat hippocampus. Stress. 1996;1:51–65. doi: 10.3109/10253899609001095. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods. 1999;93:149–162. doi: 10.1016/s0165-0270(99)00142-9. [DOI] [PubMed] [Google Scholar]
- Kalman BA, Spencer RL. Rapid corticosteroid-dependent regulation of mineralcorticoid receptor protein expression in rat brain. Endocrinology. 2002;143:4184–4195. doi: 10.1210/en.2002-220375. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. The pituitary-adrenal system and the developing brain. Prog Brain Res. 1970;32:79–85. doi: 10.1016/S0079-6123(08)61521-6. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Benett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-1 ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, Abrous DN. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur J Neurosci. 2003;18:3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- Nichols NR, Zieba M, Bye N. Do glucocorticoids contribute to brain aging? Brain Res Rev. 2001;37:273–286. doi: 10.1016/s0165-0173(01)00131-x. [DOI] [PubMed] [Google Scholar]
- Olariu A, Dayer AG, Kleaver KM, Abouantoun TG, Cameron HA. Cell cycle length in young-adult and middle-aged rat dentate gyrus granule cell precursors. Soc Neurosci Abs 617.3. 2003 [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci. 2002;22:614–618. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Montaron MF, Petry KG, Aurousseau C, Marinelli M, Premier S, Rougon G, le Moal M, Abrous DN. Complex regulation of the expression of the polysialylated form of the neural cell adhesion molecule by glucocorticoids in the rat hippocampus. Eur J Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- Roy M, Sapolsky RM. The exacerbation of hippocampal excitotoxicity by glucocorticoids is not mediated by apoptosis. Neuroendocrinology. 2003;77:24–31. doi: 10.1159/000068337. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Do glucocorticoid concentrations rise with age in the rat? Neurobiol Aging. 1992;13:171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:159–176. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. NeuroReport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Sollas AL, Den E, Neubort S. Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: characterization of an in vivo model of controlled neuronal death. J Comp Neurol. 1993;330:324–336. doi: 10.1002/cne.903300304. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Bennett SA, Khan AS, Thornton PL, Cooney PT, Ingram RL, McShane T, Brunso-Bechtold JK. Alterations in insulin-like growth factor-1 gene and protein expression and type insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88:269–279. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Young EA, Choo PH, McEwen BS. Adrenal steroid type I and II receptor binding: estimates of in vivo receptor number, occupancy, and activation with varying level of steroid. Brain Res. 1990;514:37–48. doi: 10.1016/0006-8993(90)90433-c. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens BA, Sapolsky RM. Stress, glucocorticoids, and aging. Aging. 1992;4:197–210. doi: 10.1007/BF03324092. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor 1 mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez DM, Lopez JF, Morano MI, Kwak SP, Watson SJ, Akil H. α, β, and γ mineralocorticoid receptor messenger ribonucleic acid splice variants: differential expression and rapid regulation in the developing hippocampus. Endocrinology. 1998;139:3165–3177. doi: 10.1210/endo.139.7.6095. [DOI] [PubMed] [Google Scholar]
- Walker CD, Akana SF, Cascio CS, Dallman MF. Adrenalectomy in the neonate: adult-like adrenocortical system responses to both removal and replacement of corticosterone. Endocrinology. 1990;127:832–842. doi: 10.1210/endo-127-2-832. [DOI] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Watts AG, Tanimura S, Sanchez-Watts G. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinology. 2004;145:529–540. doi: 10.1210/en.2003-0394. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Sakai RR, Spencer RL, McEwen BS. Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res. 1991;554:312–335. doi: 10.1016/0006-8993(91)90207-c. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]