Abstract
The phytohormone auxin is transported through the plant body either via vascular pathways or from cell to cell by specialized polar transport machinery. This machinery consists of a balanced system of passive diffusion combined with the activities of auxin influx and efflux carriers. Synthetic auxins that differ in the mechanisms of their transport across the plasma membrane together with polar auxin transport inhibitors have been used in many studies on particular auxin carriers and their role in plant development. However, the exact mechanism of action of auxin efflux and influx inhibitors has not been fully elucidated. In this report, the mechanism of action of the auxin influx inhibitors (1-naphthoxyacetic acid (1-NOA), 2-naphthoxyacetic acid (2-NOA), and 3-chloro-4-hydroxyphenylacetic acid (CHPAA)) is examined by direct measurements of auxin accumulation, cellular phenotypic analysis, as well as by localization studies of Arabidopsis thaliana L. auxin carriers heterologously expressed in Nicotiana tabacum L., cv. Bright Yellow cell suspensions. The mode of action of 1-NOA, 2-NOA, and CHPAA has been shown to be linked with the dynamics of the plasma membrane. The most potent inhibitor, 1-NOA, blocked the activities of both auxin influx and efflux carriers, whereas 2-NOA and CHPAA at the same concentration preferentially inhibited auxin influx. The results suggest that these, previously unknown, activities of putative auxin influx inhibitors regulate overall auxin transport across the plasma membrane depending on the dynamics of particular membrane vesicles.
Keywords: Auxin efflux carrier, auxin influx carrier, auxin transport, auxin transport inhibitor, membrane dynamics, tobacco BY-2 cells
Introduction
Differential distribution of the plant growth regulatory substance auxin is known to mediate many fundamental processes in plant development, such as the formation of the embryogenic apical–basal axis, pattern formation, tropisms, and organogenesis (reviewed in Vanneste and Friml, 2009). The distribution of auxin and the formation of auxin gradients in the tissues is directed by the activity of the plasma membrane-localized auxin influx carriers AUXIN RESISTANT 1/LIKE AUXIN RESISTANT (AUX1/LAX) and auxin efflux carriers PIN-FORMED (PIN) as well as the ATP-Binding Cassette subfamily B (ABCB)/multidrug resistance (MDR)/phosphoglycoprotein (PGP)-type transporters (Noh et al., 2001; Swarup et al., 2001; Friml et al., 2002a, b, 2003; Benková et al., 2003; Blilou et al., 2005; Vieten et al., 2007; Pernisová et al., 2009). Auxin influx carriers (AUX1/LAX), belonging to the family of plasma membrane amino acid permeases (AAP family), mediate the uptake of auxin into the cell (auxin influx). AUX1 was characterized at the molecular level in Arabidopsis by Bennett et al. (1996) and its transport function was shown by Yang et al. (2006). In addition, the function of LAX3 was characterized in Arabidopsis as well (Swarup et al., 2008).
Although most of the native auxin indole-3-acetic acid (IAA) is transported into cells by diffusion, the importance of AUX1/LAX carriers could be clearly demonstrated in many developmental processes. They are involved in embryogenesis (Ugartechea-Chirino et al., 2010), hypocotyl apical hook development (Vandenbussche et al., 2010), root gravitropism (Bennett et al., 1996), lateral root development (Swarup et al., 2001), root hair development (Jones et al., 2009), phloem loading and unloading (Marchant et al., 2002), and phyllotaxis (Bainbridge et al., 2008). Generally, the importance of auxin uptake carriers lies mainly in their role in the pumping of auxin against its concentration gradient. Mathematical modelling further supports the role of AUX1/LAX proteins in the creation of local auxin maxima as demonstrated for phyllotaxis (Smith et al., 2006).
Over the last two decades, auxin efflux inhibitors such as 2,3,5-triiodobenzoic acid (TIBA) or 1-naphthylphthalamic acid (NPA) (see a review by Rubery, 1990; Lomax et al., 1995; Morris et al., 2004) have significantly contributed to the present knowledge about auxin efflux transporters and their involvement in the control of physiological and developmental processes in plants. Compounds that inhibit auxin efflux include synthetic phytotropins (NPA and 2-(1-pyrenoyl)benzoic acid (PBA) being the most prominent representatives), cyclopropyl propane dione (CPD) and TIBA, and also natural flavonoids (e.g. quercetin). Moreover, inhibitors of intracellular protein trafficking, such as monensin and brefeldin A (BFA), also affect auxin efflux (Wilkinson and Morris, 1994; Morris and Robinson, 1998). BFA operates through plasma membrane localization of PINs and ABCBs but not AUX1/LAXes (Titapiwatanakun and Murphy, 2009).
The synthetic compounds 1-naphthoxyacetic acid (1-NOA), 2-naphthoxyacetic acid (2-NOA), and 3-chloro-4-hydroxyphenylacetic acid (CHPAA) have been used to disrupt auxin influx (uptake). The inhibitors were originally selected on the basis of their structural similarities with 2,4-dichlorophenoxyacetic acid (2,4-D), an auxin influx carrier substrate, or with naphthalene-2-acetic acid (2-NAA), a substance that inhibits auxin influx carriers (Imhoff et al., 2000). The synthetic inhibitors share some structural features with auxins in containing an aromatic moiety substituted with an acidic side-chain with a carboxyl group, which is, among other things, believed to bind specifically a particular region of the putative auxin receptor (Edgerton et al., 1994). Importantly, the naturally occurring substance chromosaponin (Rahman et al., 2001) was found to be a possible endogenous analogue to synthetic inhibitors such as 1-NOA (Parry et al., 2001).
Although the mechanism of action of auxin transport inhibitors is still far from being fully understood, recent data (Dhonukshe et al., 2008) suggest that some of these compounds (e.g. PBA, TIBA, but not NPA) can act through actin-mediated vesicle trafficking processes in eukaryotic cells. As shown in tobacco BY-2 cells, actin dynamics seems to be at both the genomic and the non-genomic levels under the control of auxin itself and NPA interferes with this process (Maisch and Nick, 2007; Nick et al., 2009). The modulation of intracellular trafficking of both AUX1 and PIN auxin transporters (Kleine-Vehn et al., 2006) by auxin transport inhibitors is thus critical for their overall abundance at the plasma membrane. Obviously, besides the action of (native) auxin transport inhibitors, many other processes regulate the overall performance of auxin transporters as well (for a review see Titapiwatanakun and Murphy, 2009).
In this paper, the function and mechanism of action of the synthetic auxin influx inhibitors 1-NOA, 2-NOA, and CHPAA were investigated. Assays of auxin transport in tobacco BY-2 cells showed that, in contrast to 2-NOA and CHPAA, the mode of action of 1-NOA is not entirely specific for auxin influx. Changes in the subcellular distribution of both AtEYFP:AUX1 and AtPIN1:GFP fusion proteins expressed heterologously in tobacco BY-2 cells, and the resulting defects in the polarity of cell division, indicate that 1-NOA acts largely through changes in membrane dynamics. 2-NOA was less effective and CHPAA was almost ineffective in this respect. On the basis of these findings the mechanism of action of these inhibitors was proposed related to the dynamics of membrane vesicles transporting particular auxin carriers.
Materials and methods
Chemicals
Unless stated otherwise, all chemicals were supplied by Sigma Aldrich (St Louis, MO, USA).
Construction of transformation vectors
The estradiol-inducible XVE::PIN1:GFP construct was prepared using DNA sequence PIN1:GFP which was PCR amplified from plasmid DNA (Benková et al., 2003). The primers containing the attB1 and attB2 Gateway recombination sites were used. The purified PCR product was placed into the Gateway pDONR 221 donor vector (BP reaction). Recombination (LR reaction) was then made with the pMDC7 binary destination vector (Curtis and Grossniklaus, 2003) containing the estradiol-inducible transactivator XVE. The resulting plasmid was verified by sequencing from the left to the right borders.
The estradiol-inducible XVE::EYFP:AUX1 construct was prepared using AUX1 and EYFP DNA sequences which were PCR amplified separately from plasmid DNA (Yang et al., 2006). The primers contained attB multisite Gateway recombination sites. For EYFP and AUX1 amplification, attB1, attB5r sites, and attB5, attB2 sites were used in this respect. The purified PCR products were then placed into Gateway pDONR 221 P1-P5r and pDONR 221 P5-P2 donor vectors (BP reactions). Multisite recombination (LR reaction) was then made with the pMDC7 binary destination vector (Curtis and Grossniklaus, 2003) containing the estradiol-inducible transactivator XVE. The resulting plasmid was verified by sequencing from the left to the right borders.
Primers used: PIN1:GFP forward (attB1), GGGGACAAGTTTGTACAAAAAAGCAGGCTCAACAATGATTACGGCGGCGGACTTCTAC, PIN1:GFP reverse (attB2), GGGGACCACTTTGTACAAGAAAGCTGGGTGTGTTTTGGTAATATCTCTTCA; AUX1 forward (attB5), GGGGACAACTTTGTATACAAAAGTTGGCTCCGCGGCCGCCCCCTTCACC, AUX1 reverse (attB2), GGGGACCACTTTGTACAAGAAAGCTGGGTATCAAAGACGGTGGTGTAAAG; EYFP forward (attB1), GGGGACAAGTTTGTACAAAAAAGCAGGCTCAACAATGGGCAAGGGCGAGGAGCTG, EYFP reverse (attB5r), GGGGACAACTTTTGTATACAAAGTTGTTGATGATCCCGGGCCCGCGG.
Plant material
Cells of tobacco line BY-2 (Nicotiana tabacum L., cv. Bright-Yellow 2) (Nagata et al., 1992) were cultured in liquid medium [3% (w/v) sucrose, 4.3 g l−1 Murashige and Skoog salts, 100 mg l−1 inositol, 1 mg l−1 thiamine, 0.2 mg l−1 2,4-D, and 200 mg l−1 KH2PO4 (pH 5.8)] in darkness at 27 °C on an orbital incubator (Sanyo Gallenkamp, Schoeller Instruments Inc., Prague, Czech Republic; 150 rpm, 32 mm orbit) and subcultured weekly. Stock tobacco BY-2 calli were maintained on the same media solidified with 0.6% (w/v) agar and subcultured monthly. BY-2 cell lines expressing GFP fusion with ABCB4 (PGP4:GFP) were described in Jelínková et al. (2010).
Stock solutions of 1-NOA (5 mM), 2-NOA (5 mM), CHPAA (10 mM), and NPA (10 mM) in ethanol were added to the BY-2 cell suspension to a final concentration of 10 μM at each subculturing. Samples of cells were taken regularly for microscopy and determination of cell density.
Expression of EYFP:AUX1 and PIN1:GFP genes was induced by the addition of estradiol (β-estradiol, 1 μM, 24 h) at the beginning of the subculture interval. Stock solutions of 1-NOA (20 mM), 2-NOA (20 mM), CHPAA (20 mM), and NPA (20 mM) in DMSO were added to reach a final concentration of 20 μM for the 3 h and 24 h treatments, and of 50 μM for the 48 h treatments. The same amount of the solvent was added to controls. FM 4-64 (4 μM) (Molecular Probes) was applied to 1 ml of 3-d-old BY-2 cells that had been pretreated with 20 μM or 50 μM 1-NOA for 4 h, and incubated for 1 min under continuous shaking.
Transformation of BY-2 cells
The basic transformation protocol of An (1985) was used. Three-day-old BY-2 cells were co-incubated with Agrobacterium tumefaciens (Petrášek et al., 2003) strain GV2260 carrying XVE::EYFP:AUX1 or XVE::PIN1:GFP constructs and transformed BY-2 cells were maintained in culture media containing 100 μg ml−1 hygromycin and 100 μg ml−1 cefotaxim.
Microscopy and image analysis
Nomarski DIC microscopy was performed using Nikon Eclipse E600 (Nikon, Japan) and images were recorded with colour digital camera (DVC 1310C, USA) using LUCIA image analysis software (Laboratory Imaging, Prague, Czech Republic). To determine cell length, a population of 400 cells was meausured using LUCIA image analysis software and the average values were expressed in μm. Confocal microscopy was performed using Zeiss LSM 5 DUO confocal microscope equipped with a ×40 C-Apochromat objective (NA=1.2 W). Fluorescence signals were obtained for GFP or YFP (excitation 488 nm, emission 505–550 nm) and FM 4-64 (excitation 561 nm, emission >575 nm).
Cell densities were determined by counting cells in at least 10 aliquots of each sample using Fuchs–Rosenthal haemocytometer slide.
Auxin accumulation measurements
Auxin accumulation in 2-d-old cells was measured using radioactively labelled auxins according to Delbarre et al. (1996), as modified by Petrášek et al. (2006). Treatments were replicated at least three times and the average values (± standard errors) were expressed as pmols of the particular auxin accumulated per million cells. 1-NOA, 2-NOA, CHPAA, NPA, or BFA were added as required from ethanolic stock solutions to give a final concentration of 10 μM at the beginning of the accumulation assay (together with the addition of radioactively labelled auxin). In the combined experiments, BFA was added to a final concentration of 10 μM at the beginning of the accumulation assay together with the addition of [3H]NAA (25 Ci mmol−1; Isotope Laboratory of the Institute of Experimental Botany, Prague, Czech Republic). Individual inhibitors 1-NOA, 2-NOA, or NPA were added after 10 min to a final concentration 10 μM.
For time-course experiments, aliquots of cell suspension were removed at specified intervals from 0–30 min after the addition of radioactively labelled auxin. Accumulation of [3H]2,4-D (20 Ci mmol−1; American Radiolabeled Chemicals, Inc., St Louis, MO, USA) in response to particular concentrations of 1-NOA or 2-NOA was determined after 20 min uptake period.
Results
Phenotypic changes in tobacco BY-2 cells after treatments with various auxin transport inhibitors
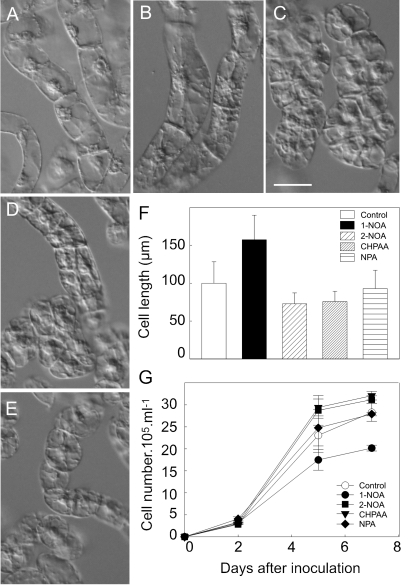
To evaluate possible differential effects of auxin influx and efflux inhibitors on the overall course of the BY-2 cell line subculture interval, the culture media were supplemented with 10 μM concentrations of 1-NOA, 2-NOA, CHPAA, or NPA. Treatments with 1-NOA resulted in phenotypic changes different from those caused by 2-NOA or CHPAA. Thus, in the presence of 1-NOA the cells divided less frequently than controls, contained amyloplasts with starch, and cell elongation was significantly enhanced (Fig. 1A, B, F), in contrast to 2-NOA or CHPAA which even stimulated cell division slightly (Fig. 1C, D, G). Treatments with NPA under the same conditions had no significant effect on cell phenotype or cell division (Fig. 1E, G).
Fig. 1.
Effects of auxin influx and efflux inhibitors on cell division activity and phenotype of tobacco BY-2 cells. The cells were grown (A) in a standard medium for 2 d, in the presence of auxin influx inhibitors (B) 1-NOA, (C) 2-NOA, (D) CHPAA, or (E) auxin efflux inhibitor NPA (10 μM each). Measurements of cell length (F) and cell density (G) showed that cells treated with 1-NOA were more elongated (F) and that cell division was reduced in contrast to cells treated with 2-NOA or CHPAA (G). Values in (F) and (G) represent means ±SE (n=10). (A–E) Scale bar, 50 μm.
Altogether, the action of 1-NOA, a putative inhibitor of auxin influx, resulted in a distinct phenotype in comparison with the phenotype caused by 2-NOA, CHPAA, which likewise block mainly auxin influx, and NPA, which blocks mainly auxin efflux.
Auxin influx inhibitors 1-NOA, 2-NOA, and CHPAA have distinct impact on the accumulation of different types of auxin
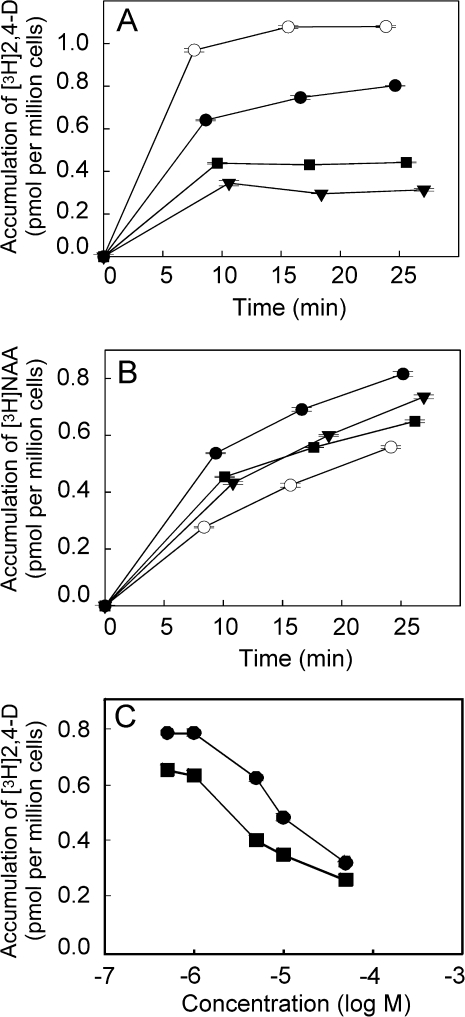
The accumulation kinetics of radioactively labelled NAA and 2,4-D has been shown to reflect the activity of auxin efflux and influx carriers, respectively, and therefore both these synthetic auxins can be used as markers for measuring the activity of these carriers, namely in tobacco cells (Delbarre et al., 1996; Petrášek and Zažímalová, 2006). The effects of the synthetic inhibitors of auxin influx on the accumulation of 2,4-D and NAA using 2-d-old tobacco BY-2 cells were investigated (Fig. 2A, B). At 10 μM concentration, 1-NOA, 2-NOA, and CHPAA all reduced the accumulation of [3H]2,4-D, CHPAA being the most effective. The amount of [3H]2,4-D that accumulated after 20 min in the presence of CHPAA was reduced by 3.5-times relative to the controls, by 2.5-times in the presence of 2-NOA, and by 1.4-times in the presence of 1-NOA (Fig. 2A). A comparison of the concentration dependence of the positional analogues 1-NOA and 2-NOA revealed that 1-NOA reduced the accumulation of [3H]-2,4-D with a 10-times lower efficiency than 2-NOA (Fig. 2C).
Fig. 2.
The effect of the putative auxin influx inhibitors on intracellular accumulation of [3H]2,4-D and [3H]NAA in tobacco BY-2 cells. The kinetics of accumulation of 2 nM [3H]2,4-D (A) and [3H]NAA (B) in the presence of 10 μM 1-NOA (filled circles), 2-NOA (filled squares) or CHPAA (filled inverted triangles). Note the difference between the efficiency of 2-NOA, CHPAA, and 1-NOA in the inhibition of auxin influx as shown by reduced accumulation of [3H]2,4-D (A) compared with control cells. Increased accumulation of [3H]NAA (reflecting preferentially the activity of auxin efflux carriers) in the presence of 1-NOA is significantly higher than that in the presence of 2-NOA and CHPAA (10 μM each). (C) Concentration dependence of [3H]2,4-D (2 nM) in response to specified concentrations of 1-NOA (filled circles) or 2-NOA (filled squares) was determined after 20 min uptake period. Accumulation of [3H]2,4-D is suppressed more effectively with 2-NOA compared with 1-NOA. Error bars represent SE (n=4).
Surprisingly, after the application of 1-NOA the accumulation of [3H]NAA significantly increased, whereas application of 2-NOA or CHPAA raised the accumulation of this labelled auxin only slightly (Fig. 2A, B). Since NAA is taken up only passively (and is a good substrate for the active auxin efflux) in tobacco cells, these results suggest that 1-NOA markedly inhibits the auxin efflux carrier(s) activity.
Taken together, the putative auxin influx inhibitor 1-NOA is much less effective in blocking the active auxin influx compared with 2-NOA or CHPAA and it also modifies the activity of auxin efflux carriers.
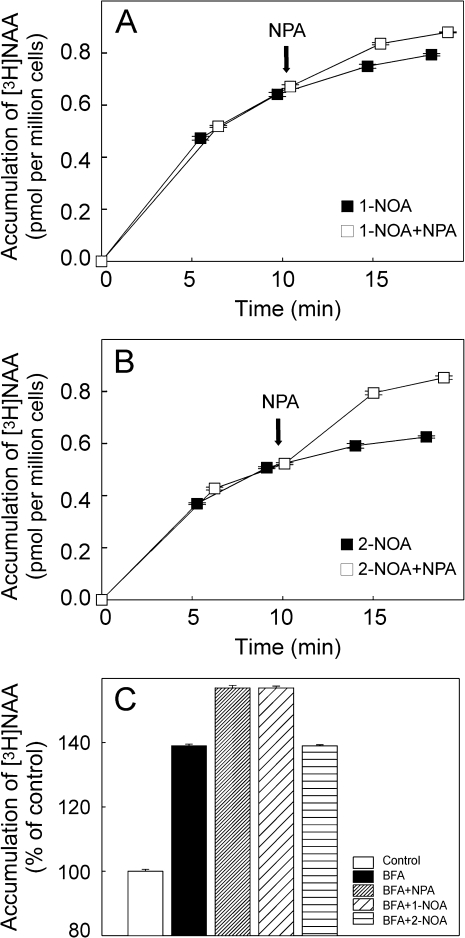
Effect of NPA on [3H]NAA accumulation in tobacco BY-2 cells treated with 1-NOA or 2-NOA
To check whether 1-NOA or 2-NOA are able to inhibit auxin efflux activity, accumulation assays were performed with BY-2 cells using [3H]NAA in the presence of 1-NOA (10 μM) or 2-NOA (10 μM). The established auxin efflux inhibitor NPA was applied at 10 μM concentration (Petrášek et al., 2003, 2006) in-flight 10 min after the addition of [3H]NAA together with 1-NOA or 2-NOA. In the presence of 1-NOA the NPA had almost no effect on the accumulation of [3H]NAA (Fig. 3A), indicating that auxin efflux carriers were already blocked by the preceding application of 1-NOA. By contrast, in the presence of 2-NOA, the in-flight addition of NPA during the accumulation of [3H]NAA caused elevation of auxin accumulation in the cells (Fig. 3B), suggesting that auxin efflux carriers were still active before the NPA treatment.
Fig. 3.
The effect of NPA on the intracellular accumulation of [3H]NAA in tobacco BY-2 cells treated with 1-NOA or 2-NOA. In-flight application of NPA (10 μM) to cells treated with 1-NOA (10 μM) (A) had very low impact on the accumulation of [3H]NAA (2 nM). In-flight application of NPA to cells treated with 2-NOA (10 μM) (B) increased auxin accumulation, suggesting that auxin efflux carriers were still active before NPA treatment (note also the higher absolute values of auxin accumulation in the case of 1-NOA in contrast to 2-NOA even before NPA treatment). Error bars represent SE (n=4). (C) Effects of the auxin influx and efflux inhibitors 1-NOA, 2-NOA, and NPA on intracellular accumulation of [3H]NAA in cells treated with the inhibitor of vesicle trafficking BFA. 2-NOA did not change the accumulation of [3H]NAA in cells treated with BFA, while both NPA and 1-NOA increased it. Values are percentages of control at 20 min after application of [3H]NAA and inhibitors. Error bars represent SE (n=4).
These results, therefore, indicate that 1-NOA, in contrast to 2-NOA, is effective in blocking auxin efflux in a way similar to NPA.
1-NOA, but not 2-NOA, increases the accumulation of [3H]NAA in tobacco BY-2 cells treated with BFA
BFA, an inhibitor of Golgi-mediated vesicle trafficking, has been shown to reduce the plasma membrane pool of PIN auxin efflux carriers within 30 min (Geldner et al., 2001). To obtain further insight into the mode of action of 1-NOA, the effect of 1-NOA, 2-NOA, and NPA on the accumulation of [3H]NAA was investigated in BY-2 cells treated with BFA. The inhibitors were added to the cells after pre-treatment with 10 μM BFA. Similarly to the previous experiments with in-flight additions of NPA, both 1-NOA and NPA increased the accumulation of [3H]NAA in cells pretreated with BFA; in contrast, 2-NOA had no additional effect (Fig. 3C).
These results provide further evidence for the role of the putative auxin influx inhibitor 1-NOA in modulating the auxin efflux carrier(s) activity. Moreover, they indicate that this inhibition is independent of anterograde membrane trafficking, suggesting either a direct influence on efflux carrier activity and/or their enhanced endocytosis.
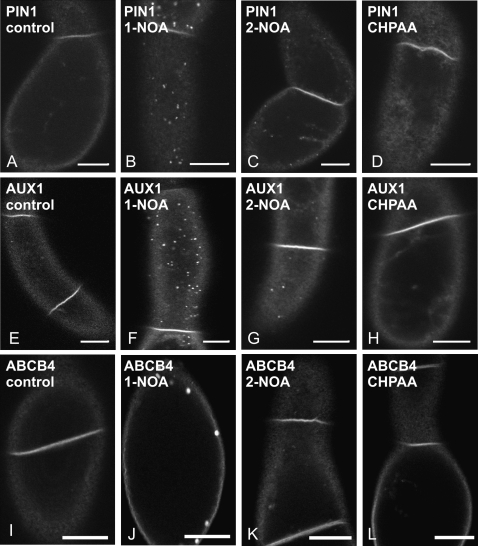
NOAs affect subcellular distribution of AUX1, PIN1, and ABCB4
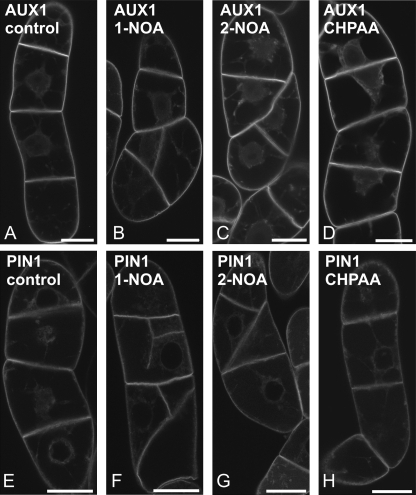
To determine whether the putative auxin influx inhibitors can influence the distribution of AUX1 or PIN1 proteins, XVE::EYFP:AUX1 or XVE::PIN1:GFP constructs were expressed in tobacco BY-2 cells. After 24 h induction with β-estradiol (1 μM), the plasma membrane fluorescence of PIN1:GFP or EYFP:AUX1 fusion proteins was observed and no vesicles in the cortical cytoplasm were seen (Fig. 4A, E). After a subsequent 3 h in the presence of 20 μM 1-NOA, both PIN1:GFP (Fig. 4B) and EYFP:AUX1 (Fig. 4F) were observed in vesicles reminiscent of endosomes and located in the cortical cytoplasm. Similar treatment with 2-NOA induced these patches as well, but the effect was weaker (Fig. 4C, G). A 3 h exposure to 20 μM CHPAA did not induce any redistribution (Fig. 4D, H), although patches were occasionally observed in a few cells (data not shown). NOA-induced vesicles of EYFP:AUX1 and PIN1:GFP did not show any movement, and partially colocalized with the membrane-selective endocytic tracer FM 4-64 as shown for EYFP:AUX1 in Supplementary Fig. S1 at JXB online. Furthermore, the localization of another plasma membrane auxin transporter ABCB4, reported to be more stable in the plasma membrane (Titapiwatanakun and Murphy, 2009), after NOA treatment was different. ABCB4 proteins (PGP4:GFP) appeared only after 24 h in a form of few membrane aggregates (Fig. 4I–L).
Fig. 4.
The effects of the putative auxin influx inhibitors on subcellular distribution of PIN1:GFP, EYFP:AUX1, and ABCB4:GFP fusion proteins in tobacco BY-2 cells. (A, E, I) non-treated controls. The effects of 1-NOA (20 μM, 3 h) (B), 2-NOA (20 μM, 3 h) (C), and CHPAA (20 μM, 3 h) (D) on the subcellular distribution of PIN1:GFP. The effects of 1-NOA (20 μM, 3 h) (F), 2-NOA (20 μM, 3 h) (G), and CHPAA (20 μM, 3 h) (H) on the subcellular distribution of EYFP:AUX1. The effects of 1-NOA (20 μM, 24 h) (J), 2-NOA (20 μM, 24 h) (K), and CHPAA (20 μM, 24 h) (L) on the subcellular distribution of ABCB4:GFP. Single confocal sections through the cortical cytoplasm (C, Apochromat ×40 1.2 W water immersion objective with a 1 Airy Unit pinhole). Scale bars, 10 μm.
These results show that both NOAs (1-NOA being more effective) can induce the formation of endosomes containing auxin carriers. At least partly this may be the reason for the decrease in auxin influx or efflux after the 1-NOA or 2-NOA treatments.
Long-term treatment with NOAs affect processes of membrane trafficking, leading to disruption of the polarity of cell division
To address the consequences of the effects of NOA, BY-2 cells transformed with XVE::EYFP:AUX1 or XVE::PIN1:GFP constructs were incubated for 24 h with 1-NOA, 2-NOA, or CHPAA at 20 μM concentration, two times higher than that used for the cell phenotypic study. The most remarkable effect was disruption of cell plate formation (Fig. 5; see Supplementary Fig. S2 at JXB online). The cell plate did not expand in the normal centrifugal manner, but often had a problem with the correct anchorage in the parental plasma membrane, leading to apolar cell division (Fig. 5B, C for AUX1 and F, G for PIN1) or even incomplete cell plates. Treatments with CHPAA did not affect the polarity of cell division at all (Fig. 5D, H). Interestingly, when the concentration of the inhibitors was further increased to 50 μM, the changes in the dynamics of the membrane appeared to be too extensive and after 48 h in 1-NOA and 2-NOA the overall fluorescence of EYFP:AUX1 and PIN1:GFP decreased substantially (see Supplementary Fig. S3B, C, F, G at JXB online). These cells were not dividing (data not shown). In contrast to 1-NOA or 2-NOA, a 48 h exposure to 50 μM CHPAA had only a weak effect on the AUX1 or PIN1 signals (see Supplementary Fig. S3D, H at JXB online).
Fig. 5.
Long-term treatments of EYFP:AUX1 or PIN1:GFP tobacco BY-2 cells with the putative auxin influx inhibitors. (A, E) non-treated controls. The effects of 1-NOA (20 μM, 24 h) (B), 2-NOA (20 μM, 24 h) (C), and CHPAA (20 μM, 24 h) (D) on cell division in BY-2 cells transformed with the EYFP:AUX1 construct. The effects of 1-NOA (20 μM, 24 h) (F), 2-NOA (20 μM, 24 h) (G), and CHPAA (20 μM, 24 h) (H) on cell division in BY-2 cells transformed with the PIN1:GFP construct. Single confocal sections through the perinuclear region (C, Apochromat ×40 /1.2 W water immersion objective with a 1 Airy Unit pinhole). Scale bars, 20 μm.
These experiments indicate that treatments with increased concentrations of 1-NOA and 2-NOA completely block the dynamics of membranes, with subsequent detrimental effects on cell division. By contrast, similar treatments with CHPAA had no such dramatic effects, suggesting either shifted efficiency of this inhibitor or different mode of its action.
Discussion
Previously, Delbarre et al. (1996) described the specificity of cellular auxin uptake and efflux towards the auxins IAA, NAA, and 2,4-D using suspension-cultured cells of tobacco cv. Xanthi XHFD8. Using the same system to screen for compounds structurally similar to IAA and competing for the auxin influx carrier, 1-NOA and CHPAA were characterized as inhibitors of active auxin influx (Imhoff et al., 2000). It was further shown that 2-NAA inhibits auxin influx carriers, but that it might also affect the auxin efflux activity (Delbarre et al., 1996; Imhoff et al., 2000). Frequent use of these compounds for physiological and developmental studies postulated the need to understand modes of action of particular auxin transport inhibitors. Our study was aimed at the mode of action of inhibitors of carrier-mediated auxin influx using the well-established model for the auxin transport studies on cellular level, tobacco BY-2 cells (Petrášek et al., 2006).
The effect of all three inhibitors, similarly to Xanthi tobacco cells (Imhoff et al., 2000), reduced the activity of auxin influx carriers in the tobacco BY-2 cells. This was demonstrated firstly by decreased accumulation of [3H]2,4-D, which is a preferential substrate for auxin influx carriers in tobacco cells. However, in contrast to 2-NOA and CHPAA, it was found that 1-NOA was less effective in reducing [3H]2,4-D accumulation. Secondly, significant differences were also found in the effects of 1-NOA, 2-NOA, and CHPAA on the accumulation of [3H]NAA, a preferential substrate for active auxin efflux which is, however, taken up into cells only passively (Delbarre et al.,1996; Petrášek et al., 2003). As expected, neither 2-NOA nor CHPAA had any significant effect on the accumulation of this auxin in BY-2 cells in contrast to the application of 1-NOA, which significantly increased its accumulation. This unexpected finding suggests that 1-NOA also influences auxin efflux. Thirdly, the contrasting effects of 1-NOA compared with 2-NOA or CHPAA also appeared in the accumulation of [3H]IAA (data not shown), which as a natural auxin is a good substrate for both auxin influx and efflux carriers. In this case 1-NOA had only a relatively minor impact on the accumulation of [3H]IAA, again suggesting its action on both auxin influx and efflux carriers. The fourth piece of evidence for the action of 1-NOA on auxin efflux came from our experiments with NPA, an established inhibitor of auxin efflux. NPA was able to increase accumulation of [3H]NAA in the presence of 2-NOA. Conversely, in the presence of 1-NOA the accumulation of [3H]NAA did not change significantly after in-flight application of NPA. In this case, the auxin efflux carriers may have already been blocked by 1-NOA before the application of NPA, again supporting the idea that 1-NOA has a significant impact on the auxin efflux activity.
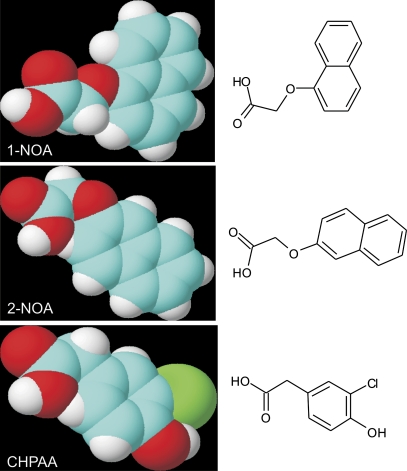
Collectively, these four pieces of evidence point to the conclusion that 1-NOA, in contrast to 2-NOA and CHPAA, also influences auxin efflux (besides the auxin influx). Interestingly, there are differences in the molecular structure of 1-NOA and the other two inhibitors (Fig. 6). All three molecules share typical features of auxin influx inhibitors. However, in contrast to 2-NOA and CHPAA, the shape of the 1-NOA molecule is not linear because of the position of the side-chain in relation to the fused aromatics rings. It is speculated that this unique structural feature of the 1-NOA molecule may be responsible for the observed physiological effects, suggesting a distinct interaction with the plasma membrane or other membranes.
Fig. 6.
Molecular structure of synthetic auxin influx inhibitors 1-NOA, 2-NOA, and CHPAA. Blue, carbon; red, oxygen; white, hydrogen; green, chlorine.
To elucidate the mechanism of how 1-NOA may affect the activity of auxin efflux carriers, experiments were performed with BFA. Generally, BFA application leads to inhibition of anterograde protein trafficking so that the plasma membrane pool of auxin efflux carriers of the PIN family (and some other proteins) is reduced (Geldner et al., 2001). Major auxin influx carriers of the AUX1/LAX family were shown to be much less sensitive or almost insensitive to BFA (Kleine-Vehn et al., 2006). In BFA-treated BY-2 cells, 2-NOA had no effect on NAA accumulation, whereas both 1-NOA and NPA increased auxin accumulation. It seems that 1-NOA and NPA act independently of BFA-dependent vesicle trafficking processes and so their effect(s) are additive to the effect of BFA alone. It could be concluded that 1-NOA either directly affects the auxin efflux carrier activity and/or somehow stimulates endocytosis; this would result in the depletion of the carriers from the plasma membrane and, consequently, in the reduction of auxin efflux. To test this hypothesis, it was necessary to follow the dynamics of membrane vesicles containing either the auxin influx carrier (AUX1) or the auxin efflux carrier (PIN1) directly.
Advantage was taken of the system of β-estradiol-inducible expression of fluorescently tagged fusion proteins in tobacco BY-2 cells. Upon induction of gene expression in control cells, the EYFP:AUX1 and PIN1:GFP signals were localized at the plasma membrane. No changes have been observed after 30 min treatment with inhibitors (see Supplementary Fig. S4 at JXB online). Surprisingly, after 3 h and more with 1-NOA both PIN1 and AUX1 were gradually redistributed into vesicles in the cortical cytoplasm, whereas 2-NOA was much less effective and CHPAA almost ineffective. However, more patches were observed after increasing the concentration of 2-NOA, but the effect was not very homogenous. In the population, there were always cells with stronger and weaker response. In contrast, increasing concentrations of CHPAA did not increase the number of patches formed (data not shown) pointing to the fact that CHPAA is probably the most reliable auxin inhibitor already acting at low concentrations and having the effect on the auxin influx carrier as depicted in the scheme (see Supplementary Fig. S5 at JXB online).
The colocalization of EYFP:AUX1 with the endocytic tracer FM 4-64 confirmed that the observed vesicles are at least partly of plasma membrane origin. Although the subcellular trafficking of AUX1 and PIN1 follows distinct pathways (Kleine-Vehn et al., 2006), 1-NOA could affect the subcellular distribution of both carriers by acting upstream of these pathways, probably by changing the composition of plasma membrane. It has been suggested that 1-NOA could influence sterol composition of the membrane, which could lead to aberrant AUX1 targeting and influence the polar localization of PIN1 in Arabidopsis roots (Kleine-Vehn et al., 2006). It was also shown that 1-NOA was effective in reducing the responses to 2,4-D in wild-type Arabidopsis but had no effect in hyd1 and hyd2/fk mutants, which are defective in genes encoding Δ8-Δ7 sterol isomerase and sterol C14 reductase, respectively (Souter et al., 2002). Thus, there may be a link between potential effects of 1-NOA on sterol composition in the plasma membrane and consequent defects in AUX1 targeting that lead to aggregation of AUX1 (Kleine-Vehn et al., 2006).
The fact that NOA was not able to induce fast redistribution of ABCB4 (as observed for AUX1 and PIN1) and that this ABCB4 appeared in membrane aggregates after longer treatment suggests a membrane composition-dependent NOA effect. The most important in this respect seems to be sterol composition, namely the presence of sterols and sterol-associated proteins (Borner et al., 2005); since ABCB4 was reported to be present in these compartments (Titapiwatanakun and Murphy, 2009), thus the ABCB4 could be more resistant to 1-NOA treatment.
The changes in the polarity of cell division, visible after long-term treatment with NOAs, point also to the general effect of both 1-NOA and 2-NOA on the plasma membrane dynamics. As shown by Dhonukshe et al. (2006) the cell plate is mainly derived from the cell surface material (cell wall components and plasma membrane proteins); therefore, disruption of cell plate formation seems to be caused by defects in the plasma membrane. Moreover, the effect of NOAs on the polarity of cell plate formation and cell polarity establishment point to the possible involvement of actin filament dynamics that has been reported to reflect the changed levels of auxin (Maisch and Nick, 2007; Nick et al., 2009).
In contrast to our results, Kleine-Vehn et al. (2006) did not observe any effects of high concentration of 1-NOA on the trafficking of PIN1 and AUX1 in Arabidopsis roots. It is speculated that, in contrast to the complex tissue of the root, the effect in single tobacco BY-2 cells may be more robust and readily visible, and thus redistribution of the carrier was observed even with a much lower concentration of the inhibitor.
Increased concentrations of 1-NOA and 2-NOA completely blocked the dynamics of membranes, with subsequent detrimental effects on cell division. In contrast, no such dramatic effects were seen following treatment with CHPAA, which therefore appears to be the most reliable auxin influx inhibitor that already acts at low concentrations and has no obvious influence on membrane dynamics.
Nevertheless, the possibility that higher concentrations of the inhibitors tested could affect vesicles that are transported towards the plasma membrane by a mechanism dependent on actin dynamics (Dhonukshe et al., 2008) cannot be excluded.
The analysis of the phenotype and growth of the tobacco BY-2 cells revealed distinct effects of 1-NOA compared with 2-NOA and CHPAA. The action of 1-NOA resulted in decreased cell division activity and in increased cell elongation. These defects could also possibly be attributed to a decreased dynamics of the plasma membrane and an observed ‘auxin starvation’ phenotype might be of secondary character. Interestingly, auxin influx inhibitors 2-NOA and CHPAA decreased the accumulation of [3H]2,4-D (see Fig. 2), a good substrate for auxin influx carriers and their application even slightly stimulated cell division. This is consistent with the finding that their action might stimulate the synthesis of endogenous auxin, as reported after auxin deprivation in another tobacco cell line VBI-0 (Zažímalová et al., 1995). Although 10 μM NPA had no significant effect in the experiments performed under conditions used in this study, at higher concentrations it may produce defects in the polarity of cell division in tobacco BY-2 cells as well as in another auxin-dependent tobacco cell line VBI-0 (Dhonukshe et al., 2005; Petrášek et al., 2002; respectively).
The effects of auxin transport inhibitors are summarized in the scheme shown in Supplementary Fig. S5 at JXB online. It also highlights the importance of the rate of the particular vesicle trafficking process that may determine the extent of the action of 1-NOA, 2-NOA, and CHPAA on the membrane dynamics. The potential importance of the distinct sensitivity of the membrane domains containing particular transport proteins to the action of inhibitors cannot be excluded. Generally, our results point to the so far unknown effect on vesicle trafficking of the putative auxin influx inhibitors.
Supplementary Material
Acknowledgments
The authors thank Professor Cris Kuhlemeier (University od Bern) for support and Dr Jan Marc (University of Sydney) for critical reading of the manuscript. We also thank Markéta Pařezová for transformation of BY-2 cell cultures. The work was supported by the Grant Agency of the Czech Republic, project KJB600380702, by the Ministry of Education, Youth and Sports of the Czech Republic, project LC06034 and by the European Fund for Regional Development, the Operational Programme Prague – Competitiveness, project no.: CZ.2.16/3.1.00/21159.
References
- An G. High efficiency transformation of cultured tobacco cells. Plant Physiology. 1985;79:568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge K, Guyomarc'h S, Bayer E, Swarup R, Bennett M, Mandel T, Kuhlemeier C. Auxin influx carriers stabilize phyllotactic patterning. Genes and Development. 2008;22:810–823. doi: 10.1101/gad.462608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Borner GHH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, MacAskill A, Napier JA, Beale MH, Lilley KS, Dupree P. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiology. 2005;137:104–116. doi: 10.1104/pp.104.053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A, Müller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198:532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Baluška F, Schlicht M, Hlavacka A, Šamaj J, Friml J, Gadella T. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Developmental Cell. 2006;10:137–150. doi: 10.1016/j.devcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, et al. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proceedings of the National Academy of Sciences, USA. 2008;105:4489–4494. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Mathur J, Hülskamp M, Gadella TW. Microtubule plus-ends reveal essential links between intracellular polarization and localized modulation of endocytosis during division-plane establishment in plant cells. BMC Biology. 2005;3:11. doi: 10.1186/1741-7007-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton MD, Tropsha A, Jones AM. Modelling the auxin-binding site of auxin-binding protein 1 of maize. Phytochemistry. 1994;35:1111–1123. [Google Scholar]
- Friml J, Benkova E, Blilou I, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002b;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002a;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Friml J. Auxin transport: shaping the plant. Current Opinion in Plant Biology. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Imhoff V, Muller P, Guern J, Delbarre A. Inhibitors of the carrier-mediated influx of auxin in suspension-cultured tobacco cells. Planta. 2000;210:580–588. doi: 10.1007/s004250050047. [DOI] [PubMed] [Google Scholar]
- Jelínková A, Malínská K, Simon S, et al. Probing plant membranes with FM dyes: tracking, dragging or blocking? The Plant Journal. 2010;61:883–892. doi: 10.1111/j.1365-313X.2009.04102.x. [DOI] [PubMed] [Google Scholar]
- Jones AR, Kramer EM, Knox K, Swarup R, Bennett MJ, Lazarus CM, Leyser HMO, Grierson CS. Auxin transport through non-hair cells sustains root-hair development. Nature Cell Biology. 2009;11:78–84. doi: 10.1038/ncb1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. The Plant Cell. 2006;18:3171–3181. doi: 10.1105/tpc.106.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant hormones: physiology, biochemistry and molecular biology. Dordrecht, Netherlands: Kluwer; 1995. pp. 509–530. [Google Scholar]
- Maisch J, Nick P. Actin is involved in auxin-dependent patterning. Plant Physiology. 2007;143:1695–1704. doi: 10.1104/pp.106.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA, Robinson JS. Targeting of auxin carriers to the plasma membrane: differential effects of brefeldin A on the traffic of auxin uptake and efflux carriers. Planta. 1998;205:606–612. [Google Scholar]
- Morris DA, Friml J, Zažímalová E. The transport of auxins. In: Davies PJ, editor. Plant hormones: biosynthesis, signal transduction, action! Dordrecht, Boston, London: Kluwer Academic Publishers; 2004. pp. 437–470. [Google Scholar]
- Nagata T, Nemoto Y, Hasezava S. Tobacco BY-2 cell line as the ‘Hela’ cell in the cell biology of higher plants. International Review of Cytology. 1992;132:1–30. [Google Scholar]
- Nick P, Han M, An G. Auxin stimulates its own transport by shaping actin filaments. Plant Physiology. 2009;151:155–167. doi: 10.1104/pp.109.140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. The Plant Cell. 2001;13:2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Delbarre A, Marchant A, Swarup R, Napier R, Perrot-Rechenmann C, Bennett MJ. Novel auxin transport inhibitors phenocopy the auxin influx carrier mutation aux1. The Plant Journal. 2001;25:399–406. doi: 10.1046/j.1365-313x.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- Pernisová M, Klíma P, Horák J, et al. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proceedings of the National Academy of Sciences, USA. 2009;106:3609–3614. doi: 10.1073/pnas.0811539106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek J, Elčkner M, Morris DA, Zažímalová E. Auxin efflux carrier activity and auxin accumulation regulate cell division and polarity in tobacco cells. Planta. 2002;216:302–308. doi: 10.1007/s00425-002-0845-y. [DOI] [PubMed] [Google Scholar]
- Petrášek J, Černá A, Schwarzerová K, Elčkner M, Morris DA, Zažímalová E. Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiology. 2003;131:254–263. doi: 10.1104/pp.012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek J, Mravec J, Bouchard R, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- Petrášek J, Zažímalová E. The BY-2 cell line as a tool to study auxin transport. In: Nagata T, Matsuoka K, Inzé D, editors. Biotechnology in agriculture and forestry 58, Tobacco BY-2 cells: from cellular dynamics to omics. Berlin, Heidelberg: Springer-Verlag; 2006. pp. 107–117. [Google Scholar]
- Rahman A, Ahamed A, Amakawa T, Goto N, Tsurumi S. Chromosaponin I specifically interacts with AUX1 protein in regulating the gravitropic response of arabidopsis roots. Plant Physiology. 2001;125:990–1000. doi: 10.1104/pp.125.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery PH. Phytotropins: receptors and endogenous ligands. Symposia of the Society for Experimental Biology. 1990;44:119–146. [PubMed] [Google Scholar]
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. A plausible model for phyllotaxis. Proceedings of the National Academy of Sciences, USA. 2006;103:1301–1306. doi: 10.1073/pnas.0510457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter M, Topping J, Pullen M, Friml J, Palme K, Hackett R, Grierson D, Lindsey K. hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. The Plant Cell. 2002;14:1017–1031. doi: 10.1105/tpc.001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme G, Bennett MJ. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes and Development. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- Titapiwatanakun B, Murphy AS. Post-transcriptional regulation of auxin transport proteins: cellular trafficking, protein phosphorylation, protein maturation, ubiquitination, and membrane composition. Journal of Experimental Botany. 2009;60:1093–1097. doi: 10.1093/jxb/ern240. [DOI] [PubMed] [Google Scholar]
- Ugartechea-Chirino Y, Swarup R, Swarup K, Péret B, Whitworth M, Bennett M, Bougourd S. The AUX1 LAX family of auxin influx carriers is required for the establishment of embryonic root cell organization in Arabidopsis thaliana. Annals of Botany. 2010;105:277–289. doi: 10.1093/aob/mcp287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Petrášek J, Žádníková P, et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development. 2010;137:597–606. doi: 10.1242/dev.040790. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends in Plant Science. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Morris DA. Targeting of auxin carriers to the plasma membrane: effects of monensin on transmembrane auxin transport in Cucurbita pepo L. tissue. Planta. 1994;193:194–202. [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. High-affinity auxin transport by the AUX1 influx carrier protein. Current Biology. 2006;16:1123–1127. doi: 10.1016/j.cub.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Zažímalová E, Opatrný Z, Březinová A, Eder J. The effect of auxin starvation on the growth of auxin-dependent tobacco cell culture: dynamics of auxin reception and endogenous free IAA content. Journal of Experimental Botany. 1995;46:1205–1213. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.