Abstract
The relationships between damage-induced electropotential waves (EPWs), sieve tube occlusion, and stop of mass flow were investigated in intact Cucurbita maxima plants. After burning leaf tips, EPWs propagating along the phloem of the main vein were recorded by extra- and intracellular microelectrodes. The respective EPW profiles (a steep hyperpolarization/depolarization peak followed by a prolonged hyperpolarization/depolarization) probably reflect merged action and variation potentials. A few minutes after passage of the first EPW peak, sieve tubes gradually became occluded by callose, with maximum synthesis occurring ∼10 min after burning. Early stop of mass flow, well before completion of callose deposition, pointed to an occlusion mechanism preceding callose deposition. This obstruction of mass flow was inferred from the halt of carboxyfluorescein movement in sieve tubes and intensified secretion of aqueous saliva by feeding aphids. The early occlusion is probably due to proteins, as indicated by a dramatic drop in soluble sieve element proteins and a simultaneous coagulation of sieve element proteins shortly after the burning stimulus. Mass flow resumed 30–40 min after burning, as demonstrated by carboxyfluorescein movement and aphid activities. Stop of mass flow by Ca2+-dependent occlusion mechanisms is attributed to Ca2+ influx during EPW passage; the reversibility of the occlusion is explained by removal of Ca2+ ions.
Keywords: Callose, EPW, membrane potential, phloem, sieve tube occlusion, two-step sieve plate blockage
Introduction
Sieve elements (SEs) are elongate, enucleate cells lacking most cell components (van Bel, 2003). They are lined by a thin mictoplasmic layer containing a parietally located smooth SE endoplasmic reticulum, SE plastids, a few inactive mitochondria, and clusters of structural proteins (van Bel, 2003). SEs are arranged end-to-end and connected by modified walls perforated by sieve pores (van Bel, 2003). A mixture of metabolites, minerals, phytohormones, soluble phloem-specific proteins (e.g. Giavalisco et al., 2006), and several forms of RNA (e.g. Kehr and Buhtz, 2008) is driven by mass flow through the wide SE lumina.
The mass flow makes plants vulnerable to the consequences of sieve tube damage. Therefore, injury events induce sieve tube occlusion to prevent loss of sieve tube sap (Evert, 1982; Schulz, 1998) and to impede invasion of phytopathogens through the injured site (van Bel, 2003). Sieve tubes may be occluded by callose deposition and by proteins (e.g. Furch et al., 2007).
Callose deposition is a universally observed mode of sieve plate (SP) occlusion (Kudlicka and Brown, 1997; Nakashima et al., 2003). Callose is a β-1,3-glucan polymer which is produced enzymatically and deposited extracellularly around plasmodesmata and sieve pores in the form of collars (Blackman et al., 1998; Zabotin et al., 2002) as a reaction to chemical or mechanical stress (Kudlicka and Brown, 1997; Nakashima et al., 2003; Levy et al., 2007). As for protein plugging, the numerous aggregation forms of phloem-specific proteins (Cronshaw and Sabnis, 1990) promise an immense variation between plant species. However, reports on protein plugging of sieve tubes are scarce. In cucurbits, phloem protein 1 (PP1) produce insoluble aggregates in response to oxidation (Kleinig, 1975; Alosi et al., 1988) and they plug injured sieve tubes (Read and Northcote, 1983). In legumes, spindle-like protein bodies (forisomes) disperse upon wounding and occlude the sieve pores (Knoblauch and van Bel, 1998).
Callose deposition and protein plugging can operate in parallel in a time-shifted fashion in the same species. Burning stimuli elicit distant occlusion in Vicia faba by rapid forisome dispersion and slower callose deposition (Furch et al., 2007, 2009). These occlusion reactions are reversible; forisome dispersion is reversed by the time that callose deposition comes to full development (Furch et al., 2007). It has been speculated therefore that plants may dispose of a universal safety design for sieve tube occlusion, a quick one executed by phloem-specific proteins and a slower one executed by callose (Furch et al., 2007).
As both occlusion mechanisms are Ca2+ dependent (Kauss et al., 1983; Colombani et al., 2004), remote-controlled occlusion was associated with Ca2+ influx during passage of damage-induced electropotential waves (EPWs) (Furch et al., 2007, 2009; Hafke et al., 2009). EPWs communicate sudden and profound physiological changes over long distances (Stankovic et al., 1998; Stahlberg et al., 2006; Grams et al., 2009). They have been recorded in response to stimuli such as wounding, cold, heat, and electrical shocks (Fromm and Spanswick, 1993; Rhodes et al., 1996; Mancuso, 1999; Furch et al., 2007).
The question arises as to whether the ‘safety design’ in sieve tube occlusion is also functioning in plant families without forisomes. Since burning is the most effective stimulus to trigger remote sieve tube occlusion in intact plants, the relationship between remote burning and mass flow was investigated in sieve tubes of intact Cucurbita maxima plants. To this end, intra- and extracellular electrophysiology was used to record the propagation of EPWs, SDS–PAGE separation to determine soluble protein content, aniline blue staining to follow callose deposition, and carboxyfluorescein transport and aphid behaviour to monitor mass flow.
Materials and methods
Plant material
Cucurbita maxima (cv. Gele Reuzen; Enza Zaden, The Nederlands) plants were cultivated in pots in a greenhouse under standard conditions (21 °C, 60–70% relative humidity, and a 14/10 h light/dark regime). Supplementary lamp light (model SONT Agro 400 W; Phillips Eindhoven, The Nederlands) led to an irradiance level of 200–250 μmol−2 s−1 at the plant apex. Plants were taken in the vegetative phase just before flowering, 21–28 d after germination. For experiments, mature leaves with a size of ∼15×15 cm were used.
Extracellular electrophysiology
Extracellular voltage measurements were carried out on a vibration-stabilized bench with a Faraday cage. Borosilicate microelectrodes (tip diameter 1–2 μm; Hilgenberg GmbH, Malsfeld, Germany), filled with 0.5 M KCl in 1% agar in the tip, were pierced blindly into the main vein of a mature leaf of an intact plant, by means of a micromanipulator (model ST 35; Brinkmann Instrumentenbau, Mannheim, Germany). The reference electrode, filled with 0.5 M KCl, was inserted into the soil. Electrodes were connected with a high-impedance amplifier (KS-700, World Precision Instruments Inc., New Haven, CT, USA). After the resting potential had settled, the leaf tip was burnt (2–3 s) and EPWs were recorded at 4 cm and 8 cm from the burning site.
Exposure of phloem tissue in intact plants
For in vivo observation of sieve tubes, cortical cell layers were removed locally down to the phloem from the lower side of the main vein of mature leaves, attached to intact plants. While avoiding damage to the phloem, cell layers were locally removed by manual paradermal slicing with a razor blade to excise an observation window (Knoblauch and van Bel, 1998). The distance between the observation window and the leaf tip was between 3 cm and 9 cm. The leaf was mounted on a microscope slide with two-sided adhesive tape, fixed onto the stage of a confocal laser scanning microscope (CLSM), and the free-lying phloem tissue was submerged in a phloem physiological buffer. This medium, containing 2 mol m−3 KCl, 1 mol m−3 CaCl2, 1 mol m−3 MgCl2, 50 mol m−3 mannitol, and 2.5 mol m−3 MES/NaOH buffer, pH 5.7 (Hafke et al., 2005), was also used for dilution of dyes. Integrity of phloem tissue, as indicated by non-swollen SPs, was checked under water immersion objectives.
Intracellular electrophysiology
Intracellular electrophysiology on intact phloem tissue was described in detail previously (Hafke et al., 2005). After submersion of the exposed phloem tissue in the above-mentioned medium for 1 h, SEs were impaled by a microelectrode by an micromanipulator (model LN SM-1; Luigs and Neumann, Ratingen, Germany) under microscopic surveillance. After stabilization of the SE potential, the leaf tip was burnt at a distance of 4 cm and 8 cm from the observation window, and SE membrane potential profile was recorded.
Fluorescent dyes and confocal laser scanning microscopic observation
To visualize callose deposition, a 0.005% solution of aniline blue (Merck, Darmstadt, Germany) was employed of which the stock solution (0.5 M phosphate buffer, pH 7.4) contained 0.05 mg ml−1 aniline blue. The phloem-mobile fluorochrome 5(6)carboxyfluorescein diacetate (CFDA)-mixed isomers (Invitrogen, Karlsruhe, Germany) was used to visualize stop of mass flow in sieve tubes. Stock solutions of CFDA (1 mg solubilized in 1 ml of dimethylsulphoxide) were diluted (1 μl of the stock solution dissolved in 2 ml of medium) to a final concentration of ∼1 μM. Sulphorhodamine 101 (10 μM) (Molecular Probes Europe BV, Leiden, The Netherlands) dissolved in medium was employed to visualize protein coagulation.
A CLSM (Leica TCS 4D; Leica Microsystems, Heidelberg, Germany) equipped with a 75 mW argon/krypton laser (Omnichrome, Chino, CA, USA) was used to detect CFDA fluorescence by the 488 nm line and sulphorhodamine 101 fluorescence by the 564 nm line. A multiphoton CLSM (Leica TCS SP2/MP; Leica Microsystems, Bensheim, Germany) equipped with a multiphoton laser was used to detect aniline blue fluorescence. Aniline blue was excited at 800 nm and emission was recorded in the spectral window between 450 nm and 510 nm.
After incubation in medium for at least 1 h, the exposed phloem tissue was stained with aniline blue or sulphorhodamine 101 for 15–20 min. Subsequently, the tissue was washed with the medium and distant damage was inflicted by a burning stimulus (careful burning by a match for 2 s) at the major vein near the tip at a distance of 9 cm from the observation window. Every 5–10 min, events in sieve tubes after diverse remote stimuli were documented in real-time during 1–2 h after the stimulus. Fluorescence was quantified with ImageJ 1.38 (Wayne Rosband, National Institute of Health, USA).
CFDA was applied to a loading window, 3.5 cm upstream from the observation window. CFDA permeates the plasma membrane in the non-fluorescent acetate form and is cleaved by cytosolic enzymes producing membrane-impermeant fluorescent carboxyfluorescein which is transported by mass flow inside SEs. After the carboxyfluorescein had reached the observation window situated at 9 cm from the tip, the leaf tip was burnt.
Observations were performed without a coverslip by means of a water immersion objective (HCX APO L40×0.80 W U-V-l, Leica, Heidelberg, Germany). Digital image processing was executed using Adobe® PhotoShop 8.0 and Adobe® Illustrator 11.0 (Adobe Systems Inc., USA) to optimize brightness, contrast, and colouring.
Aphid cultivation
Macrosiphum euphoribiae was reared on 20- to 28-d-old plants of C. maxima in a controlled-environment room at 25 °C and a 17/7 h light/darkness regime in Perspex cages with large gauze-covered windows (cf. Will et al., 2009).
Electrical penetration graph recording of aphid behaviour
Aphid activities were recorded by the electrical penetration graph (EPG) technique (Tjallingii, 1978, 1985; Will et al., 2007). Aphids (M. euphorbiae) were placed on the main vein of a mature leaf of C. maxima, at various distances from the leaf tip. After the aphids started phloem sap ingestion, aphid activities in response to EPW passage triggered by burning the leaf tip were analysed according to Prado and Tjallingii (1994). For recording and analysis of EPGs, the software package PROBE 3.5 (EPG Systems, Wageningen, The Netherlands) was adopted. Mann–Whitney rank sum tests were done using SigmaStat 3.0 software (SPSS Inc., Chicago, IL, USA) and SigmaPlot 8.0 software (SPSS Inc.).
Collection of phloem sap followed by one-dimensional SDS–PAGE
Phloem sap was collected from the stump of cut petioles by glass pipettes and transferred to 4-fold concentrated reducing sample buffer (Roti-Load 1; Carl-Roth, Karlsruhe, Germany) in the proportion of 1:3.
One-dimensional SDS–PAGE of phloem sap was carried out according to Laemmli (1970) by using a 4% stacking gel and a 12% separation gel in a MiniProtean 3 Electrophoresis System (Bio-Rad Laboratories, Hercules, CA, USA) with Precision Plus Protein Standard–All Blue (Bio-Rad) as a protein size marker. The gels were stained by Coomassie blue (Roti-Blue; Carl-Roth, Karlsruhe, Germany), scanned with the Gel Doc XR documentation system (Bio-Rad), and proteins in the respective lanes were quantified by means of Quantity One 1-D Analysis Software (Bio-Rad). Statistical significance was analysed by the Holm–Sidak method using SigmaStat 3.0 software (SPSS Inc.)
Results
Time course of extracellular EPWs after burning the leaf tip
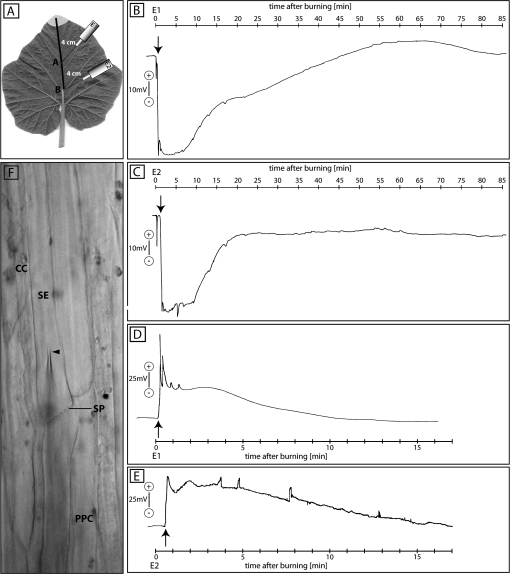
Microelectrodes were inserted blindly into the apoplast of the main vein. Following stabilization of the apoplastic electrical potential, the leaf tip was burnt. EPWs were recorded extracellulary at 4 cm (Fig. 1B) and 8 cm (Fig. 1C) from the burning site (Fig. 1A).
Fig. 1.
Apoplastic (B, 4 cm; and C, 8 cm from the leaf tip) and intracellular (D, 4 cm; and E, 8 cm from the leaf tip) electrical recordings of EPW passage after burning the leaf tip of intact Cucurbita maxima plants (n=6 for extracellular, n=3 for intracellular measurements). (A) Experimental set-up of the electrophysiological measurements. Electrode E1 represents the site recording in B and D, and E2 the site of recording in C and E. The grey region at the leaf tip indicates the burning area. The leaf tip was burnt at t=0 s. The first action potential-like transients are marked by an arrow. (F) Bright-field image of phloem tissue showing the insertion of a microelectrode into an SE. The arrowhead marks the microelectrode tip. SE, sieve element; CC, companion cell; SP, sieve plate; PPC, phloem parenchyma cell.
At both recording points, the passage of EPWs was observed. A minute short hyperpolarization of –20 mV to –10 mV directly after burning was observed ahead of EPWs in all extracellularly conducted experiments. Since they reflect the sudden, transient turgor disbalance of the system, these hyperpolarizations are most probably irrelevant for the present studies.
At 4 cm, a fast and short hyperpolarization (66 mV) 27 s after burning was followed by a fast short repolarization for 18 s (Fig. 1B). Subsequently, a second hyperpolarization commenced (65 mV) which was followed by a prolonged repolarization including a plateau phase of 48 min and an undulating profile, before finally reaching the resting potential.
At 8 cm, the first hyperpolarization (46 mV) arose after 72 s (Fig. 1C). The second phase of hyperpolarization (46 mV) started after 99 s and was much shorter (19.5 min duration) than the prolonged one at 4 cm distance (Fig. 1C). The variability in strength and duration of the EPWs at both recording sites is summarized in Table 1.
Table 1.
Comparison of the two extracellularly recorded EPWs dependent on the distance
| Distance | Wave 1 |
Wave 2 |
||||
| Time frame (s) | Voltage change (mV) | Duration (s) | Time frame (s) | Voltage change (mV) | Duration (min) | |
| 3–5 cm (n=6) | 9–36 | 66–115 | Sharp, ∼18 | 45–90 | 62–105 | Long lasting, ∼81 |
| 7.5–12 cm (n=7) | 36–72 | 40–103 | Sharp, ∼36 | 72–144 | 37–99 | Long lasting, ∼49.5 |
Time course of intracellular EPWs after burning the leaf tip
Microelectrodes were inserted under microscopic surveillance into a submersed SE of the main vein. Following stabilization of the resting membrane potential, the leaf tip was burnt and EPWs were recorded intracellularly at 4 cm (Fig. 1D) and 8 cm (Fig. 1E) from the burning site.
At 4 cm (Fig. 1D), a transient depolarization after 9 s (142 mV) was followed by a second depolarization (106 mV) 24 s after burning, with a prolonged repolarization (52 mV) including small transients (60 mV) over 13.5 min.
At 8 cm (Fig. 1E), a fast depolarization (87 mV) developed 30 s after burning with a duration of 30 s. Subsequently, a second depolarization (79 mV) occurred after 78 s and was followed by a sustained repolarization of 16 min.
Stop of mass flow induced by leaf tip burning
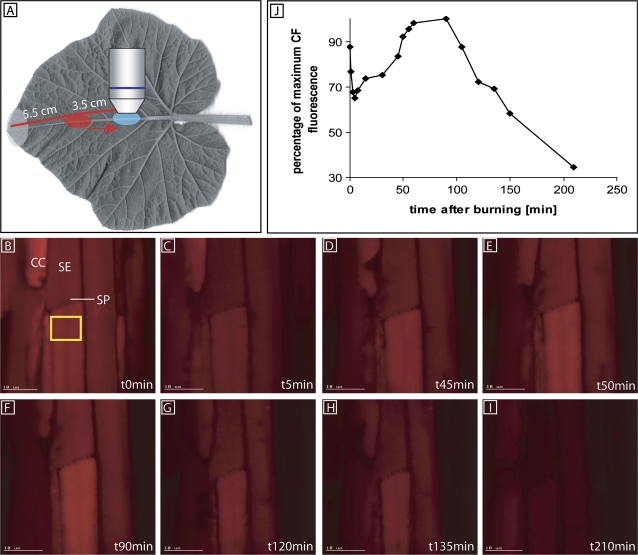
Stop of mass flow in sieve tubes of C. maxima induced by leaf tip burning (at a distance of 9 cm from the observation window) was observed using CFDA, as recorded using CLSM (Fig. 2).
Fig. 2.
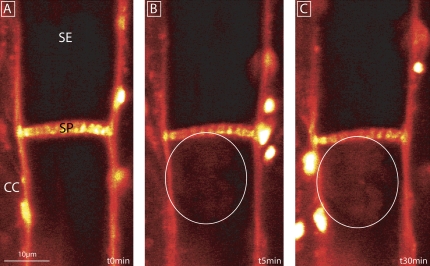
Stop of mass flow in sieve tubes of Cucurbita maxima induced by leaf tip burning (at a distance of 9 cm from the observation window) as recorded using a CLSM in intact plants (n=3). The direction of mass flow is from top (leaf tip) to bottom (leaf base). (A) Observation of carboxyfluorescein mass flow. CFDA was applied to a loading window (red circle), 3.5 cm upstream of the observation window (blue circle). (B) The membrane-impermeant carboxyfluorescein (CF) was transported by mass flow. (C) After burning of the leaf tip at t=0 s, carboxyfluorescein was photobleached at the observation window. Fluorescence did not emerge during the next 40 min probably due to sieve tube occlusion. The sieve tube at the other end of the SP is bending downwards and is therefore out of focus. Carboxyfluorescein transport through the SP was optically checked, but is not presented here. (D–F) Increasing fluorescence between 45 min and 90 min demonstrated recovery of carboxyfluorescein supply from the upstream loading window which inferred the lifting of the sieve tube blockage. (G–I) Repeated photobleaching of carboxyfluorescein at each observation revealed a second sieve tube occlusion after ∼90 min. The leaf tip is towards the top and the leaf base is towards the bottom in all photomicrographs. (J) Quantification of CF fluorescence (given on an arbitrary scale) in a sieve tube (yellow box) after burning the leaf tip. Each replicate was executed with different plants. SE, sieve element; CC, companion cell; SP, sieve plate.
CFDA was applied onto the loading window located at 3.5 cm from the observation window (Fig. 2A) and transported downstream in the form of carboxyfluorescein through the sieve tubes. After carboxyfluorescein fluorescence had reached a saturation level at the observation window, the leaf tip was burnt (Fig. 2B). Non-recovery of carboxyfluorescein photobleaching (Fig. 2C, J) indicated that the SPs were fully occluded ∼1 min after burning. The rationale is that fluorescence will not recover unless the SPs are unblocked and mass flow resumes. Between 45 min and 90 min after burning (Fig. 2D–F, J) the fluorescence increased again, indicating that mass flow had recovered. A second decrease of fluorescence took place between 100 min and 120 min (Fig. 2G–I, J).
Distant callose deposition onto sieve plates after burning and cutting the leaf tip/major vein
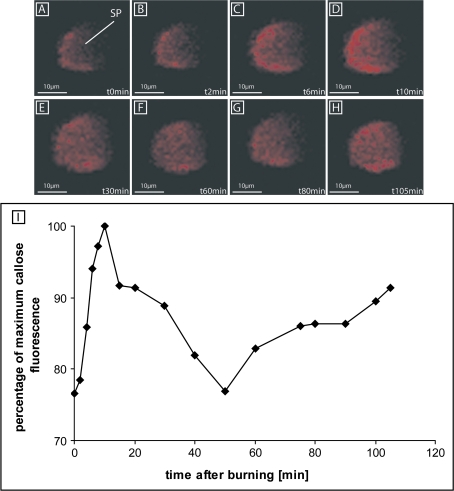
The time course of callose deposition onto SPs in response to burning the leaf tip (Fig. 3) was investigated by means of multiphoton microscopy. Ten minutes before the burning stimulus at 9 cm distance, a non-toxic concentration of 0.005% aniline blue was applied onto the observation window (Furch et al., 2007). In control plants, the amounts of callose at the SPs were stable and low after the same preparation procedure without stimulus (results not shown). After burning the leaf tip, aniline blue fluorescence gradually rose, with a maximum at 10 min (Fig. 3A–D, I), after which callose appeared to be degraded. After the original low level of fluorescence had been reached (45–50 min; Fig. 3E–F, I), callose deposition recommenced, but increased at a slower rate than before (60–105 min; Fig. 3G, H). The second wave of callose deposition may explain the second inhibition phase of mass flow as found with carboxyfluorescein (Fig. 2G–I).
Fig. 3.
Time course of the average aniline blue fluorescence as an indication of callose deposition and degradation at the sieve plates (SPs) of sieve elements after burning the leaf tip (at a distance of 9 cm from the observation window) as recorded using a multiphoton CLSM (n=2) in intact Cucurbita maxima plants. (A–H) Between 10 min and 15 min after burning, a strong increment of aniline blue fluorescence (A–D) is followed by a decline which passes into a second longer-lasting, but slower increase in fluorescence. (I) Quantification of callose deposition and degradation (given on an arbitrary scale) at the SP (dashed green circle) after burning the leaf tip.
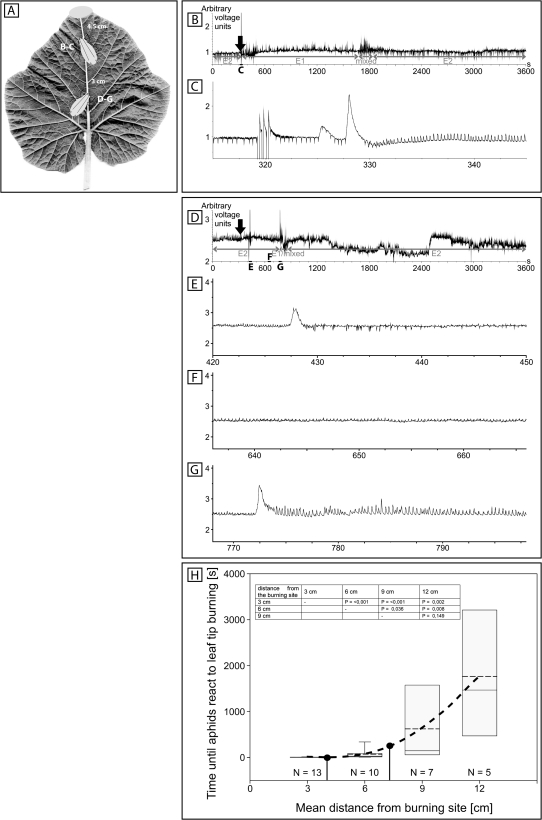
EPG recording at different distances after burning the leaf tip
The activities of aphids (M. euphorbiae), feeding on the main vein at distances of 4.5 cm (Fig. 4A–C) and 7.5 cm (Fig. 4A, D–G) from the leaf tip, were monitored by EPG profiles before, during, and after the passage of burning-induced EPWs. Prior to the burning stimulus, the aphids show an E2 profile (Fig. 4B, D) representing ingestion of SE sap accompanied by rhythmic secretion of small amounts of watery saliva that is resorbed together with phloem sap (Tjallingii and Hogen Esch, 1993). The first depolarizations shortly after burning did not influence aphid activities and may therefore reflect a general turgor imbalance of the system in response to burning.
Fig. 4.
EPG (electropenetogram) recordings of sieve tube-related activities of ingesting aphids (Macrosiphum euphorbiae) at different distances [B, C, 4.5 cm; D–G, 7.5 cm; H, 3, 6, 9, and 12 cm (mean values)] after leaf tip burning of intact Cucurbita maxima plants (n=3). (A) Experimental set-up for EPG recording. Two aphids are placed in line on the main vein at distances of 4.5 cm (B, C) and 7.5 cm (D–G) from the leaf tip. The grey region represents the burning area. (B) A 1 h overview EPG profile showing changes in the activities of the aphid at 4.5 cm from the leaf tip. Leaf tips were burnt between 321 s and 324 s after the start of the recording as indicated by three artificial signals. (C) Detail of the EPG during 30 s after burning. (D) A 1 h overview EPG profile showing changes in the activities of the aphid at 7.5 cm from the leaf tip. (E–G) Details of the EPG profile after burning (indicated in D). (H) Box–whisker plot of the lag period until aphids react to leaf tip burning dependening on the distance from the burning site. Grey boxes show the range of 25–75% of all measurements in one group. Whiskers represent 5–25% and 75–95% of the data range. The dashed line indicates the mean, and the solid line the median. The inset shows statistical comparison of data groups from H by Mann–Whitney rank sum test. P <0.05 indicates significant differences between two groups. A line of best fit (black dashed) was calculated to connect the four measuring distances of 3–12 cm. Black dots indicate measuring distances in B–C and D–G. N is the number of replicates for a given distance.
At 4.5 cm distance from the burning site, aphids switched their activities from E2 to E1 behaviour [representing secretion of watery saliva into the SE lumen (Prado and Tjallingii, 1994)] 10 s after leaf tip burning. They stayed for ∼25 min in the E1 phase, showed a mixed E1/E2 profile for ∼2.5 min, and subsequently returned to ingestion characterized by E2 activities (Fig. 4B). Aphids located at 7.5 cm from the leaf tip changed their behaviour from E2 to E1 after 7.5 min. At this site, the E1 and mixed E1/E2 profiles lasted for a much shorter time (E1, 66 s; mixed E1/E2, 27.5 s) than those at 4.5 cm before the aphids switched back to the E2 behaviour (Fig. 4D). A box–whisker plot (Fig. 4H) displays the time until aphids reacted to leaf tip burning by switching from E2 to E1 behaviour. With increasing distance from the burning site, the time until aphids changed their behaviour increased in a non-linear fashion (Fig. 4H), indicating a declining EPW propagation velocity along the phloem. Statistical comparisons by Mann–Whitney rank sum test of data groups are presented as a table inset in Fig. 4H. P-values <0.05 indicate significant differences between two groups.
Distant sieve tube occlusion by protein plugs after burning the leaf tip/major vein
The previous results (Fig. 3, 4) suggest ready occlusion of SPs at the time that callose deposition has hardly started. In analogy to the events in V. faba (Furch et al., 2007, 2009), proteins may be engaged in sieve tube occlusion. Therefore, the response of SE proteins to burning the leaf tip/major vein at a distance of 9 cm was observed using sulphorhodamine 101 (Fig. 5) and separation by 1-D SDS-PAGE (Fig. 6). Twenty minutes prior to burning, 10 μM sulphorhodamine 101 (cf. Peters et al., 2010), which preferentially associates with membranes, was applied onto the observation window. From 5 min after burning onwards, a cloud of fluorescence was observed at the SPs (Fig. 5B, C) indicative of protein clogging. It was technically impossible to further shorten the period between burning and observation.
Fig. 5.
Distant protein plugging at the sieve plate after burning the leaf tip/major vein (at a distance of 9 cm from the observation window) as recorded using CLSM in intact Cucurbita maxima plants. The direction of mass flow is from the top (leaf tip) to the bottom (leaf base) of the picture. The bright spots are autofluorescent chloroplasts. Phloem tissue was pre-stained with 20 μM sulphorhodamine 101 for 20 min. (A) Control image at t=0 s showing the staining of plasma membranes. (B and C) Emergence of a fluorescent cloud (within the white circles) in the vicinity of the SP after 5 min and 30 min, respectively. SE, sieve element; CC, companion cell; SP, sieve plate.
Fig. 6.
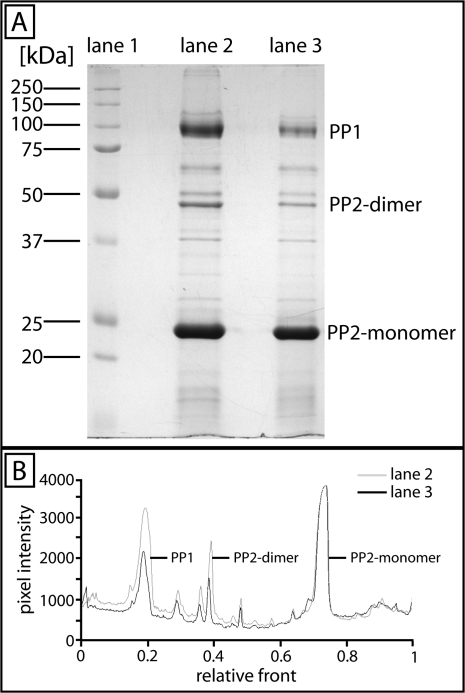
(A) 1-D SDS–PAGE of Cucurbita maxima phloem sap proteins stained with Coomassie blue (n=7). Lane 1, marker proteins; lane 2, protein bands of 0.5 μl of phloem sap collected from an untreated plant; lane 3, protein bands of 0.5 μl of phloem sap collected 5 min after burning the leaf tip/main vein at 9 cm distance. Note the changes of staining intensity of the major protein bands for PP1 and the PP2-dimer. (B) Comparison of the pixel intensity of the bands in lane 2 (grey curve) and lane 3 (black curve) by use of Quantity One 1-D Analysis Software.
SE proteins of untreated (Fig. 6A, lane 2; B, grey curve) and treated (collection of phloem sap 5 min after burning; Fig. 6A, lane 3; B, black curve) plants were separated by 1-D SDS–PAGE, Coomassie stained (Fig. 6A), and quantified (Fig. 6B). Quantities of the three major proteins of phloem sap samples—PP1 (95 kDa), phloem protein 2 (PP2)-dimer (48 kDa), and PP2-monomer (24 kDa)—differ considerably (Fig. 6A, B). In treated samples, the quantities of PP1 and PP2-dimer are strongly reduced (P <0.001), which indicates a disappearance of the water-soluble forms. PP2-monomer protein bands are equally stained under both conditions (Fig. 6A, B).
Discussion
Nature of EPWs along sieve tubes
As in other plants (e.g. Wildon et al., 1989; Stankovic and Davies, 1996; Stankovic et al., 1998; Furch et al., 2007, 2008, 2009), burning triggers EPWs along the phloem of cucurbits (Fig. 1). The intracellular EPW profiles—a steep transient of depolarization, followed by a long-lasting repolarization (Fig. 1)—hint at the merging of a rapid action potential and a slower variation potential (Stankovic et al., 1998; Hafke et al., 2009). A similar assessment was made for EPWs induced by burning leaf tips of other species (Stankovic et al., 1998; Hlavackova et al., 2006; Hafke et al., 2009). In keeping with this interpretation, the first depolarization peak and the onset of the second depolarization wave drift apart with increasing distance from the burning site (Fig. 1, Table 1).
The decline of the putative action potential depolarization between the points of recording (Fig. 1) seems to contradict the definition of an action potential (Zawadzki et al., 1991; Dziubinska, 2003). In contrast to the prevailing opinion, however, we agree with Stahlberg et al. (2006) that action potentials can fade along the sieve tubes, in particular when, as in Cucurbita, symplasmic continuity with surrounding phloem parenchyma cells is high (Kempers et al., 1998). Leakage of electrical current also explains the occurrence of reduced EPWs in phloem cells adjacent to the sieve tubes in tomato leaf veins (Rhodes et al., 1996). In species specialized in the transmission of action potentials such as Mimosa pudica, sieve tubes are electrically isolated to prevent current efflux to adjoining cells (Fleurat-Lessard and Roblin, 1982).
It should be noted that analysis of different kinetic components is complex; for example, apoplasmic recordings (Fig. 1B,C) express the overlapping reactions of different cell types. The high variability of both kinetic phases here in terms of duration and strength is in agreement with the variability of electrical signals recorded by microelectrodes blindly pierced into the main vein of tomato leaves (Herde et al., 1998).
Ca2+ influx in response to remote stimuli
The initial depolarization of an action potential is thought to involve activation of plasma membrane-localized voltage-sensitive Ca2+ channels in the plasma membrane (e.g. Felle and Zimmermann, 2007). Depolarization of the plasma membrane may be communicated to SE endoplasmic reticulum-located voltage-sensitive Ca2+ channels leading to supplementary Ca2+ influx into the SE mictoplasm (Hafke et al., 2009). Action potentials do not seem to mediate substantial Ca2+ influx (Hafke et al., 2009). In contrast, synergistic Ca2+ channel activation in plasma membrane and SE endoplasmic reticulum is proposed to result in massive Ca2+ influx in sieve tubes during variation potentials (Hafke et al., 2009). Variation potentials depend upon direct activation of mechano-sensitive Ca2+ channels or indirect activation of ligand-activated Ca2+ channels via signalling cascades (Stahlberg and Cosgrove, 1997; Stahlberg et al., 2006).
In V. faba, Ca2+ influx into SEs, which probably corresponds to the intensity of the stimulus, is decisive for the successive occlusion by forisomes and callose (Furch et al., 2007). As in Vicia, EPWs in Cucurbita represent merged potential waves, so that the proportional contribution of action potential (if any) and variation potential to Ca2+ influx and, hence, their individual effects on sieve tube occlusion cannot possibly be distinguished.
Mass flow stoppage triggered by EPWs
Bleached carboxyfluorescein fluorescence failed to recover within the first 1–2 min after burning. That the dye was not replaced and thus failed to reach the site of observation indicated cessation of mass flow by blockage of the pathway. About 40 min later, fluorescence re-emerged and phloem transport appeared to have resumed (Fig. 3D).
In an independent approach, aphids were used as biosensors for sieve tube occlusion, because they start secreting massive amounts of watery saliva in response to SE occlusion (Will et al., 2007, 2009). This saliva aims to prevent Ca2+-induced sieve tube occlusion by supplying Ca2+-binding proteins (Will et al., 2007). While feeding on SEs, aphids switch from phloem sap ingestion (E2) to secretion of watery saliva (E1) in response to the stop of mass flow triggered by leaf tip burning (Will et al., 2007, 2009). The fact that aphids resume E2 behaviour much faster further away from the burning site (Fig. 4) is consistent with the idea that variation potentials strongly dissipate along the propagation pathway (Davies, 2006). Accordingly, aphid saliva would be able to bind more rapidly to the lower amount of Ca2+ ions flowing into SEs further away from the leaf tip.
Indications for an occlusion mechanism supplementary to callose
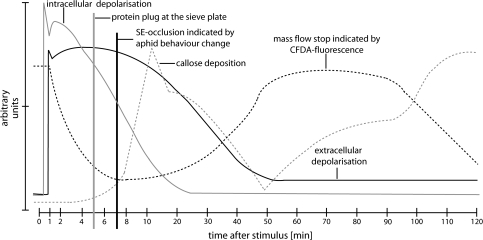
Like carboxyfluorescein (Fig. 2), aphids provide compelling physiological evidence in favour of a stop of mass flow due to occlusion in response to remote damage. However, the time delay between stop of mass flow and callose deposition is difficult to interpret. Aphids feeding on the main vein showed an abrupt change in behaviour (Fig. 4) well after the time point when mass flow had stopped (Fig. 7). It may take some time before sieve tube occlusion is perceived by aphids as a loss of pressure (Will et al., 2008). If the aphid response indeed presents a delayed reaction to sieve tube occlusion, aphid behaviour could reflect stoppage of mass flow long before callose deposition is being completed (Fig. 7). Similarly, carboxyfluorescein movement stops (Fig. 2) well before callose deposition is completed (Fig. 3). That stoppage of mass flow does not need maximal deposition of callose could explain this time-incongruence, but the following alternative may be attractive.
Fig. 7.
Graphic overlay of EPWs measured by independent methods and their impact on sieve tube occlusion and mass flow after burning the leaf tip. The black solid line shows extracellular electrical potentials, the grey solid line the membrane potentials of SEs, the black dashed line sieve tube occlusion as indicated by carboxyfluorescein fluorescence, the grey vertical line the time point of protein plug formation at the SP, the black vertical line the time point of sieve tube occlusion indicated by aphid behaviour change, and the grey dashed line callose deposition and degradation.
In V. faba, sieve tube occlusion by callose was preceded by fast forisome dispersion (Furch et al., 2007) triggered during the initial action potential-like phase. Such a safety design may also operate in a modified form in cucurbits: an immediate protein reaction precedes the slower callose deposition (Fig. 5). Candidates for sieve tube occlusion are PP1 and PP2 (Fig. 6), two major proteins from cucurbit exudates (Read and Northcote, 1983) which easily coagulate in the presence of oxygen (Alosi et al., 1988; Golecki et al., 1998). Moreover, PP1 and PP2-dimers are covalently cross-linked by disulphide bonds, forming high molecular weight polymers (Read and Northcote, 1983). These filaments were released from the plasma membrane and the SE endoplasmic reticulum and washed towards the SP in the case of wounding (Smith et al., 1987). The loss of the water-soluble forms of PP1 and PP2 (Fig. 6) in response to burning indicates that part of these proteins have become water insoluble due to polymerization. This conformational transition is in accordance with the visual appearance of protein plugs (Fig. 5). Most probably, therefore, the protein plugs observed represent polymerized PP1 and PP2. Given the Ca2+ dependence of the occlusion mechanisms in V. faba (Furch et al., 2009; Hafke et al., 2009), potential Ca2+-binding sites on PP1 (McEuen et al., 1981; Arsanto, 1986) make this compound a prime candidate for proteinaceous occlusion prior to callose occlusion in cucurbits.
Acknowledgments
We acknowledge the guidance of Alexander Schulz and Helle Martens (University of Copenhagen, Denmark) in using multiphoton microscopy. Furthermore, we are grateful to Steffanie V. Buxa, Aline Koch, and Sarah R. Kornemann (Justus-Liebig-University Gießen, Germany) for technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft in the frame of the Schwerpunktprogramm 1108 (BE1925/8-2, 8-3, 15-1).
Glossary
Abbreviations
- CC
companion cell
- CFDA
carboxyfluorescein diacetate
- CLSM
confocal laser scanning microscope
- EPG
electrical penetration graph
- EPW
electropotential wave
- PPC
phloem parenchyma cell
- PP1/PP2
phloem protein 1/2
- SE
sieve element
- SP
sieve plate
References
- Alosi MC, Melroy DL, Park RB. The regulation of gelation of phloem exudates from Cucurbita fruit by dilution, glutathione, and glutathione reductase. Plant Physiology. 1988;86:1089–1094. doi: 10.1104/pp.86.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsanto JP. Ca2+-binding sites and phosphatase activities in sieve element reticulum and P-protein of chick-pea phloem. A cytochemical and X-ray microanalysis survey. Protoplasma. 1986;132:160–171. [Google Scholar]
- Blackman LM, Gunning BES, Overall RL. A 45 kDa protein isolated from the nodal walls of Chara coralline is localised to plasmodesmata. The Plant Journal. 1998;15:401–411. [Google Scholar]
- Colombani A, Djerbi S, Bessueille L, Blomqvist K, Ohlsson A, Berglund T, Teeri TT, Bulone V. In vitro synthesis of (1→3)-β-d-glucan (callose) and cellulose by detergent extracts of membranes from cell suspension cultures of hybrid aspen. Cellulose. 2004;11:313–327. [Google Scholar]
- Cronshaw J, Sabnis DD. Phloem proteins. In: Behnke HD, Sjolund RD, editors. Sieve elements. Comparative structure, induction and development. Berlin: Springer-Verlag; 1990. pp. 257–283. [Google Scholar]
- Davies E. Electrical signals in plants: facts and hypotheses. In: Volkov AG (ed) Plant electrophysiology. 2006 Berlin: Springer-Verlag, 407–422. [Google Scholar]
- Dziubinska H. Ways of signal transmission and physiological role of electrical potentials in plants. Acta Societatis Botanicorum Poloniae. 2003;72:309–318. [Google Scholar]
- Evert RF. Sieve-tube structure in relation to function. Bioscience. 1982;32:789–795. [Google Scholar]
- Felle HH, Zimmermann MR. Systemic signalling in barley through action potentials. Planta. 2007;226:203–214. doi: 10.1007/s00425-006-0458-y. [DOI] [PubMed] [Google Scholar]
- Fleurat-Lessard P, Roblin G. Comparative histocytology of the petiole and the main pulvinus in Mimosa pudica L. Annals of Botany. 1982;50:83–92. [Google Scholar]
- Fromm J, Spanswick R. Characteristics of action potential in willow (Salix viminalis L.) Journal of Experimental Botany. 1993;44:1119–1125. [Google Scholar]
- Furch ACU, Hafke JB, Schulz A, van Bel AJE. Ca2+-mediated remote control of reversible sieve tube occlusion in Vicia faba. Journal of Experimental Botany. 2007;58:2827–2838. doi: 10.1093/jxb/erm143. [DOI] [PubMed] [Google Scholar]
- Furch ACU, Hafke JB, van Bel AJE. Plant- and stimulus-specific variations in remote-controlled sieve-tube occlusion. Plant, Signaling and Behavior. 2008;3:858–861. doi: 10.4161/psb.3.10.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furch ACU, van Bel AJE, Fricker MD, Felle HH, Fuchs M, Hafke JB. Sieve element Ca2+channels as relay stations between remote stimuli and sieve tube occlusion in Vicia faba. The Plant Cell. 2009;21:2118–2132. doi: 10.1105/tpc.108.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavalisco P, Kapitza K, Kolasa A, Buhtz A, Kehr J. Towards the proteome of Brassica napus phloem sap. Proteomics. 2006;6:896–909. doi: 10.1002/pmic.200500155. [DOI] [PubMed] [Google Scholar]
- Golecki B, Schulz A, Carstens-Behrens U, Kollmann R. Evidence for graft transmission of structural phloem proteins or their precursors in heterografts of Cucurbitaceae. Planta. 1998;206:630–640. [Google Scholar]
- Grams TEE, Lautner S, Felle HH, Matyssek R, Fromm J. Heat-induced electrical signals affect cytoplasmic and apoplasmic pH as well as photosynthesis during propagation through the maize leaf. Plant, Cell and Environment. 2009;32:319–326. doi: 10.1111/j.1365-3040.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- Hafke JB, Furch ACU, Fricker MD, van Bel AJE. Forisome dispersion in Vicia faba is triggered by Ca2+ hotspots created by concerted action of diverse Ca2+ channels in sieve elements. Plant, Signaling and Behavior. 2009;4:968–972. doi: 10.4161/psb.4.10.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafke JB, van Amerongen J-K, Kelling F, Furch ACU, Gaupels F, van Bel AJE. Thermodynamic battle for photosynthate acquisition between sieve tubes and adjoining parenchyma in transport phloem. Plant Physiology. 2005;138:1527–1537. doi: 10.1104/pp.104.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herde O, Pena-Cortés H, Willmitzer L, Fisahn J. Remote stimulation by heat induces characteristic membrane-potential responses in the veins of wild-type and abscisic acid-deficient tomato plants. Planta. 1998;206:146–153. [Google Scholar]
- Hlavackova V, Krchnak P, Naus J, Novak O, Spundova M, Strnad M. Electrical and chemical signals in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta. 2006;225:235–244. doi: 10.1007/s00425-006-0325-x. [DOI] [PubMed] [Google Scholar]
- Kauss H, Höhle H, Jeblick W. Proteolytic activation and stimulation by Ca2+ of glucan stopcocks from soybean cells. FEBS Letters. 1983;158:84–88. [Google Scholar]
- Kehr J, Buhtz A. Long distance transport and movement of RNA through the phloem. Journal of Experimental Botany. 2008;59:85–92. doi: 10.1093/jxb/erm176. [DOI] [PubMed] [Google Scholar]
- Kempers R, Ammerlaan A, van Bel AJE. Symplasmic constriction and ultrastructure features of the sieve element/companion cell complex in the transport phloem of apoplasmically and symplasmically phloem-loading species. Plant Physiology. 1998;116:271–278. [Google Scholar]
- Kleinig H. Filament formation in vitro of a sieve tube protein from Cucurbita maxima and Cucurbita pepo. Planta. 1975;127:163–170. doi: 10.1007/BF00388377. [DOI] [PubMed] [Google Scholar]
- Knoblauch M, van Bel AJE. Sieve tubes in action. The Plant Cell. 1998;10:35–50. [Google Scholar]
- Kudlicka K, Brown RM. Cellulose and callose biosynthesis in higher plants. Plant Physiology. 1997;115:643–656. doi: 10.1104/pp.115.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy A, Guenoune-Gelbart D, Epel BL. β-1,3-glucanases. Plasmodesmal gate keepers for intercellular communication. Plant, Signaling and Behavior. 2007;5:288–290. doi: 10.4161/psb.2.5.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso S. Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Australian Journal of Plant Physiology. 1999;26:55–61. [Google Scholar]
- McEuen AR, Hart JW, Sabnis DD. Calcium-binding protein in sieve tube exudate. Planta. 1981;151:531–534. doi: 10.1007/BF00387430. [DOI] [PubMed] [Google Scholar]
- Nakashima J, Laosinchai W, Cui X, Brown RM., Jr. New insight into the mechanism of cellulose and callose biosynthesis: proteases may regulate callose biosynthesis upon wounding. Cellulose. 2003;10:369–389. [Google Scholar]
- Peters WS, Haffer D, Hanakam CB, van Bel AJE, Knoblauch M. 2010. Legume phylogeny and the evolution of a unique contractile apparatus that regulates phloem transport. American Journal of Botany97, 797–808. [DOI] [PubMed] [Google Scholar]
- Prado E, Tjallingii WF. Aphid activities during sieve element punctures. Entomologia Experimentalis et Applicata. 1994;72:157–165. [Google Scholar]
- Read SM, Northcote DH. Subunit structure and interactions of the phloem proteins of Cucurbita maxima (pumpkin) European Journal of Biochemistry. 1983;134:561–569. doi: 10.1111/j.1432-1033.1983.tb07603.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JD, Thain JF, Wilson DC. The pathway for systemic electrical signal conduction in the wounded tomato plant. Planta. 1996;200:50–57. [Google Scholar]
- Schulz A. The phloem. Structure related to function. Progress in Botany. 1998;59:429–475. [Google Scholar]
- Smith LM, Sabnis DD, Johnson RPC. Immunochemical localization of phloem lectin from Cucurbita maxima using peroxidase and colloidal-gold labels. Planta. 1987;170:461–470. doi: 10.1007/BF00402980. [DOI] [PubMed] [Google Scholar]
- Stahlberg R, Cosgrove DJ. The propagation of slow wave potentials in pea epicotyls. Plant Physiology. 1997;113:209–217. doi: 10.1104/pp.113.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlberg R, Stephens NR, Cleland RE, Van Volkenburgh E. Shade-induced action potentials in Helianthus annuus L. originate primarily from the epicotyl. Plant, Signaling and Behavior. 2006;1:15–22. doi: 10.4161/psb.1.1.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic B, Davies E. Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. FEBS Letters. 1996;390:275–279. doi: 10.1016/0014-5793(96)00672-2. [DOI] [PubMed] [Google Scholar]
- Stankovic B, Witters DL, Zawadzki T, Davies E. Action potentials and variation potentials in sunflower: an analysis of their relationships and distinguishing characteristics. Physiologia Plantarum. 1998;103:51–58. [Google Scholar]
- Tjallingii WF. Electronic recording of penetration behaviour by aphids. Entomologia Experimentalis et Applicata. 1978;24:721–730. [Google Scholar]
- Tjallingii WF. Electrical nature of recorded signals during stylet penetration by aphids. Entomologia Experimentalis et Applicata. 1985;38:177–186. [Google Scholar]
- Tjallingii WF, Hogen Esch T. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiological Entomology. 1993;18:317–328. [Google Scholar]
- van Bel AJE. The phloem, a miracle of ingenuity. Plant, Cell and Environment. 2003;26:125–149. [Google Scholar]
- Wildon DC, Doherty HM, Eagles G, Bowles DJ, Thain JF. Systemic responses arising from localized heat stimuli in tomato plants. Annals of Botany. 1989;64:691–695. [Google Scholar]
- Will T, Hewer A, van Bel AJE. A novel perfusion system shows that aphid feeding behaviour is altered by decrease of sieve-tube pressure. Entomologia Experimentalis et Applicata. 2008;127:237–245. [Google Scholar]
- Will T, Kornemann SR, Furch ACU, Tjallingii WF, van Bel AJE. Aphid watery saliva counteracts sieve-tube occlusion: a universal phenomenon? Journal of Experimental Biology. 2009;212:3305–3312. doi: 10.1242/jeb.028514. [DOI] [PubMed] [Google Scholar]
- Will T, Tjallingii WF, Thönnessen A, van Bel AJE. Molecular sabotage of plant defence by aphid saliva. Proceedings of the National Academy of Sciences, USA. 2007;104:10536–10541. doi: 10.1073/pnas.0703535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabotin AI, Barysheva TS, Trofimova OI, Lozovaya VV, Widholm J. Regulation of callose metabolism in higher plant cells in vitro. Russian Journal of Plant Physiology. 2002;49:792–798. [Google Scholar]
- Zawadzki T, Davies E, Dziubinska H, Trebacz K. Characteristics of action potentials in Helianthus annuus L. Physiologia Plantarum. 1991;83:601–604. [Google Scholar]