Abstract
Stomatal movement results in large and repetitive changes in cell volume and consequently surface area. While endocytosis has been extensively studied and is thought to be a major mechanism for accommodating the volume changes as evidenced mainly by fluorescent labelling and confocal imaging, studies at the ultrastructural level in intact guard cells of stomata regulated by natural factors have never been reported. Here, it is reported that excretion and folding of the plasmalemma are critical for accommodating the volume alterations in intact guard cells in Vicia faba L. Using transmission electron microscopy in combination with laser confocal microscopy, it was observed that in fully opened stomata the plasmalemma was smooth and tightly adhered to the cell walls while a whole large vacuole appeared in the cell. In the closed stomata, besides vacuole fragmentation, endocytosis of the tonoplast rather than the plasmalemma commonly occurred. Importantly, in stomata where pore closure was induced by circadian rhythm or CO2, numerous tiny vesicles were found outside the plasmalemma and, moreover, extensive folding of the plasmalemma could also be found in some regions of the cells. Additionally, an unknown structure was found at the interface between the plasmalemma and cell walls, especially in those areas of the cell where extensive folding occurred, suggesting that plasmalemma turnover is possibly associated with an interaction between the plasmalemma and cell walls. Collectively, the results strongly indicate that excretion and folding of the plasmalemma are critical for the accommodation of the cell volume alterations during stomatal movement.
Keywords: Excretion, folding, guard cell, plasmalemma, stomata
Introduction
Guard cells control stomatal movement thereby regulating gas exchange in plants. Swelling of guard cells causes stomatal opening, and shrinkage of the cells causes stomatal closure. During stomatal movement, guard cells undergo considerable and repetitive variations in cell volume and consequently in surface area over a period of minutes. It was estimated that the changes in plasmalemma surface area caused by stomatal movement could be up to 40% (Raschke, 1979). Because the maximum possible stretching of membranes is limited to only ∼2% (Wolfe et al., 1986), such a large change in the plasmalemma surface area has raised the issue of how the plasmalemma is able to accommodate the volume changes during stomatal movement. Although many studies have tried to address this issue, its exact mechanisms are still poorly understood (Gradmann and Robinson, 1989; Homann, 1998; Homann and Thiel, 1999; Kubitscheck, 2000; Shope et al., 2003; Sano et al., 2008)
Theoretically, the mechanisms for the volume change accommodation may not be complex because changes in plasmalemma surface area can be achieved only through two possible means: one is membrane folding and unfolding, and the other is the removal and addition of membrane material from and to the plasma membrane, respectively. A prevailing opinion is that the changes in plasmalemma surface area cannot possibly be achieved through membrane folding and unfolding due to the presence of a relatively high turgor pressure in both opened and closed stomata (Homann, 2005; Meckel et al., 2005). Therefore, the removal and addition of membrane material from and to the plasmalemma have been paid particular attention, and, in particular, endocytosis and exocytosis have been proposed to be a major mechanism for the plasmalemmal turnover (Homann, 2005; Meckel et al., 2005).
Endocytosis is defined as the uptake of extracellular substances as well as the internalization of the plasmalemma into cells. Endocytosis starts with the plasmalemma invaginating into a pit which further develops into a bud and finally pinches off as a vesicle into the cytoplasm (Thiel and Battey, 1998; Battey et al., 1999; Holstein, 2002; Homann, 2005). The reverse process, that is for a vesicle to move from the cytoplasm to the plasmalemma and finally fuse with the plasmalemma, is referred to as exocytosis. To observe endocytosis and exocytosis during stomatal movement, two techniques have normally been adopted: one is confocal imaging of fluorescently labelled vesicles and the other is capacitance recordings by patch–clamp. Using the membrane-impermeable fluorescent dye Lucifer Yellow (LY), Diekmann et al. (1993) found an uptake of the dye in hyperosmotically treated guard cell protoplasts. By incubation of guard cell protoplasts with the fluorescent membrane probe FM1-43, Kubitscheck et al. (2000) observed a diffuse distribution of the FM1-43 label throughout the cytoplasm without any resolvable vesicular structures in hyperosmotically treated protoplasts, and with this they concluded that endocytosis of small vesicles occurred below the resolution limit. Importantly, a more quantitative assessment demonstrated that a decrease in surface area under hyperosmotic conditions correlated with the internalization of the membrane marker FM4-64 (Shope et al., 2003). Capacitance measurement with patch–clamp allows the examination of exocytosis and endocytosis with a much higher resolution. With this technique, many studies have found a linear relationship between membrane capacitance and surface area, suggesting that variations in membrane surface area resulted from membrane turnover (Zorec and Tester, 1992; Thiel et al., 1994; Carroll et al., 1998; Homann, 1998).
While endocytosis has been reported to be a major mechanism for plasmalemma turnover in guard cells, it should be noted that most of the studies were carried out in protoplasts and, moreover, cellular shrinking was commonly induced by artificial osmotic regulation. Because protoplasts differ from intact guard cells in many aspects, and the process of osmotically induced cellular shrinking is also not the same as that occurring during stomatal closure induced by natural factors, such as light, CO2, and so on, it is still unclear whether the endocytosis, as observed in protoplasts or in intact guard cells but induced by osmotic regulation, may be able to play a major role in the plasmalemma turnover in intact guard cells under natural conditions. Additionally, before endocytosis can be accepted as the major cause of changes in membrane turnover, two critical issues must be addressed. One is how the guard cells can precisely sort a large number of endocytotic vesicles and retrieve them to the plasmalemma during stomatal opening, and the other is how endocytosis may be able to occur against high turgor pressure in guard cells (Homann, 2005).
In view of the questions above, it is suggested that further investigations are required of intact guard cells induced to open and close by natural factors. Because membrane turnover may be quite subtle, investigations at the ultrastructural level are absolutely necessary. Using transmission electron microscopy in combination with laser confocal microscopy, the present study has demonstrated that vesicle excretion and folding of the plasmalemma rather than endocytosis are critical for the accommodation of the cell volume alterations during stomatal closure induced by circadian rhythm or CO2 in Vicia faba L.
Materials and methods
Plant materials and growth conditions
Seeds of V. faba L. were soaked in water until they were germinated, then transplanted into pots (90 mm×150 mm) containing a mixture of soil and sand (3:1). Plants were grown in a controlled environment with the following conditions: day/night temperature 27 °C and 18 °C, humidity 40%, and photoperiod 15 h with a photosynthetic photon flux density (PPFD) of 350 μmol m−2 s−1. Plants were watered daily to the drip point. When plants were 3 weeks old, the third fully expanded leaf was used as experimental material.
Manipulation of stomatal movement by circadian rhythm and CO2
For observation of opened stomata, plants were watered with 50 mM KCl solution to the drip point, then incubated under a temperature of 25 °C, humidity 60%, and a PPFD of 350 μmol m−2 s−1. About 3 h later, the stomata were fully opened as determined by light microscopy. For observation of the closed stomata induced by circadian rhythm, plants were grown in normal conditions as described above, and leaves were sampled at night. For observation of the closed stomata induced by CO2, plants were first treated with KCl until the stomata were fully opened as described above, and then a whole plant was selected and placed in an airtight box that was connected to a CO2 generator. The CO2 generator was made up of a 500 ml sealed bottle that contains 200 ml of 20% NaCO3 solution, and CO2 generation was started by injecting concentrated HCl solution into the bottle. Stomata were fully closed in <30 min after the CO2 generation was started.
Sample preparation for ultrastructural observation
Quickly after leaves were cut from plants, square pieces of leaves of ∼1 cm2 were cut in the central part of the lamina while avoiding the midvein, then further cut into small pieces of ∼2 mm2 with a razor blade, immediately transferred to fixative buffer (0.15 M phosphate buffer, pH 7.2, 1% glutaraldehyde, and 4% paraformaldehyde), sunk by vacuum pumping, fixed for 8 h at 4 °C, rinsed with three changes of the phosphate buffer, post-fixed in 1% OsQ in buffer for 1 h, washed again in buffer and distilled water, then dehydrated in a graded series of acetone solutions. Samples were embedded in Spurr's resin (Spurr, 1969) and polymerized for 8 h at 70 °C. Semi-thin sections were cut with glass knives and continuously examined until the guard cells with the profiles of interest were found, then they were used for the prepartion of ultrathin sections with diamond knives on an LKB ultratome (Lab Extreme Inc., Muskegon, MI, USA). Sections were mounted on formvar-coated single hole nickel grids, and stained with uranyl acetate and lead citrate. Ultrastructures were investigated with a JEOL 1200 EX electron microscope.
Cell labelling and imaging by laser confocal scanning microscopy
The abaxial epidermis was carefully removed from the third fully expanded leaf of 3-week-old V. faba L. plants and immediately floated on 10 mM MES buffer, pH 6.2. The strips were floated mesophyll-side down and incubated for 2 h at a PPFD of 350 μmol m−2 s−2 in Petri dishes containing 10 mM MES, pH 6.2, and 50 mM KCl, through which CO2-free air was bubbled to open the stomata fully. For labelling and imaging of guard cell pairs in opened stomata, the strips were randomly transferred to Petri dishes containing 10 mM MES, pH 6.2, 50 mM KCl, and 10 μM FM1-43 (Brumback et al., 2004; Molecular Probes, Leiden, The Netherlands) and, after staining for 2 min, the strips were transferred on a microscope slide for confocal microscopic analysis. For labelling and imaging of guard cell pairs in closed stomata, and after staining for 5 min, the strips were immediately transferred to dishes containing 10 mM MES, pH 6.2, 50 mM KCl, and 50 μM abscisic acid (ABA), and, after incubating for a further 20 min, the strips were transferred onto a microscope slide for confocal microscopic analysis. For examination of osmiophilic bodies, different staining conditions were tested by changing the dye concentration in combination with the incubation time, i.e. 5, 10, and 20 μM FM1-43 in combination with 1, 2, and 5 min. Good results were obtained for the labelling condition of 5 μM FM1-43 for 5 min. Imaging analyses were performed on a Nikon ECLIPSE TE2000-E inverted fluorescence microscope equipped with a Nikon D-ECLIPSE C1 spectral confocal laser scanning system. FM1-43 was excited at 488 nm and the emission was detected at 530–630 nm. To observe whether plasmolysis may occur when cells are alive, guard cells were labelled by fluorescein diacetate (FDA) and the imaging analysis was performed under 488 nm excitation and 530 nm emission (Mathur et al., 2003). As a control for observation of plasmolysis, and also to observe whether the plasmolysed cells were able to restore their normal status, guard cells were treated with urea and then labelled by FDA according to the method of Stadelmann and Wattendorff (1966).
Results
Ultrastructural characterization of opened stomata
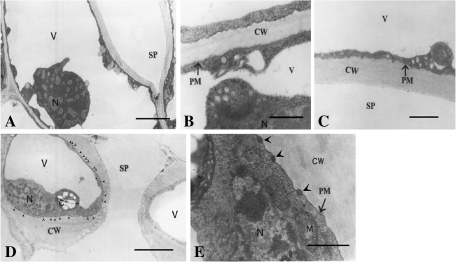
Figure 1A is a paradermal section of an opened stoma with a relatively low magnification, in which it can be seen that a large whole vacuole appears in the guard cell. The vacuole is so large that it tightly squeezes the cytoplasm against the cell walls. To show the plasmalemma associated with the walls and cytoplasm more clearly, high magnification pictures of dorsal and ventral regions are provided in Fig. 1B and C, respectively. It is clearly seen that the plasmalemma is tightly adhered to the cell walls in both the dorsal (Fig. 1B) and ventral region (Fig. 1C). Cross-sections of opened stomata show basically the same features as those in the paradermal sections, i.e. the guard cells contain only one large whole vacuole, and the plasmalemma, which is smoother than that shown in paradermal sections, is tightly adhered to the cell walls (Fig. 1D). However, interestingly, in the cross-sections, many oval osmiophilic bodies can be clearly seen outside the plasmalemma. As shown in the magnified picture (Fig. 1E), these oval osmiophilic bodies are free of structures. It is noteworthy that while these oval osmiophilic bodies can be clearly seen in the lower, upper, and ventral regions, they are seldom seen in the dorsal regions, suggesting that the distribution of these oval osmiophilic bodies seems to be regionalized (Fig. 1D, E).
Fig. 1.
Sections of opened stomata. Stomatal opening was induced as described in the Materials and methods. (A) Paradermal section showing a whole view of an opened stoma. Note that each cell contains only one large whole vacuole. Bar=3 μm. (B) A magnified paradermal section near the dorsal wall showing the stretched plasmalemma and its conjunction with the cell wall. Bar=0.5 μm. (C) A magnified paradermal section near the ventral wall showing the stretched plasmalemma and its conjunction with the cell wall. Bar=0.5 μm. (D) A cross-section showing a whole view of an opened stoma. Note the osmiophilic bodies in the interface between the plasmalemma and the walls (arrow heads). Bar=4 μm. (E) A cross-section at a much higher magnification showing the region near the ventral wall. Note the osmiophilic bodies in the interface between the plasmalemma and walls (arrowheads). Bar=0.1 μm. V, vacuole; N, nucleus; SP, stomatal pore; CW, cell wall; PM, plasmalemma; M, mitochondria.
Membrane turnover during stomatal closure induced by circadian rhythm
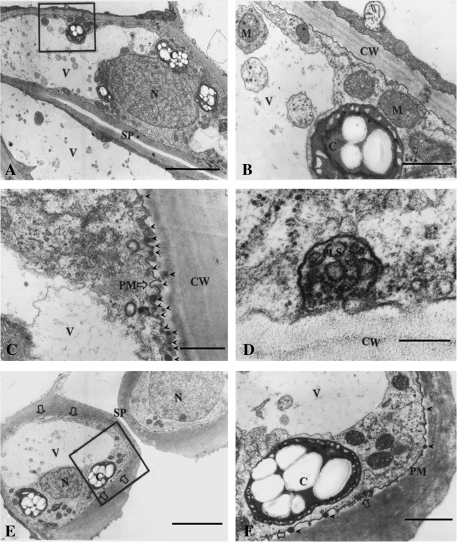
Stomatal movement is regulated by circadian rhythm, i.e. stomata are regularly open during the day and closed during the night. To observe the membrane turnover during stomatal movement in natural conditions, leaves were sampled and immediately fixed at night. In the paradermal sections it is clearly seen that numerous clumps of cytoplasmic materials appear in the vacuole (Fig. 2A). To show the structures more clearly, a magnified image of the framed region in Fig. 2A is shown in Fig. 2B, from which it can seen that the cytoplasmic clumps vary not only in size but also in content, but no matter what size and content, all of them are wrapped up by a monolayer membrane, suggesting that these cytoplasmic clumps are formed by endocytosis of the tonoplast.
Fig. 2.
Sections of closed stomata. Stomatal closure was induced by circadian rhythm as described in the Materials and methods. (A) A paradermal section showing a whole view of a closed stoma. Note that the large central vacuole contains numerous round masses of endocytotic cytoplasm. Bar=2 μm. (B) Magnified image of the framed region in A, showing the rugged plasmalemma and endocytotic cytoplasm. Bar=0.15 μm. (C) Magnified paradermal section near the ventral wall showing extensive folding of the plasmalemma. Bar=0.5 μm. (D) Paradermal section with a much higher magnification near the ventral wall showing a plasmalemmasome. Bar=0.05 μm. (E) A cross-section showing a whole view of a closed stoma. Note the vesicle clusters outside the plasmalemma (large open arrows). Bar=2 μm. (F) Magnified image of the framed region in E. Note the vesicles outside the plasmalemma (large open arrow) and the osmiophilic bodies (arrowheads). Bar=0.25 μm. V, vacuole; N, nucleus; SP, stomatal pore; CW, cell wall; PM, plasmalemma; PLS, plasmalemmasome; C, chloroplasts.
In contrast to what is observed in opened stomata (Fig. 1), in the closed stomata the plasmalemma becomes folded and slightly detached from the walls. Figure 1C is a paradermal section of the ventral regions near the lower walls, in which it can be seen that the plasmalemma becomes extensively folded, and, importantly, more oval osmiophilic bodies can be seen in this region. The plasmalemmasome is a type of structure formed in many plant cells due to plasmalemma invagination, and this type of structure could also be found in some regions of the guard cells (Fig. 2D). The typical features observed in the cross-sections (Fig. 2E, F) are basically the same as that shown in the paradermal sections (Fig. 2A–D), but, besides these features, the cross-section has shown another rather important feature, i.e. numerous tiny vesicles that appear in clusters in the plasmolysed spaces (open arrows). Figure 2F is the magnified image of the framed region in Fig. 2E, from which it can be more clearly seen that the plasmalemma is folded and numerous tiny vesicles appear in clusters in the plasmolysed spaces.
Membrane turnover during stomatal closure induced by CO2
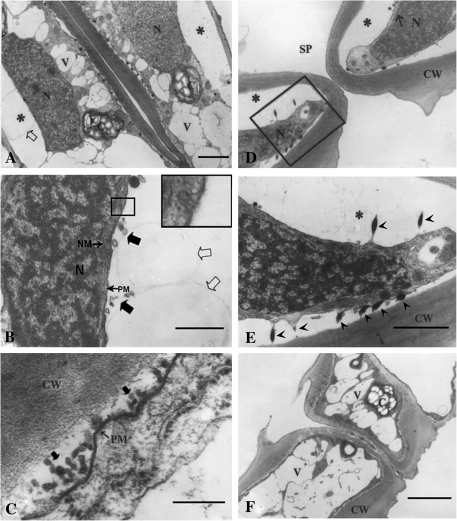
Circadian rhythm-regulated stomatal closure is a relatively slow process, which usually takes several hours or even longer. Fast stomatal closure can be induced by many natural factors such as CO2 and ABA, which may take <1 h or even several minutes when induced by high concentrations of CO2 or ABA. It was of interest to determine whether the membrane turnover in a slowly closing process may be somewhat different from that in a fast closing process. Figure 3A is a paradermal section of a stoma whose closure was induced by CO2. Surprisingly, unlike that observed in Fig. 2A, the large central vacuole fragmented into small vacuoles and, simultaneously, plasmolysis occurred more extensively, especially in the middle region of the dorsal walls (Fig. 3A). Figure 3B is a paradermal section with a higher resolution, in which it can be seen that plasmolysis occurred in the middle parts of the dorsal wall. Extensive examinations indicated that incipient plasmolysis always occurred in this place. Additionally, endocytosis can be found, but only occasionally (see the framed image). Figure 3C is a paradermal section in the dorsal wall area with an even higher resolution. Numerous tiny vesicles can again be clearly observed outside the plasmalemma. These observations demonstrate that vesicle excretion may function as an important mechanism for plasmalemma turnover. Besides plasmolysis and vesicle excretion, in cross-sections a noteworthy feature is the relationship between the osmiophilic bodies and the plasmalemma. Figure 3E is a magnified picture of the framed region in Fig. 3D. As seen in Fig. 1E, in fully opened stomata the osmiophilic bodies have oval shapes in the plasmalemma–wall interface, but in closed stomata these osmiophilic bodies have become spindly with each end connected to plasmalemma and cell wall, respectively (Fig. 3E), which appears to suggest that these osmiophilic bodies may function to adhere the plasmalemma to the cell walls, thus regulating plasmolysis. As shown in Fig. 3D, the plasmolysis is quite extensive, but in-depth examinations suggested that such an extensive plasmolysis is only confined to the central regions of the dorsal walls and sometimes the upper and lower walls. Interestingly, extensive plasmolysis never occurs in ventral walls, suggesting that the plasmolysis is regionalized. To indicate this, a cross-section taken away from the central area has been provided as Fig. 3F, from which it can be seen that the fragmented vacuoles are full of the whole cells and the plasmalemma is basically connected to the walls, although slight plasmolysis can be seen in some areas.
Fig. 3.
Sections of closed stomata. The stomatal closure was induced by CO2 as described in the Materials and methods. (A) A paradermal section showing a whole view of a closed stoma. Note the vacuolar fragmentation and plasmolysis (*). Bar=2 μm. (B) Magnified image of the heavily plasmolysed area. Note the small (large filled arrows) and large vesicles (large open arrows) outside the plasmalemma. The image with a large frame in the upper right corner is a enlarged view of the region with a small frame showing endocytosis. Bar=0.25 μm. (C) A paradermal section with a much higher magnification near the ventral wall showing a large number of vesicles outside the plasmalemma (large filled arrows). Bar=0.05 μm. (D) A cross-section near the central line (see Fig. 1) showing a whole view of a closed stoma. Note the plasmolysis (*). Bar=2 μm. (E) Magnified image of the framed region in D showing the relationship among plasmalemma, osmiophilic bodies, and the cell wall. Bar=0.15 μm. (F) A cross-section in the position between the central line and stomatal ledge (see Fig. 1) showing that no serious plasmolysis occurred around the cell walls. Bar=2 μm. V, vacuole; N, nucleus; SP, stomatal pore; CW, cell wall; NM, nuclear membrane; PM, plasmalemma. Bar=0.25.
Characterization of the osmiophilic bodies and membrane turnover with confocal microscopy
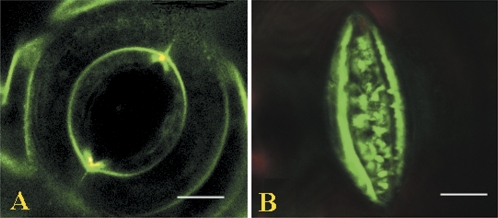
The osmiophilic bodies, as observed by electron microscopy (Figs 1–3), have never been described before in guard cells. In view of their possible roles in stomatal movement or membrane turnover, their possible chemical nature was characterized further. Guard cells contain numerous lipid bodies in the cytoplasm; based on their structural features, the osmiophilic bodies look somewhat like lipid bodies, although they are much smaller. To understand their chemical properties, guard cells were labelled with fluorescent styryl dye, and investigated with confocal microscopy. Consistent with the observations using electron microscopy, in fully opened stomata the FM1-43-labelled plasmalemma was quite smooth and taught (Fig. 4A). However, in the closed stomata, while no endocytotic vesicles were clearly observed, the labelled plasmalemma became thick and folded in the ventral areas, and, simultaneously, numerous masses of fluorescence appeared outside the plasmalemma (Fig. 4B), which strongly suggests that processes of membrane folding, extrusion, and excretion occurred. As shown in Fig. 4, in normal cases it was not possible to find any fluorescent labels for the osmiophilic bodies. However, when successive sections were examined, it was observed that some brightly labelled spots appeared in the plasmalemma, suggesting that the osmiophilic bodies can be labelled by FM1-43 (Fig. 5C, E). Because lipid bodies could not be labelled by styryl dyes (Meckel et al., 2004), these osmiophilic bodies cannot be lipid bodies, and they are more likely to contain materials with similar properties to plasmalemma.
Fig. 4.
Confocal images of stomata labelled by FM1-43. Guard cell pairs of stomata were labelled by FM1-43 and the imaging was performed as described in the Materials and methods. (A) Opened stoma. Note that the plasmalemma is quite smooth and taught. (B) Closed stoma. Note that the ventral plasmalemma becomes folded with numerous extrusions, and excreted vesicles appear nearby, whereas the dorsal plasmalemma becomes invisible.
Fig. 5.
Confocal images of successive sections of stomata labelled by FM1-43. Guard cell pairs of stomata were labelled by FM1-43 and the imaging was performed as described in the Materials and methods. (A–E) Successive sections. Only ventral plasmalemma is clearly labelled. Note that many brightly labelled tiny spots appear in the plasmalemma in D and E.
Discussion
Electron microscopy is a powerful tool to study the fine structures of cells. Membrane turnover during stomatal movement has been extensively investigated in the past, but nearly all studies have used confocal microscopy. However, it is most likely that membrane turnover with stomatal movement is based on changes of some structures that are too subtle to be observed by confocal microscopy. Using transmission electron microscopy in combination with confocal microscopy, this study has observed excretion and folding of plasmalemma that function as the mechanisms for plasmalemma turnover. Indeed, the structures involved in membrane turnover are so delicate that even using electron microscopy some of them can only be observed at a relatively high magnification. It has been suggested that the transmission electron microscope technique may possibly produce some artificial images due to inappropriate sample preparation, but it is thought that new insight may be gained with a combination of techniques used carefully. In the present study, the focus was on the membrane system, and in all sections it is clearly shown that the membrane system was well preserved, and, more importantly, the study only focuses on comparisons between different states of the cells, and hence the question of artificial images should not be an issue here. The present study demonstrates that the adoption of transmission electron microscopy is absolutely necessary for studies on plasmalemma turnover in stomatal movement.
Tonoplast turnover has been described in some studies (Diekmann et al., 1993; Gao et al., 2005). Vacuolar fragmentation is believed to be responsible for the tonoplast turnover to accommodate the vacuolar volume alterations during stomatal closure.
Interestingly, in the present study another mechanism underlying the tonoplast turnover was found, i.e. invagination of the cytoplasm into the vacuole, which can also be referred to as an endocytic process of the tonoplast. Endocytosis of the tonoplast was found to be an important mechanism for tonoplast turnover in circadian rhythm-induced stomatal closure (Fig. 2A), whereas in fast closure of stomata induced by CO2 it was observed that vacuolar fragmentation is a major mechanism for tonoplast turnover (Fig. 3A). Regulation of stomatal movement on a rhythmic basis is a relatively slow process compared with movement induced by osmotic regulation or some other regulators such as ABA and CO2. It is not clear whether the different mechanisms for tonoplast turnover are associated with the speed or degree of stomatal closure. The relationship between endocytosis and fragmentation is likely to be similar to the interaction between water and oil. When mixing oil with water, depending on the ratio of water to oil, a change in the muffling relationships between the oil and water must occur. Accordingly, it appears that tonoplast turnover is more likely to be associated with the degree of closure. Consistent with this proposition, compared with movement induced by circadian rhythm (Fig. 2A), the degrees of closure and plasmolysis are much more severe in the stomata induced to close by CO2 (Fig. 3A).
Endocytosis is believed to play a major role in plasmalemma turnover in guard cells. Given the fact that the changes in plasmalemma surface area may be up to 40% (Shope et al., 2003; Homann, 2005; Meckel et al., 2005), a considerable number of endocytotic vesicles should be observed. In the present study, however, it was not possible to find the expected number of endocytotic vesicles, but instead numerous vesicles outside the plasmalemma as well as membrane folding in some regions in the closed stomata were found. Endocytosis of the plasmalemma has been reported in many studies, but most of the studies were carried out in protoplasts (Lambrechts et al., 1992; Diekmann, 1993; Homann, 1998; Kubitscheck et al., 2000; Bick et al., 2001). Protoplasts have no turgor pressure, and it may not be possible to extrapolate the results to intact guard cells. Shope et al. (2003) observed endocytosis in intact guard cells and suggested that changes in surface area of guard cells are correlated with membrane internalization. However, in this study changes in guard cell volume were induced by artificial osmotic regulation and, moreover, the related assessments were based on observations with the confocal microscope. Changes in guard cell volume induced by artificial osmotic regulation may also be different from those induced by natural factors (e.g. light, ABA, and CO2), because the natural factor-induced stomatal movement is a positively driven process (Morris and Homann, 2001). In the present study, while a large number of vesicles were found outside the plasmalemma, the expected number of endocytotic vesicles was not observed. Consistent with the present observation, while Diekmann et al. (1993) were able to find endocytosis in protoplasts, they were not able to find the endocytotic vesicles in intact guard cells. Accordingly, the mechanisms for plasmalemma turnover in intact guard cells are likely to be different from that in protoplasts, and from that in guard cells induced by artificial osmotic regulation.
There are two issues that are difficult to address in studies of endocytosis in guard cells. One is that endocytosis must occur against high turgor pressures in guard cells and the other is that a large number of endocytotic vesicles must be completely retrieved to the plasmalemma during stomatal opening. It is believed that the turgor pressure can be quite high in guard cells (Franks et al., 1995, 1998; Meckel et al., 2004). Endocytosis against high turgor pressure means that there is a substantial energy requirement. It has been proposed that lipid asymmetry may function as a driving force for endocytosis, but the exact mechanism for this remains unclear (Homann et al., 2005; Meckel et al., 2005). Given the fact that stomatal closure and opening can occur repeatedly in a day, retrieval of endocytotic vesicles is particularly important. Changes in plasmalemma surface area can be quite large (Raschke, 1979; Meckel et al., 2007); therefore, stomatal closure should produce a large number of endocytotic vesicles. It is difficult to imagine how such a large number of vesicles can be precisely sorted and totally retrieved to the plasmalemma while stomatal closure and opening occurs repeatedly. In accordance with this, while Diekmann et al. (1993) had demonstrated endocytosis in shrunken protoplasts, they found that the endocytotic vesicles could not be retrieved to the plasmalemma in response to the protoplast swelling. In the present study, it was demonstrated that excretion and folding of the plasmalemma can be mechanisms for accommodating the alterations in guard cell volume during stomatal closure. More importantly, with the mechanisms demonstrated in the present study, the two issues above can be more reasonably addressed.
Folding of plasmalemma has long been thought to be impossible due to the presence of turgor pressure in guard cells. This opinion has actually overlooked the possible role of a structural basis in the regulation of the membrane turnover. The plasmalemma is not an isolated membrane; rather it is delicately connected with both cytoplasmic structures and the walls. During stomatal closure, outflow of water from guard cells is most likely to occur unevenly, and this would possibly give rise to the plasmalemma pulling away from the wall in a few specific locations. In accordance with this supposition, plasmolysis that occurs quite unevenly in guard cells has been observed (Figs 2, 3). The observed plasmolysis could not be an artificial result because in live cells, as evidenced by strong FDA fluorescence, the plasmolysis could also be seen (Fig. 6A, B) and, furthermore, the plasmolysed cells could restore their normal status as evidenced by the experiment using urea treatment (Fig. 6C, D). Plasmolysis has created the condition for plasmalemma excretion and folding to occur. Besides, stomatal movement is known to be a process driven by positive forces of guard cells themselves, which has provided a prerequisite for the plasmalemma folding occurring under turgor pressure. No matter how the vesicle excretion and plasmalemma folding occur, the delicate structural basis should be an important determinant. Interestingly, in this study, a distinct structure, the oval-shaped osmiophilic bodies, was found outside the plasmalemma. Labelling with a fluorescent styryl dye demonstrates that their chemical nature is somewhat like that of the plasmalemma. This is consistent with ultrastructural observations where it was shown that these osmiophilic bodies are tightly adhered to or even fused with the plasmalemma. Besides adhering to the plasmalemma, the osmiophilic bodies also appear to be tightly adhered to the cell walls, suggesting they may function to regulate the reaction between the plasmalemma and cell walls. Indeed, it was observed that incipient plasmolysis always started in dorsal walls where no osmiophilic bodies could be found (Figs 3A, 6B, C). Interestingly, in places where extensive membrane folding occurs, there exist more osmiophilic bodies.
Fig. 6.
Confocal images of stomata labelled by FAD. Guard cell pairs of stomata were labelled by FAD and the imaging was performed as described in the Materials and methods. (A) Open stoma. The red and yellow dotted lines in the magnified picture represent the locus of the plasmalemma and cell wall, respectively. (B) Closing stoma. Stomatal closure was induced by CO2 treatment as described in the Materials and methods. Note that incipient plasmolysis occurred, particularly, in the middle region of the dorsal wall (arrowheads). The red and yellow dotted lines in the magnified picture represent the locus of the plasmalemma and cell wall, respectively. (C) A guard cell treated by urea as described in the Materials and methods. Note that serious plasmolysis occurred in the middle region of the dorsal wall. (D) A guard cell showing deplasmolysis after urea treatment.
While the present study has demonstrated that plasmalemma excretion together with membrane folding is a critical mechanism for the accommodation of the alterations in cell volumes during stomatal movement, it should be noted that the phenomenon of endocytosis is not in question because some endocytotic structures (e.g. Fig. 3B) as well as plasmalemmasomes (e.g. Fig. 2D), a feature of endocytosis (Verbelen, 1979; Herman and Lamb, 1992), can occasionally be found in the present study. The problem is whether endocytosis plays a major role in plasmalemma turnover in intact guard cells under natural conditions. This isssue needs further clarification in future studies.
Acknowledgments
This work was supported by grants from National Crops Transgenic Special Grant (2009ZX08009-0738 and 2009ZX08003-009B), a grant from the National Natural Science Foundation (50939005), a grant from the Doctoral Fund of the Ministry of Education of China (200800190019), and a grant from Beijing Natural Science and Education Commission Foundation (KZ200910020001).
References
- Battey NH, James NC, Greenland AJ, Brownlee C. Exocytosis and endocytosis. The Plant Cell. 1999;11:643–659. doi: 10.1105/tpc.11.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick I, Thiel G, Homann U. Cytochalasin D attenuates the desensitisation of pressure-stimulated vesicle fusion in guard cell protoplasts. European Journal of Cell Biology. 2001;80:521–526. doi: 10.1078/0171-9335-00189. [DOI] [PubMed] [Google Scholar]
- Brumback AC, Lieber JL, Angleson JK, Betz WJ. Using FM1-43 to study neuropeptide granule dynamics and exocytosis. Methods. 2004;33:287–294. doi: 10.1016/j.ymeth.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Carroll AD, Moyen C, van Kesteren WJP, Tooke F, Battey NH, Brownlee C. Ca21, annexins, and GTP modulate exocytosis from maize root cap protoplasts. The Plant Cell. 1998;10:1267–1276. doi: 10.1105/tpc.10.8.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann W, Hedrich R, Raschke K, Robinson DG. Osmocytosis and vacuolar fragmentation in guard cell protoplasts: their relevance to osmotically-induced volume changes in guard cells. Journal of Experimental Botany. 1993;44:1569–1577. [Google Scholar]
- Franks PJ, Cowan IR, Faquhar GD. A study of stomatal mechanics using the cell pressure probe. Plant, Cell and Environment. 1998;21:94–100. [Google Scholar]
- Franks PJ, Cowan IR, Tyerman SD, Cleary AL, Lloyd J, Farqhuar GD. Guard cell pressure/aperture characteristics measured with the pressure probe. Plant, Cell and Environment. 1995;18:795–800. [Google Scholar]
- Gao XQ, Li CG, Wei PC, Zhang XY, Chen J, Wang XC. The dynamic changes of tonoplasts in guard cells are important for stomatal movement in Vicia faba. Plant Physiology. 2005;139:1207–1216. doi: 10.1104/pp.105.067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradmann D, Bobinson DG. Does turgor prevent endocytosis in plant cells? Plant, Cell and Environment. 1989;12:151–154. [Google Scholar]
- Herman EM, Lamb CJ. Arabinogalactan-rich glycoproteins are localized on the cell surface and in intravacuolar multivesicular bodies. Plant Physiology. 1992;98:264–272. doi: 10.1104/pp.98.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SHE. Clathrin and plant endocytosis. Traffic. 2002;3:614–620. doi: 10.1034/j.1600-0854.2002.30903.x. [DOI] [PubMed] [Google Scholar]
- Homann U. Fusion and fission of plasma-membrane material accommodates for osmotically induced changes in the surface area of guard-cell protoplasts. Planta. 1998;206:329–333. [Google Scholar]
- Homann U. Endocytosis in guard cells. In: Samaj J, Bluska F, Menzal D, editors. Plant endocytosis. Plant Cell Monographs. Vol. 1. Berlin: Springer; 2005. [Google Scholar]
- Homann U, Thiel G. Unitary exocytotic and endocytotic events in guard-cell protoplasts during osmotically driven volume changes. FEBS Letters. 1999;460:495–499. doi: 10.1016/s0014-5793(99)01396-4. [DOI] [PubMed] [Google Scholar]
- Kubitscheck U, Homann U, Thiel G. Osmotic evoked shrinking of guard cell protoplasts causes retrieval of plasma membrane into the cytoplasm. Planta. 2000;210:423–431. doi: 10.1007/PL00008151. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Schroeder JI, Verbelen JP. The influence of osmolarity on the surface properties of the plasma membrane of isolated guard cell protoplasts from Vicia faba L. Plant Physiology. 1992;11:25–32. [Google Scholar]
- Mathur J, Mathur N, Kernebeck B, Hülskamp M. Mutations in actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. The Plant Cell. 2003;15:1632–1645. doi: 10.1105/tpc.011676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckel T, Gall L, Semrau S, Homann U, Thiely G. Guard cells elongate: relationship of volume and surface area during stomatal movement. Biophysical Journal. 2007;92:1072–1080. doi: 10.1529/biophysj.106.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckel T, Hurst AC, Thiel G, Homann U. Guard cells undergo constitutive and pressure-driven membrane turnover. Protoplasma. 2005;226:23–29. doi: 10.1007/s00709-005-0106-6. [DOI] [PubMed] [Google Scholar]
- Meckel T, Hurst AC, Thiel G, Homann U. Endocytosis against high turgor: intact guard cells of Vicia faba consititutively endocytose fluorescently labeled plasma membrane and GFP-tagged K+-channel KAT1. The Plant Journal. 2004;39:182–193. doi: 10.1111/j.1365-313X.2004.02119.x. [DOI] [PubMed] [Google Scholar]
- Morris CE, Homann U. Cell surface area regulation and membrane tension. Journal of Membrane Biology. 2001;179:79–102. doi: 10.1007/s002320010040. [DOI] [PubMed] [Google Scholar]
- Raschke K. Movements of stomata. In: Haupt W, Feinleb ME, editors. Encyclopedia of plant physiology. Physiology of movements. Vol. 7. Berlin: Springer; 1979. pp. 382–441. [Google Scholar]
- Sano T, Kutsuna N, Hasezawa S, Tanaka Y. Membrane trafficking in guard cells during stomatal movement. Plant Signaling and Behavior. 2008;3:233–235. doi: 10.4161/psb.3.4.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope JC, DeWald DB, Mott KA. Changes in surface area of intact guard cells are correlated with membrane internalization. Plant Physiology. 2003;133:1314–1321. doi: 10.1104/pp.103.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stadelmann EJ, Wattendorff J. Plasmolysis and permeability of alpha-irradiated epidermal cells of Allium cepa L. Protoplasma. 1966;62:86–116. [Google Scholar]
- Thiel G, Battey N. Exocytosis in plants. Plant Molecular Biology. 1998;38:111–125. [PubMed] [Google Scholar]
- Thiel G, Rupnik M, Zorec R. Raising the cytosolic Ca2+ concentration increases the membrane capacitance of maize coleoptile protoplasts: evidence for Ca2+-stimulated exocytosis. Planta. 1994;195:305–308. [Google Scholar]
- Verbelen JP. A tubular type of plasmalemmasome in xylem of Phaseolus. Protoplasma. 1979;93:363–367. [Google Scholar]
- Wolfe J, Dowgert MF, Steponkus PL. Mechanical study of the deformation of the plasma membranes of protoplasts during osmotic expansions. Journal of Membrane Biology. 1986;93:63–74. [Google Scholar]
- Zorec R, Tester M. Cytoplasmic calcium stimulates exocytosis in a plant secretory cell. Biophysical Journal. 1992;63:864–867. doi: 10.1016/S0006-3495(92)81662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]