Abstract
The cytokines IL-10 and TGF-β regulate immunity and inflammation. IL-10 is known to suppress the extent of hepatic damage caused by parasite ova during natural infection with Schistosoma mansoni, but the role of TGF-β is less clear. Cytokine blockade studies in mice revealed that anti-IL-10R mAb treatment during acute infection modestly increased cytokine production and liver damage, whereas selective anti-TGF-β mAb treatment had marginal effects. In contrast, mice administered both mAbs developed severe hepatic inflammation, with enlarged, necrotic liver granulomas, cachexia, and >80% mortality by 8 wk postinfection, despite increased numbers of CD4+CD25+Foxp3+ T regulatory cells. Blocking both IL-10 and TGF-β at the onset of egg production also significantly increased IL-4, IL-6, TNF, IFN-γ, and IL-17 production and markedly increased hepatic, peritoneal, and splenic neutrophilia. In contrast, coadministration of anti-IL-10R and TGF-β mAbs had little effect upon parasite ova-induced intestinal pathology or development of alternatively activated macrophages, which are required to suppress intestinal pathology. This suggests that inflammation is controlled during acute S. mansoni infection by two distinct, organ-specific mechanisms: TGF-β and IL-10 redundantly suppress hepatic inflammation while intestinal inflammation is regulated by alternatively activated macrophages.
Although inflammation is fundamental to immune-mediated protection against most pathogens, counterregulatory mechanisms are required to limit collateral damage to host tissues (1, 2). In the case of schistosomiasis, a tropical disease affecting over 250 million persons worldwide, excessive inflammation and cytokine production is associated with severe immunopathology and mortality (3–5). Ova produced by these parasites are highly immunogenic and cause TH2 differentiation, granulomatous inflammation, and marked hepatic and intestinal fibrosis (6, 7). In mice, TH2-derived cytokines (IL-4, IL-5, and IL-13) can produce morbidity during the chronic phase of infection (16–20 )-wk postinoculation) but are less rapidly harmful than TH1 (IFN-γ or TH17 (IL-17)-derived cytokines, which can cause fatal disease during the acute phase (7–9 wk postinoculation) (8–11). Thus, mechanisms that restrict parasite egg-driven inflammation during schistosomiasis are critical for host survival.
We have previously demonstrated that alternatively activated macrophages, which are induced by IL-4 and IL-13, are required to regulate the intestinal inflammation that develops once Schistosoma mansoni worms begin to lay eggs (12). Chimeric mice that selectively fail to express IL-4R on bone marrow-derived cells and transgenic mice that selectively fail to express IL-4R on macrophages and neutrophils die of severe intestinal inflammation during the acute phase of infection (12, 13). Surprisingly, although hepatic inflammation is pronounced in both mice and humans infected with S. mansoni (14, 15), it was not increased to the same extent as intestinal inflammation in infected chimeric or transgenic mice (13). This led us to investigate whether an unrelated mechanism is primarily responsible for regulating hepatic inflammation.
Our studies evaluated two cytokines, IL-10 and TGF-β, that are both well known for their immunosuppressive properties; each can independently regulate the differentiation, proliferation, and activation of immune and nonimmune cells (2, 16–18). Both cytokines are produced in increased quantity during S. mansoni infection of humans, nonhuman primates, and mice (19, 20). IL-10 has been well studied in murine models of S. mansoni infection and has been shown to control excessive TH1 or TH2 responses, suppress macrophage and dendritic cell activation, and limit worm ova-induced hepatotoxicity during the acute phase of infection (9, 21, 22). In contrast, although macrophages and T cells produce TGF-β during parasitic worm infections and this cytokine is known to suppress Ag-specific immunity and promote the differentiation and suppressive function of CD4+CD25+Foxp3+ T regulatory cells (Tregs),3 TGF-β has not been shown to suppress S. mansoni-induced inflammation (23–25).
We now demonstrate that IL-10 and TGF-β redundantly promote host survival by suppressing proinflammatory cytokine production and liver injury during acute schistosomiasis. Although treatment of S. mansoni-infected mice with anti-TGF-β mAb induces only marginal changes, the combination of anti-TGF-β and anti-IL-10R mAbs profoundly increases cytokine production, hepatic inflammation, and mortality without having a major effect on alternatively activated macrophages or intestinal pathology.
Materials and Methods
Mice
Male BALB/c mice were purchased from Taconic. All mouse experiments were approved by the Institutional Animal Care and Use Committee at the Cincinnati Veterans Affairs Medical Center.
mAbs for neutralization and flow cytometry
Anti-IL-10R receptor (1B1.3), anti-TGF-β (1D11 16.8), and isotype control mAb (GL113) (IgG1) were produced in Pristane-primed (Acros Organic) athymic nude mice as ascites and purified by means of ammonium sulfate precipitation and diethyl-amino-ethyl-cellulose column chromatography. Endotoxin values of all mAb were determined to be <200 pg/ml (13). Spleen and PBMC were stained with fluorochrome-labeled rat mAbs to mouse CD11b (M1/70) (26), Gr-1 (RB6-8C5) (27), CD4 (RM4-5), CD25 (PC-61), and Foxp3 (FJK-16s).
Infection of mice with S. mansoni
Mice were anesthetized and percutaneously infected with 50–70 S. mansoni cercariae as previously described (12). Parasites were provided by the National Institute of Allergy and Infectious Diseases Schistosomiasis Resource Center at the Biomedical Research Institute, through National Institute of Allergy and Infectious Diseases Contract N0-AI-30026.
Flow cytometry
Splenocytes were isolated washed in FACS buffer (HBSS, 1% FBS, and 0.2% sodium azide) and incubated with anti-FcγRII/RIII mAb (2.4G2). Cells were then stained with the mAbs described and analyzed with a BD FACSCalibur and Cellquest software.
Evaluation of cytokine production and morbidity
IL-17A levels were measured by ELISA (eBioscience). IL-4, TNF, IL-6, IL-10, and IFN-γ were measured by in vivo cytokine capture assay (IVCCA) (28). A TGF-β1 ELISA kit was obtained from R&D Systems. Serum AST concentration was measured by the Veterans Affairs Medical Center clinical pathology laboratory. Serum LPS concentration was measured as described (13).
S. mansoni granuloma measurement
Histologically processed sections of liver tissue (5 μm) were stained with H&E or Masson’s trichrome. Individual granuloma areas were measured on coded slides; only granulomas that possessed a central egg were evaluated. Quantitation of area was performed using a SPT Diagnostics imaging system and Simple PCI C-Imaging systems software. Data shown are mean ± SE of 150 granulomas per group from two independent experiments.
Real-time PCR
RNA was obtained from hepatic tissue, DNase I-treated, and cDNA was generated using SuperScript II Reverse Transcriptase (Invitrogen). Real-time PCR was conducted on a Gene Amp 7500 instrument (PE Biosystems) with the Syber Green detection reagent. Cycle threshold values for Arginase I and Relm-α (FIZZ-1) were determined and fold induction was compared with vimentin using the 1/Δcycle threshold method (12).
Vimentin For: 5′-TGA CCG GCT TGT ATG CTA TC-3′
Vimentin Rev: 5′-CAG TGT GAG CCA GGA TAT AG-3′
Arginase I For: 5′-CAG AAG AAT GGA AGA GTC AG-3′
Arginase I Rev: 5′-CAG ATA TGC AGG GAG TCA CC-3′
Relm-α/Fizz-1 For: 5′-AGA TGG GCC TCC TGC CCT GCT GGG-3′
Relm-α/Fizz-1 Rev: 5′-ACC TGG TGA CGG GCG ACG ACG GTT-3′
Determination of hydroxyproline content
Hepatic collagen content was measured as hydroxyproline concentration (12, 29).
Statistical analysis
Statistical significance was assessed by either one-tailed Student’s t test (two groups) or ANOVA for multiple groups and a post hoc Bonferroni’s test to determine significance, all performed using Prism GraphPad software.
Results
IL-10 and TGF-β production are induced by worm eggs and promote host survival during acute S. mansoni infection
The inflammatory response to worm ova during murine schistosomiasis can be separated into acute (6–9 wk) and chronic (<13 wk) phases (15). Because chronic schistosomiasis is characterized by T cell anergy and reduced production of inflammatory cytokines (30, 31), we evaluated whether IL-10 and TGF-β levels increase as the disease progresses toward chronicity. IL-10 production, as detected by IVCCA, changed from undetectable levels in serum at 5 wk postinoculation to 3 ± 1 ng/ml by 7.5 wk and 9 ± 2 ng/ml by 13 wk postinfection (Fig. 1A). TGF-β serum levels increased 3-fold between 5 and 7.5 wk postinoculation and plateaued afterward (Fig. 1B).
FIGURE 1.
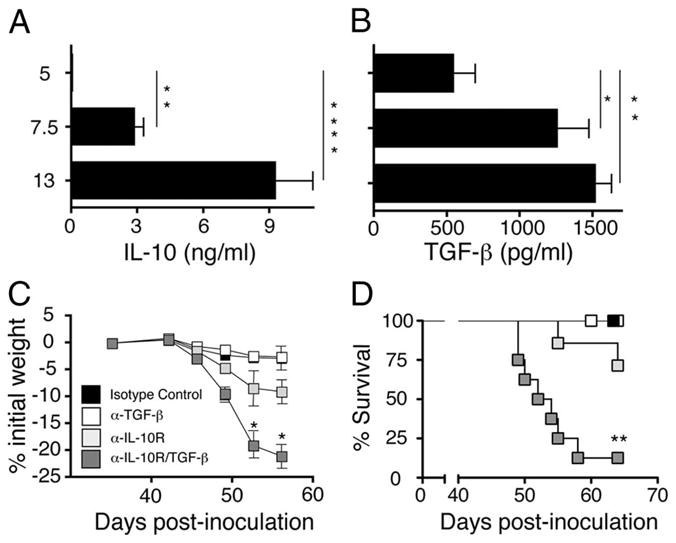
IL-10 and TGF-β redundantly protect against lethal schistosomiasis. Serum levels of IL-10 (A) and TGF-β (B) determined by IVCCA and ELISA, respectively, at the indicated time points in mice infected with 60–70 S. mansoni cercariae. Means ± SE of 8–10 mice/group. Experiment performed twice. Weight change (C) and survival kinetics (D) of BALB/c mice infected with 60–70 S. mansoni cercariae were injected i.p on days 38, 42, and 46 with 1 mg of anti-TGF-β mAb (clone 1D11), 1 mg of anti-IL-10 receptor mAb (1B1.3), 1 mg each of both mAbs, or 2 mg of an isotype control mAb (clone J1.2). Data shown as means ± SE of eight mice/group. Experiment performed four times (*, p < 0.05; **, p < 0.01; and ***, p < 0.0001 compared with isotype control treated group). Significance determined by ANOVA.
Ab blockade of IL-10 and/or TGF-β was performed to determine whether these cytokines are host-protective during the acute phase of infection. Treatment of S. mansoni-inoculated mice with anti-IL-10R mAb caused a significant increase in weight loss (Fig. 1C) and a trend toward increased mortality that was not statistically significant ( p < 0.068) (Fig. 1D), while anti-TGF-β mAb, by itself, had little effect. Treatment of worm-inoculated mice with both anti-mouse IL-10R and TGF-β mAbs (α-IL-10R/α-TGF-β) caused more than twice the weight loss induced by anti-IL-10 mAb alone and increased mortality to greater than 80% by 9 wk postinoculation. In contrast, treatment of uninoculated mice with α-IL-10R, α-TGF-β, or the combination of α-IL-10R/α-TGF-β mAbs did not cause significant weight loss (data not shown).
Combined blockade of IL-10 and TGF-β exacerbates the acute inflammatory response
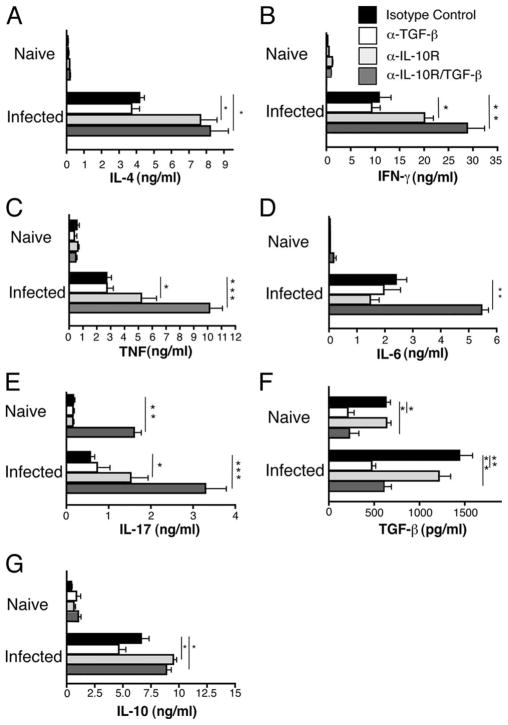
Because cytokine perturbations are associated with increased mortality during schistosomiasis (9), we used the IVCCA to evaluate whether neutralization of IL-10 and TGF-β modifies proinflammatory cytokine production (28). At 7.3 wk postinoculation, treatment of S. mansoni-inoculated mice with α-TGF-β mAb had little or no effect on IL-4, IL-6, IL-17, IFN-γ, or TNF production, whereas anti-IL-10R mAb significantly increased secretion of IL-4, IFN-γ, TNF, and IL-17 and combined α-IL-10R/α-TGF-β treatment caused significantly greater increases in IFN-γ, TNF, and IL-17 secretion and a large, significant increase in IL-6 secretion (Fig. 2). As expected, α-TGF-β mAb treatment reduced TGF-β serum levels and anti-IL-10R mAb treatment increased serum levels of IL-10.
FIGURE 2.
Blocking IL-10 and TGF-β causes increased levels of cytokines associated with TH1, TH2, and TH17 polarization. Production of (A) IL-4, (B) IFN-γ, (C) TNF, (D) IL-6 (G), IL-10 (measured by IVCCA), (E) IL-17, and (F) TGF-β1 (measured by ELISA) in sera from naive or S. mansoni-inoculated BALB/c mice on day 52 postinfection. Animals were injected i.p. on days 38, 42, and 46 with 1 mg of anti-TGF-β (clone 1D11), 1 mg of anti-IL-10 receptor (1B1.3), 1 mg of both anti-TGF-β/anti-IL-10R, or 2 mg of isotype control (clone J1.2). Means ± SE of six to eight mice/group. Experiment performed three times (*, p < 0.05; **, p < 0.01, and ***, p < 0.0001 compared with isotype control treated group).
TGF-β neutralization increases the effects of IL-10 blockade on hepatic granuloma size, liver injury, and neutrophil accumulation
In most inbred mouse strains, S. mansoni liver granulomas are well-circumscribed, fibrotic lesions that contain lymphocytes, eosinophils, and macrophages (15). Granuloma size was increased by IL-10 blockade and even more by combined blockade of IL-10 and TGF-β (Fig. 3A). Hepatocyte injury, as indicated by serum levels of aspartate transaminase (AST), was also increased in S. mansoni-inoculated mice to some extent by blockade of IL-10 and to a considerably greater extent by combined IL-10/TGF-β blockade (Fig. 3B). In contrast, liver fibrosis, while induced by S. mansoni infection, was not significantly affected by IL-10/TGF-β blockade (Fig. 3C).
FIGURE 3.
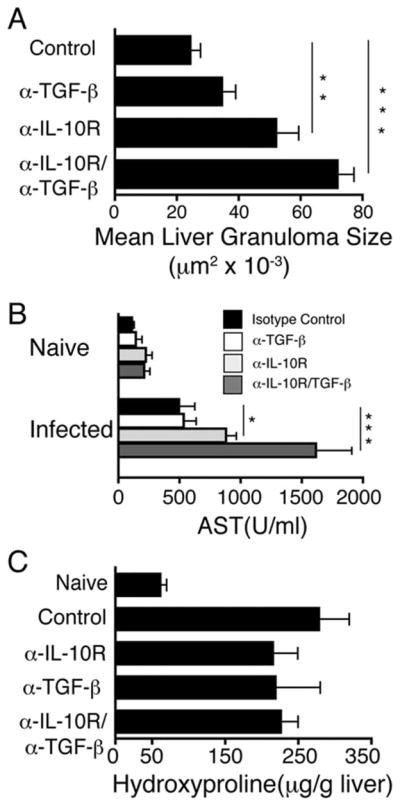
Blocking both IL-10 and TGF-β exacerbates hepatocellular damage and granulomatous pathology, but does not affect fibrosis. A, Liver granuloma cross-sectional area was determined 52 days postinoculation. 300 granulomas were measured per group. B, Serum levels of AST in naive or S. mansoni infected mice 52 days postinfection. C, Liver hydroxyproline levels 52 days postinfection. Means ± SE of six to eight mice/group. Experiments performed three times with similar results (*, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with isotype control-treated group).
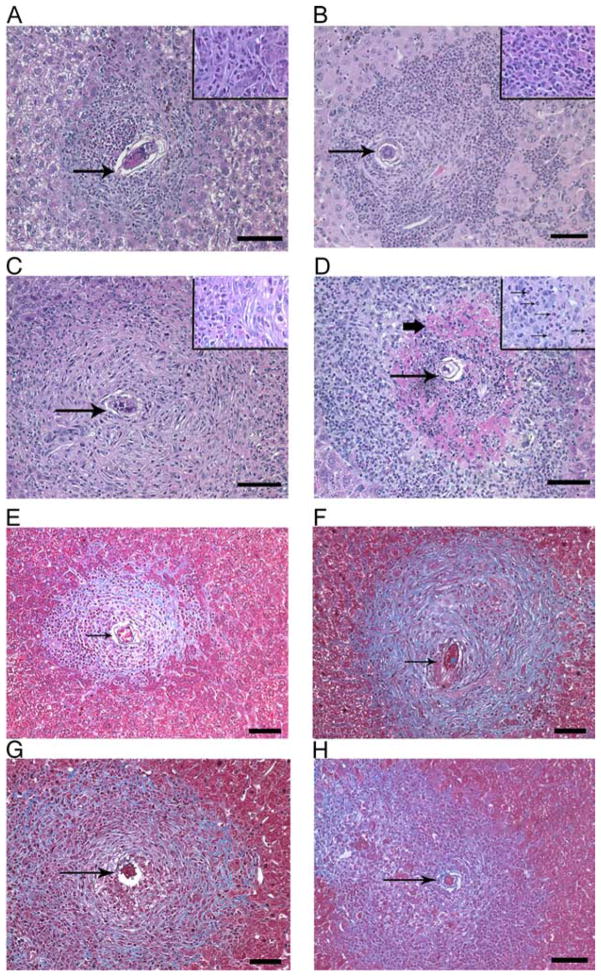
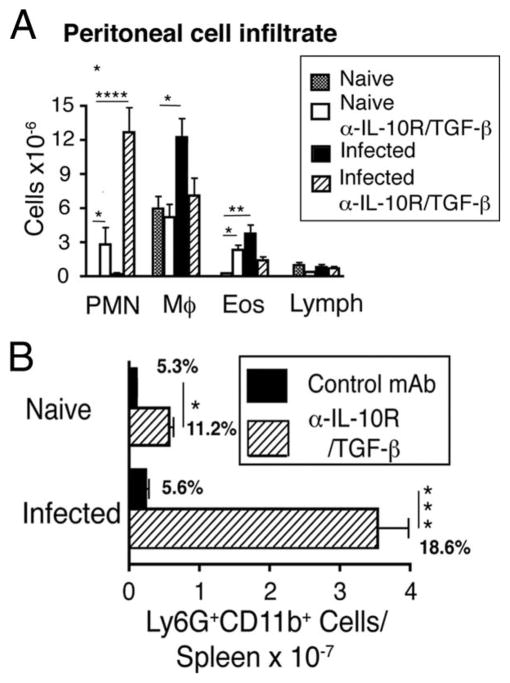
These observations were consistent with analysis of hepatic granuloma morphology in S. mansoni-inoculated mice (Fig. 4), which demonstrated severe necrosis of hepatocytes within granulomas only in mice that received both mAbs (Fig. 4D) and no increase in hepatic fibrosis beyond that induced by S. mansoni itself (Fig. 4, E–H). Although IL-10/TGF-β blockade did not increase hepatic inflammation or neutrophil number in naive mice (data not shown), tissue sections showed a remarkable increase in neutrophil infiltration at the margin of granulomas in α-IL-10R/α-TGF-β mAb-treated, S. mansoni-infected mice (Fig. 4D, inset). Consistent with this, numbers of neutrophils (CD11b+/Gr-1bright cells) were dramatically elevated in both the peritoneal cavity (Fig. 5A) and spleen (Fig. 5B) of these infected mice and smaller increases in peritoneal neutrophil number were observed in uninfected, α-IL-10R/α-TGF-β mAb-treated mice.
FIGURE 4.
Blocking both IL-10 and TGF-β causes hepatic necrosis. Liver sections from S. mansoni-inoculated mice on day 52 postinfection following treatment with isotype control (A and E), anti-TGF-β (B and F), anti-IL-10R (C and G), or anti-TGF-β/anti-IL-10R (D and H) Ab. Thin arrows point to parasite ova. Bold arrow points to area of necrosis. Representative photos are shown. A–D, H&E-stained sections. ×100 magnification (large panel) 600× (inset) Scale bar 100 μm. E–H, Masson’s trichrome-stained sections at a ×200 magnification. Staining for collagen (blue) and hepatocytes (reddish/purple). Scale bar 50 μm.
FIGURE 5.
Blocking IL-10 and TGF-β during acute schistosomiasis causes profound neutrophilia in peritoneal cavity and spleen. A, Inflammatory cells from peritoneal lavage from naive or S. mansoni-inoculated mice (day 52) treated with 1 mg each of anti-IL-10R/TGF-β mAbs or left untreated. B, Spleen cells from naive or S. mansoni-inoculated mice (day 52) were stained for Ly6G/C (GR-1+) and Mac-1 (CD11b+) following injection with 1 mg of anti-IL-10R/TGF-β or control mAbs. Means ± SE of total numbers per spleen from four mice/group. Percent gated population shown on graph. Experiments performed twice with similar results (*, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001 compared with isotype control treated group).
Anti-IL-10R/anti-TGF-β mAb treatment does not exacerbate gut immunopathology or block alternative macrophage activation
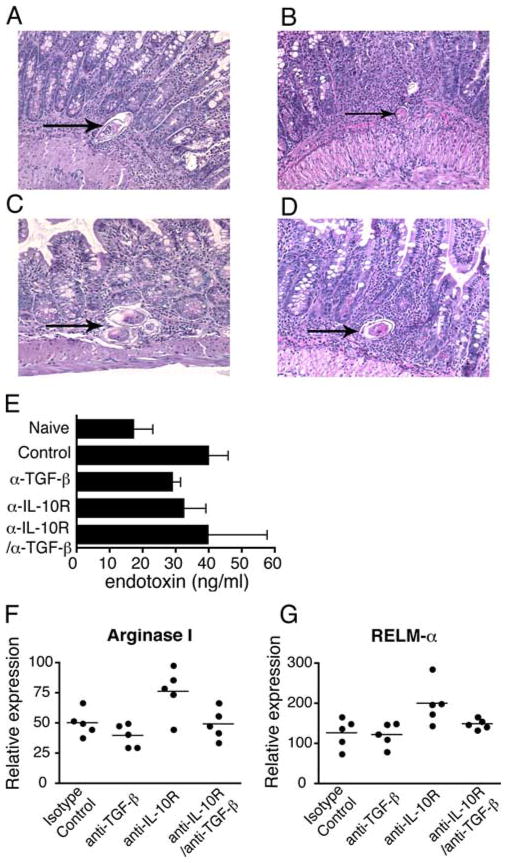
The absence of IL-4/IL-13-responsive macrophages during S. mansoni infection causes weight loss and death by increasing endotoxemia and gut pathology (12, 13). Consequently, we evaluated whether α-IL-10R/α-TGF-β treatment had similar effects. Contrary to our expectation, we observed no exacerbated gut injury or dysregulation of intestinal granuloma architecture in S. mansoni-inoculated, α-IL-10R/α-TGF-β mAb-treated mice (Fig. 6, A–D) or increase in serum levels of endotoxin (Fig. 6E). Consistent with this, alternative macrophage activation was unaffected by IL-10/TGF-β blockade, as shown by a lack of effect on intestinal mRNA transcripts for the alternatively activated macrophage proteins arginase I (Fig. 6F) and RELMα (FIZZ-1) (Fig. 6G), although anti-TGF-β mAb seemed to suppress the small increases in Arginase I and RELM-α expression that were induced by anti-IL-10R mAb (32).
FIGURE 6.
Blocking IL-10 and TGF-β does not affect intestinal pathology, alternative macrophage activation, or mucosal integrity. H&E staining of ileal tissue sections from S. mansoni infected mice on d 52 postinfection following treatment with isotype control (A), anti-TGF-β (B), anti-IL-10R (C), or anti-TGF-β/anti-IL-10R (D) mAb. Magnification 200×. Arrows point to parasite ova. Measurement of serum endotoxin levels on day 52 postinfection (E). Real-time PCR quantification of gut mRNA transcripts for (F) Arginase I and (G) RELM-α. Means ± SE of five mice/group. Experiment performed twice.
Treg generation is unaffected by IL-10/TGF-β blockade
Both IL-10 and TGF-β have been reported to promote the generation of Tregs (33, 34). Consequently, it seemed possible that blockade of both cytokines might increase immunopathology indirectly by suppressing Treg generation. Indeed, CD4+CD25+Foxp3+ cells have been linked with down-modulation of both TH1 and TH2 cell expansion during acute murine schistosomiasis (35, 36). Surprisingly, however, combined IL-10/TGF-β blockade markedly increased numbers of both regulatory (CD4+CD25+Foxp3+) (Fig. 7A) and effector (CD4+CD25+Foxp3−) (Fig. 7B) T cells in S. mansoni-inoculated mouse spleen.
FIGURE 7.
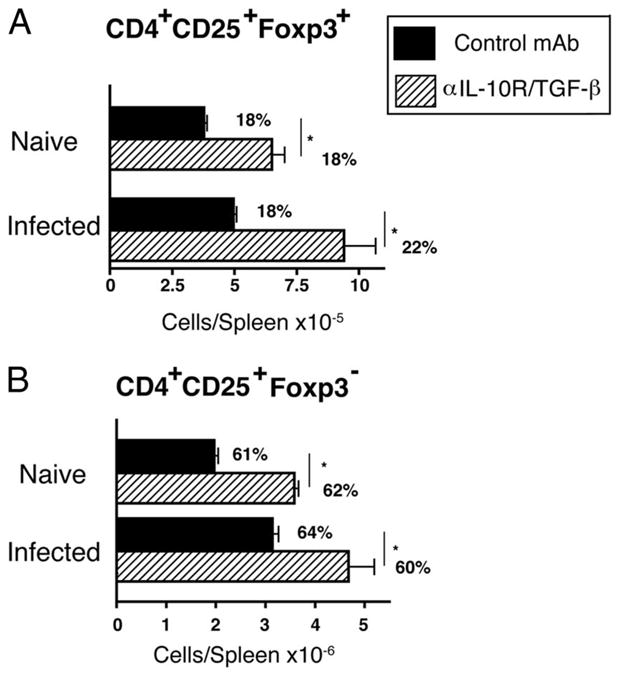
Blocking IL-10 and TGF-β during acute schistosomiasis infection increases Treg number. Spleen cells from naive or S. mansoni-inoculated mice (day 52) that received 2 mg of GL113 or were treated with 1 mg each of anti-IL-10R/TGF-β mAbs were stained with fluorochrome-labeled mAbs for CD4 (RM4-5), CD25 (PC-61), and Foxp3 (FJK-16s) and analyzed by flow cytometry for number of CD4+CD25+Foxp3+ cells. Percent of gated CD4+ cell population shown on graph. Means ± SE of four mice/group. Experiments were performed twice (A and B) with similar results (*, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with isotype control-treated group).
Discussion
This work demonstrates that TGF-β redundantly controls hepatic immunopathology and promotes survival of hosts infected with the parasitic worm S. mansoni. Although several reports have demonstrated that IL-10 limits inflammation in murine schistosomiasis (22, 30, 37), the potential role in this disease of TGF-β, a second cytokine that can limit inflammation, has not previously been explored. Our studies confirm that anti-IL-10R mAb modestly increases weight loss and appears to increase mortality in S. mansoni-inoculated mice during the acute phase of infection, but show that anti-TGF-β mAb, by itself, has little effect. Administration of anti-IL-10R mAb, but not anti-TGF-β mAb also significantly increased IL-4, IFN-γ, TNF, and IL-17 production 7.3 wk postinfection and increased liver granuloma size and exacerbated hepatocellular injury (increased serum AST). Although anti-TGF-β mAb, by itself, had little effect on S. mansoni-induced inflammation, it greatly exacerbated inflammation and mortality in anti-IL-10R mAb-treated, S. mansoni-inoculated mice. Mice administered both anti-IL-10 receptor and anti-TGF-β mAbs from 5 to 6.5 wk postinoculation developed increased TH1, TH2, and TH17 cytokine production, neutrophilia, and marked necrosis within hepatic granulomas by 7.5 wk postinoculation. These mice lost 15–20% of their body weight and >80% died by 8 wk. This demonstrates a large degree of redundancy between IL-10 and TGF-β in limiting hepatic inflammation during S. mansoni infection. This redundancy cannot be explained by a compensatory increase in TGF-β production in the absence of IL-10 because S. mansoni-infected mice treated with anti-IL-10R mAb had slightly lower levels of TGF-β and vice versa.
There is precedent for the redundant effects of IL-10 and TGF-β in limiting inflammation (38–42). Treatment of IL-10KO mice with anti-TGF-β mAb during Trichinella spiralis infection greatly exacerbates inflammatory cell recruitment to the site of parasite invasion (43). IL-10 redundancy with other cytokines is also established: S. mansoni causes much greater inflammation and mortality in the absence of IL-4 and IL-10 or IL-12 and IL-10 than when only one of these cytokines is absent (9). Even in naive mice, combined neutralization of IL-10 and TGF-β substantially increased numbers of peritoneal (but not liver) neutrophils. This may have predisposed these animals to develop lethal, neutrophilic hepatic inflammation following S. mansoni infection.
Although combined anti-IL-10/anti-TGF-β mAb treatment greatly exacerbates S. mansoni-induced inflammation in the liver, it has little effect on the intestine, the other organ that is a major site of ova-induced damage during infection with this parasite (12). In contrast, intestinal inflammation is markedly increased in the absence of IL-4Rα, a necessary component of the receptors for IL-4 and IL-13 (44). IL-4Rα-mediated limitation of intestinal inflammation depends to a great extent on alternative activation of macrophages by these cytokines (12). Thus, S. mansoni-induced inflammation is limited in the two major target organs by independent mechanisms: macrophage activation by IL-4 and/or IL-13 limits intestinal inflammation, whereas effects of IL-10 and TGF-β prevent hepatic necrosis.
The mechanism by which IL-10 and TGF-β limit hepatic inflammation is not clear and may be multifactorial. However, the remarkable increase in IL-17 production, previously observed links between increased IL-17 production and lethality during murine schistosomiasis, and the considerable, presumably IL-17-related hepatic infiltration of neutrophils in anti-IL-10R/TGF-β mAb-treated, S. mansoni-inoculated mice suggest that IL-17 may contribute importantly to hepatocellular damage when neither IL-10 nor TGF-β is available to limit inflammation (10, 45). The increased production of IL-17 in the absence of IL-10 and TGF-β, even in uninfected mice, is remarkable because TGF-β is thought to be required to stimulate naive T cells to differentiate into IL-17-secreting TH17 cells and to promote TH17 cell survival in the mouse (46, 47). Our data suggest that increased TGF-β production over baseline is not essential to induce and maintain TH17 cells and even inhibits this population, at least in the combined absence of IL-10 and the presence of a strong inflammatory stimulus. In-deed, IL-6 secretion promotes TH17 differentiation (48), and production of this cytokine was significantly increased following neutralization of IL-10 and TGF-β in naive and S. manson-infected mice. Alternatively, our anti-TGF-β mAb may efficiently suppress paracrine effects of this cytokine but not autocrine effects. Consistent with this hypothesis, the TGF-β that promotes the differentiation of naive CD4+ T cells into TH17 cells is produced by the CD4+ T cells themselves (16).
The same considerations hold for the surprising stimulatory effect of anti-TGF-β mAb on the expansion of Tregs in S. mansoni-infected mice. TGF-β is thought to be required for the differentiation of Tregs as well as TH17 cells (the combination of TGF-β and IL-6 promotes TH17 differentiation, whereas TGF-β in the absence of IL-6 induces Tregs) (46, 48). Expansion of the Treg population might, in theory, keep inflammation from becoming even worse in S. mansoni-inoculated mice that have been treated with anti-IL-10 and anti-TGF-β mAbs; however, we and others (49) have found that Treg depletion with anti-CD25 mAb has little effect on survival or immunopathology in S. mansoni-inoculated mice, regardless of whether they have been treated with anti-IL-10R/anti-TGF-β mAb (data not shown).
In sum, our observations demonstrate a partial redundancy between two anti-inflammatory cytokines during S. mansoni infection, suggest that cytokine control of TH17 and Treg differentiation may be more complex than has been previously thought, and show that separate anti-inflammatory mechanisms control inflammation in different organs. These observations have considerable practical importance, inasmuch as both anti-IL-10 and anti-TGF-β mAbs are being evaluated for the treatment of human disease (50, 51).
Acknowledgments
We thank Ariel Munitz with assistance with TGF-β1 ELISA.
Footnotes
This work was supported by the US Department of Veterans Affairs and National Institutes of Health Grants R01GM083204-01 and R01 AI052099.
Abbreviations used in this paper: Treg, T regulatory cell; IVCCA, in vivo cytokine capture assay; AST, aspartate transaminase.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Wan YY, Flavell RA. “Yin-Yang” functions of transforming growth factor-β and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunne DW, Pearce EJ. Immunology of hepatosplenic schistosomiasis mansoni: a human perspective. Microbes Infect. 1999;1:553–560. doi: 10.1016/s1286-4579(99)80095-1. [DOI] [PubMed] [Google Scholar]
- 4.Abath FG, Morais CN, Montenegro CE, Wynn TA, Montenegro SM. Immunopathogenic mechanisms in schistosomiasis: what can be learnt from human studies? Trends Parasitol. 2006;22:85–91. doi: 10.1016/j.pt.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Stadecker MJ. The shrinking schistosomal egg granuloma: how accessory cells control T cell-mediated pathology. Exp Parasitol. 1994;79:198–201. doi: 10.1006/expr.1994.1080. [DOI] [PubMed] [Google Scholar]
- 6.de Jesus AR, Magalhaes A, Miranda DG, Miranda RG, Araujo MI, de Jesus AA, Silva A, Santana LB, Pearce E, Carvalho EM. Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection. Infect Immun. 2004;72:3391–3397. doi: 10.1128/IAI.72.6.3391-3397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34:273–282. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- 8.La Flamme AC, Patton EA, Bauman B, Pearce EJ. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol. 2001;166:1903–1911. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 10.Rutitzky LI, Stadecker MJ. CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):327–330. doi: 10.1590/s0074-02762006000900052. [DOI] [PubMed] [Google Scholar]
- 11.Stadecker MJ, Asahi H, Finger E, Hernandez HJ, Rutitzky LI, Sun J. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev. 2004;201:168–179. doi: 10.1111/j.0105-2896.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 12.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 13.Herbert DR, Orekov T, Perkins C, Rothenberg ME, Finkelman FD. IL-4R α expression by bone marrow-derived cells is necessary and sufficient for host protection against acute schistosomiasis. J Immunol. 2008;180:4948–4955. doi: 10.4049/jimmunol.180.7.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today. 2000;21:465–466. doi: 10.1016/s0167-5699(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 15.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 16.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Wan YY, Flavell RA. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev. 2006;212:114–130. doi: 10.1111/j.0105-2896.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 18.Kriegel MA, Li MO, Sanjabi S, Wan YY, Flavell RA. Transforming growth factor-β: recent advances on its role in immune tolerance. Curr Rheumatol Rep. 2006;8:138–144. doi: 10.1007/s11926-006-0054-y. [DOI] [PubMed] [Google Scholar]
- 19.Farah IO, Mola PW, Kariuki TM, Nyindo M, Blanton RE, King CL. Repeated exposure induces periportal fibrosis in Schistosoma mansoni-infected baboons: role of TGF-β and IL-4. J Immunol. 2000;164:5337–5343. doi: 10.4049/jimmunol.164.10.5337. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez HJ, Rutitzky LI, Lebens M, Holmgren J, Stadecker MJ. Diminished immunopathology in Schistosoma mansoni infection following intranasal administration of cholera toxin B-immunodominant peptide conjugate correlates with enhanced transforming growth factor-β production by CD4 T cells. Parasite Immunol. 2002;24:423–427. doi: 10.1046/j.1365-3024.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 21.Oswald IP, Wynn TA, Sher A, James SL. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor α required as a costimulatory factor for interferon γ-induced activation. Proc Natl Acad Sci USA. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 23.Ince MN, Elliott DE, Setiawan T, Blum A, Metwali A, Wang Y, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus induces TLR4 on murine mucosal T cells that produce TGFβ after lipopolysaccharide stimulation. J Immunol. 2006;176:726–729. doi: 10.4049/jimmunol.176.2.726. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda E, Yoshida Y, En Shan B, Yamashita U. Suppression of macrophage interleukin-12 and tumour necrosis factor-α production in mice infected with Toxocara canis. Parasite Immunol. 2001;23:305–311. doi: 10.1046/j.1365-3024.2001.00387.x. [DOI] [PubMed] [Google Scholar]
- 25.Omer FM, Kurtzhals JA, Riley EM. Maintaining the immunological balance in parasitic infections: a role for TGF-β? Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 26.Corbi AL, Kishimoto TK, Miller LJ, Springer TA. The human leukocyte adhesion glycoprotein Mac-1 (complement receptor type 3, CD11b) α subunit: cloning, primary structure, and relation to the integrins, von Willebrand factor and factor B. J Biol Chem. 1988;263:12403–12411. [PubMed] [Google Scholar]
- 27.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6 – 8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 28.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Current Protocols in Immunolgy. Unit 6.28. Chapter 6. John Wiley and Sons; New York: 2003. [DOI] [PubMed] [Google Scholar]
- 29.Leeto M, Herbert DR, Marillier R, Schwegmann A, Fick L, Brombacher F. TH1-dominant granulomatous pathology does not inhibit fibrosis or cause lethality during murine schistosomiasis. Am J Pathol. 2006;169:1701–1712. doi: 10.2353/ajpath.2006.060346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadler CH, Rutitzky LI, Stadecker MJ, Wilson RA. IL-10 is crucial for the transition from acute to chronic disease state during infection of mice with Schistosoma mansoni. Eur J Immunol. 2003;33:880–888. doi: 10.1002/eji.200323501. [DOI] [PubMed] [Google Scholar]
- 31.Stadecker MJ, Kamisato JK, Chikunguwo SM. Induction of T helper cell unresponsiveness to antigen by macrophages from schistosomal egg granulomas: a basis for immunomodulation in schistosomiasis? J Immunol. 1990;145:2697–2700. [PubMed] [Google Scholar]
- 32.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, Thomson AW. Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868–5877. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 35.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 36.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 37.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr, Wynn TA. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol. 2002;168:2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 38.Anderson CF, Lira R, Kamhawi S, Belkaid Y, Wynn TA, Sacks D. IL-10 and TGF-β control the establishment of persistent and transmissible infections produced by Leishmania tropica in C57BL/6 mice. J Immunol. 2008;180:4090–4097. doi: 10.4049/jimmunol.180.6.4090. [DOI] [PubMed] [Google Scholar]
- 39.Oswald IP, Gazzinelli RT, Sher A, James SL. IL-10 synergizes with IL-4 and transforming growth factor-β to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 40.Toms C, Powrie F. Control of intestinal inflammation by regulatory T cells. Microbes Infect. 2001;3:929–935. doi: 10.1016/s1286-4579(01)01454-x. [DOI] [PubMed] [Google Scholar]
- 41.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-β and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 42.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-β -bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 43.Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-β. J Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- 44.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci USA. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 47.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 49.Walsh CM, Smith P, Fallon PG. Role for CTLA-4 but not CD25+ T cells during Schistosoma mansoni infection of mice. Parasite Immunol. 2007;29:293–308. doi: 10.1111/j.1365-3024.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 50.Seoane J. The TGFβ pathway as a therapeutic target in cancer. Clin Transl Oncol. 2008;10:14–19. doi: 10.1007/s12094-008-0148-2. [DOI] [PubMed] [Google Scholar]
- 51.Llorente L, Richaud-Patin Y, Garcia-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, Alcocer-Varela J, Grangeot-Keros L, Alarcon-Segovia D, Wijdenes J, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]