Abstract
The C-type Allatostatins (C-ASTs) are a family of highly pleiotropic arthropod neuropeptides. In crustaceans, transcriptomic/mass spectral studies have identified C-ASTs in the nervous systems of many species; the cellular distributions of these peptides remain unknown. Here, the distribution of C-AST was mapped in the nervous system of the copepod Calanus finmarchicus, the major contributor to the North Atlantic’s zooplanktonic biomass; C-AST-immunopositive neurons were identified in the protocerebrum, in several peripheral ganglia associated with feeding appendages, and in the ganglia controlling the swimming legs, with immunopositive axons present throughout the ventral nerve cord. In addition, axons innervating the dorsal longitudinal and ventral longitudinal muscles of the body wall of the metasome were labeled by the C-AST antibody. While the distribution of C-AST-like immunoreactivity was similar between sexes, several differences were noted, i.e. two pair of somata located at the deutocerebral/tritocerbral border in males and immunopositive fibers that surround the genital opening in females. To place the C-AST-like labeling into context with those of several previously mapped peptides, i.e. A-type allatostatin (A-AST) and tachykinin-related peptide (TRP), we conducted double-labeling studies; the C-AST-like immunopositive neurons appear distinct from those expressing either A-AST or TRP (and through extrapolation, pigment dispersing hormone). Collectively, our data represent the first mapping of C-AST in crustacean neural tissue, show that sex-specific differences in the distribution of C-AST exist in the C. finmarchicus CNS, and suggest that the peptide may be involved in the modulation of both feeding and postural control/locomotion.
Keywords: Calanus finmarchicus, copepod, C-type allatostatin, Manduca sexta-type allatostatin, PISCF-type allatostatin, neuropeptide, neurohormone
1. Introduction
Modulation of physiology and behavior is a major contributor to an organism’s ability to adapt to environmental and anthropogenic challenges. In most animals, peptides represent the largest and most diverse class of these signaling agents (Kastin, 2006). In decapod crustaceans, numerous studies have focused on elucidating the complements of and physiological roles served by native peptides (e.g. Fu et al., 2005; Fu et al., 2007; Stemmler et al., 2007; Dickinson et al., 2008; Ma et al., 2008; Dickinson et al., 2009; Ma et al., 2009a; Ma et al., 2009b; Stevens et al., 2009; Christie et al., 2010a; Ma et al., 2010). In contrast, relatively little is known about the identity or functional roles served by peptide modulators in members of the lower crustacean taxa (Christie et al., 2008; Sousa et al., 2008; Gard et al., 2009; Christie et al., 2010b), this despite the fact that these animals function as keystone species in many aquatic ecosystems (e.g. Martin-Creuzburg et al., 2005; Hill et al., 2006; Ducklow et al., 2007; Smith et al., 2007; Wagner and Benndorf, 2007; Provan et al., 2009).
Calanus finmarchicus is a copepod crustacean that serves as the primary contributor to the zooplanktonic biomass of the North Atlantic. Given its abundance and trophic position, this species is of extreme importance to the continued existence of many important fisheries, as well as to the survival of many critically endangered marine mammals, e.g. the North Atlantic right whale (e.g. Stone et al., 1988; Murison and Gaskins, 1989; Woodley and Gaskins, 1996). While much work has focused understanding the basic biology, life history and ecology of C. finmarchicus (e.g. Wishner et al., 1995; Tande and Miller, 1996; Wiebe et al., 2001), little is known about peptidergic control in this species; at present only three peptide modulators have been investigated in C. finmarchicus: A-type allatostatin (A-AST; Christie et al., 2008), pigment dispersing hormone (PDH; Sousa et al., 2008) and tachykinin-related peptide (TRP; Sousa et al., 2008). Interestingly, during an ongoing expressed sequence tag (EST) project (Christie et al., 2009), one C. finmarchicus transcript (accession no. FK867612) was identified and annotated as putatively encoding a somatostatin receptor-like protein. While no authentic somatostatins have been found in arthropods, the C-type allatostatins (C-ASTs), a family of peptides characterized by a pyroglutamine blocked amino (N)-terminus, the unblocked carboxyl (C)-terminus –PISCF, and a disulfide bridge between two internal Cys residues (Stay and Tobe, 2007), have been proposed as the invertebrate counterpart of this vertebrate family (Veenstra, 2009); both peptide groups share some sequence similarity, have been implicated in the control of growth and development, and are generally inhibitory in their bioactivity (Veenstra, 2009). Thus, given the possible existence of a somatostatin-like receptor in C. finmarchicus, we became interested in investigating whether or not C-AST-like peptides are present in the nervous system of this species, and, if present, what functional roles these molecules might serve in the physiological control of this copepod.
Here, we report the results of immunohistochemical experiments mapping the distribution of C-AST-like peptides in the C. finmarchicus nervous system. As our results will show, one or more peptide immunologically-related to the C-AST family was detected in the copepod nervous system. While the distribution of C-AST-like immunoreactivity was similar in both males and females, several sex-specific differences were noted. Moreover, double-labeling experiments suggest that the C-AST-expressing neurons of C. finmarchicus are distinct from those expressing A-AST, PDH and TRP, and, based on their location within the nervous system, may contribute to the modulation of both feeding and postural control/locomotion in this species.
2. Materials and methods
2.1. Animals
C. finmarchicus were collected during June and July (2009) using oblique net tows through the upper 100 meters of water near Mount Desert Rock in the Gulf of Maine. All animals were maintained on a 11 hour:13 hour light-dark cycle at densities of approximately 15 individuals per liter in jars of filtered seawater at 8°C, and were fed twice weekly on a diet of Phyto-Feast Premium (Reed Mariculture, Campbell, CA; catalog #PFC-MED). Animals were sorted by sex and developmental stage; sex determination and developmental staging was done using descriptions provided in Mauchline (1998). The early June tow consisted primarily of last-stage (C5) copepodids and adults, with females predominating males, whereas the July tow garnered copepodites of younger developmental stages (C2-adults).
2.2. Immunohistochemistry
2.2.1. Antibodies
2.2.1.1. Primary antibodies
For the detection of C-AST-like peptides, a rabbit polyclonal antibody generated against Manduca sexta allatostatin-C conjugated via 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) to keyhole limpet hemocyanin (Audsley et al., 1998; a kind gift from Dr. Robert Weaver [Central Science Laboratory, Sand Hutton, York, UK]) was used at final dilutions ranging from 1:100-1:300; no differences in labeling were noted between the different dilutions (data not shown). For the detection of A-AST-like peptides, a mouse monoclonal antibody (clone 5F10; purchased from the Developmental Studies Hybridoma Bank, University of Iowa, Department of Biology, Iowa City, IA) generated against the cockroach Diploptera punctata peptide APSGAQRLYGFGLamide conjugated to bovine serum albumen via glutaraldehyde (Stay et al., 1992; Woodhead et al., 1992) was used at a final dilution of 1:100. For the detection of TRPs, a rat monoclonal antibody (clone NC1/34 HL; purchased from Abcam Incorporated, Cambridge, MA; catalog # ab6338) generated against RPKPQQFFGLMamide (substance P) conjugated to bovine serum albumen via EDC (Cuello et al., 1979) was used at a final dilution of 1:500. For the detection of acetylated-α-tubulin, a mouse monoclonal antibody (clone #6-11B-1; purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA; catalog #SC-23950) generated against the outer arms of Strongylocentrotus purpuratus (sea urchin) sperm axonemes was used at a final concentration of 1:50.
2.2.1.2. Secondary antibodies
Visualization of C-AST-like labeling was accomplished using donkey anti-rabbit IgG conjugated with Alexa Fluor 488 (Molecular Probes, Eugene, OR; catalog #A-11008) or Alexa Fluor 594 (Molecular Probes; catalog #11012). Visualization of the TRP-like labeling was accomplished using donkey anti-rat IgG conjugated with either Alexa Fluor 488 (Molecular Probes; catalog # 21208) or Alexa Fluor 594 (Molecular Probes; catalog # 21209). Visualization of A-AST-like and acetylated-α-tubulin-like labeling was accomplished using donkey anti-mouse IgG conjugated with either Alexa Fluor 488 (Molecular Probes; catalog #21202) or Alexa Fluor 594 (Molecular Probes; catalog #21203).
2.2.1.3. Anti-C-AST specificity controls
The A-AST and substance P antibodies have each been used previously to map the distribution of their respective antigen (or related ones) in the C. finmarchicus CNS and their specificities for these antigens in this tissue demonstrated (Christie et al., 2008; Sousa et al., 2008). The acetylated-α-tubulin antibody has been used extensively to map the distribution of this protein in a number of crustaceans (e.g. Semmler et al., 2008; Brenneis and Richter, 2010), including C. finmarchicus (Orcine and Hartline, 2010); while not directly tested for C. finmarchicus, the specificity of this monoclonal antibody for acetylated-α-tubulin has demonstrated in many species (for a detailed description of the antibody’s specificity readers are referred to http://datasheets.scbt.com/sc-23950.pdf). However, as our study is the first to use the C-AST antibody on C. finmarchicus neural tissue, preadsorption controls were conducted to assess the specificity of its labeling. Specifically, the C-AST antibody was incubated for 2 hours at room temperature with a 10−4 M concentration of pQIRYHQCYFNPISCF (the only known crustacean C-AST; Ma et al., 2009b; Stemmler et al., 2010) prior to its application to tissue. This preadsorption blocked all C-AST-like immunoreactivity in the C. finmarchicus nervous system (n=5 adults; data not shown).
2.2.2. Wholemount labeling
Immunohistochemistry was done as wholemounts as described previously (Christie et al., 2008; Sousa et al., 2008). In brief, the dorsal cuticle, digestive tract, and musculature overlying the ventral nerve cord (VNC) were removed by manual microdissection in either chilled (approximately 4°C) physiological saline (concentration in mM: 440 NaCl; 11 KCl; 13 CaCl2; 26 MgCl2; 10 HEPES acid; pH 7.4 [adjusted with NaOH]) or filtered seawater. Dissected animals were then fixed for 12-24 hours at 4°C in a solution of 4% paraformaldehyde (EM grade; Electron Microscopy Sciences, Hatfield, PA; catalog #15710) in 0.1 M sodium phosphate (P) buffer (pH 7.4), after which they were rinsed five times at one-hour intervals in a solution of P containing 0.3% Triton X-100 (P-Triton) and then incubated for approximately 72 hours in primary antibody (see 2.2.1.1) diluted to a final working concentration in P-Triton containing 10% normal donkey serum (NDS; Jackson ImmunoResearch, West Grove, PA; catalog #017-000-121). After incubation in primary antibody, preparations were again rinsed five times at one-hour intervals in P-Triton and then incubated overnight in fluorescently-labeled secondary antibody (see 2.2.1.2) diluted to a final concentration of 1:300 in P-Triton containing 10% NDS. After secondary antibody incubation, preparations were rinsed five times at one-hour intervals in P and then mounted between a glass microscope slide and coverslip using either Vectashield Mounting Medium (Vector Laboratories, Inc., Burlingame, CA; catalog #H-1000) or Vectashield containing DAPI (Vector Laboratories; catalog #H-1200). Incubations in both primary and secondary antibody were conducted at 4°C, while all rinses were done at room temperature (approximately 18°C). Secondary antibody incubation was conducted in the dark, as was all subsequent processing.
In all double-labeling experiments, both primary antibodies were applied to the tissue as a mixture, as were the secondary antibodies used for their visualization.
2.2.3. Epifluorescense and confocal microscopy
Data were collected and digital images were generated using a Zeiss Axiovert 200 epifluorescent microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY) or an Olympus Fluoview 1000 confocal system (Olympus America, Center Valley, PA). The Axiovert 200 was equipped with EC Plan-NEOFLUAR 10x/0.3, LD Plan-NEOFLUAR 20x/0.4, and LD Plan-NEOFLUAR 40x/0.6 objective lenses (all dry), an EXFO X-Cite Series 120 halide arc lamp (EXFO Photonic Solutions Inc., Mississauga, Ontario, Canada) and standard Zeiss FITC, Rhodamine and DAPI filter sets. The Olympus Fluoview 1000 confocal system consisted of an Olympus IX-81 inverted microscope, 10x/0.4 UPlanSApo, 20x/0.5 UPlanFLN, and 40x/0.95 UPlanSApo objective lenses (all dry) and HeNe and multi-Ar lasers, as well as manufacturer-supplied Alexa Fluor 488, Alexa Fluor 594 and DAPI filter sets and image-processing software.
2.3. Figure production
For the production of figures, digital images were exported from the Olympus confocal system as .tiff files. Figures were produced from the exported files using Photoshop (version 7.0; Adobe Systems Inc., San Jose, CA) and/or CorelDraw software (version 11.0; Corel Corporation, Mountain View, CA). It should be noted that the contrast and brightness of the final figures were adjusted as needed to optimize the clarity of the printed images.
3. Results
3.1. Anatomical nomenclature of C-AST-like immunopositive structures in the C. finmarchicus nervous system
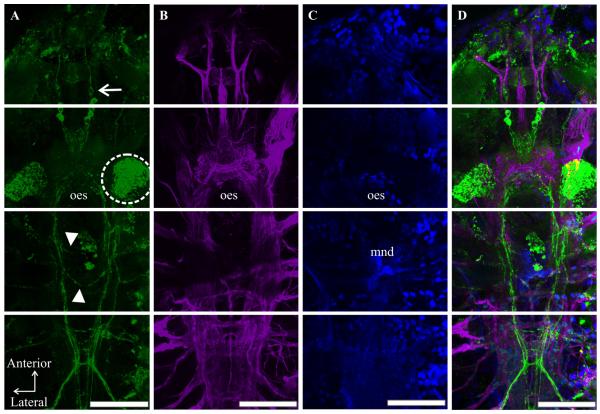
The locations of peptidergic structures in the C. finmarchicus nervous system reported in several previous studies (Christie et al., 2008; Sousa et al., 2008) were based on major structural features that were visible through the fluorescent background of the immunolabels, e.g. the mandibles and the oesophagus, and/or scaling to a map of the C. finmarchicus central nervous system produced from transverse serial sections of the animal by Lowe (1935). Recently, an antibody generated against acetylated-α-tubulin was shown to label all fiber tracts and neuropil regions of central nervous system of C. finmarchicus (Orcine and Hartline, 2010). The pairing of this antibody with others in double-labeling studies, in combination with Lowe’s schematic reconstruction of the C. finmarchicus nervous system, allows for a much more refined mapping of immunopositive structures in the nervous system of this species than was available previously, and we have used this methodology to ascribe locations to the C-AST-like labeling reported here. An example of a C-AST/acetylated-α-tubulin double-labeled preparation (counter stained with DAPI to reveal nuclei) is shown in Figure 1. With one notable exception, the identification of a maxillipedal basal ganglion (MBG), the structures labeled by the acetylated-α-tubulin antibody correspond well to those described by Lowe (1935). Interestingly, every adult possessed C-AST-immunopositive somata in the MBG; these somata projected axons into the nerve cord at the level of the maxilliped ganglion (Fig. 2C). A lack of description of the MBG by Lowe (1935) is not totally unexpected, as she did not follow all nerves laterally in her serial reconstruction of the C. finmarchicus central nervous system.
Figure 1.
C-type Allatostatin (C-AST)-like immunolabeling in the context of the overall nervous system and cell nuclei in adult Calanus finmarchicus. In order to properly characterize C-AST-like labeling (A), this immunoreactivity was complimented by processing using an antibody (acetylated-α-tubulin [B]) known to label nervous system tissue, as well as a stain (DAPI [C]) specific for cell nuclei and autofluorescent structures (e.g. mandible teeth, mnd; oesophagus, oes in [C]). (D) Merged images (A-C) revealed the location of C-AST-expressing somata and neurites/axons within the nervous system, as described in Figure 2. All nervous system features were previously characterized (Lowe, 1935), except the maxillipedal basal ganglion (shown in Fig. 2). (A) Some animals had variations in C-AST expression including an additional pair of protocerebral (PC) somata (arrow) with neurites extending anterior in the frontal nerve, dense staining dorsal to the antennal nerve (dashed circle), and commissures anterior and posterior to the mandible (arrowheads). Scale bars are 100μm. Images are all from the same preparation at the brightest pixel projections of 11, 14, and 13 optical sections (collected at 3μm intervals). For each optical section, the fluorochromes were imaged sequentially.
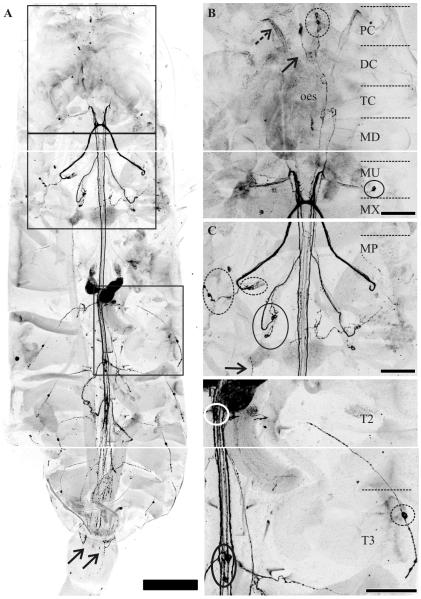
Figure 2.
Detailed characterization of C-type allatostatin (C-AST)-like immunoreactivity in the adult nervous system. (A) C-AST-like immunoreactivity in the central nervous system from the anterior protocerebrum to the mid-urosome segments in the teleson. Arrows indicate C-AST-like immunoreactive neurites that surrounded the genital openings of the female sex organs. Insets for B, C, and D are indicated by squares from top to bottom respectively. (B) C-AST-like staining in the cephalosome of C. finmarchicus reveals three pair of protocerebral (PC) neurons (dashed circle, PCC-AST) that send projections to the deutocerebrum (DC) with midline neuropil also labeled (solid arrow). In two preparations, some neurites in the antennular nerve (dashed arrow) were also C-AST-like immunoreactive. The PC neurons send projections around the oesophagus (oes) and continue posteriorly at least to the level of the maxillule (MU). (C) Posterior to the tritocerebrum (TC), several ganglia lateral to the main nerve tracts had C-AST containing cells including the maxillulary oval basal ganglion (MOBGC-AST; one cell body shown in solid circle [B], but up to 6-9 neurites), the maxillar elongated basal ganglion (MEBGC-AST; dashed circles, [C]), and the maxillipedal basal ganglion (MBGC-AST solid circle, [C]). Projections from these lateral basal ganglia come into their respective neuromeres (maxillule, MU; maxilla, MX; maxilliped, MP) and then project to various locations (see Results), but appear to mainly form a prominent commissure or extend anteriorly to the MU, then loop back posteriorly continuing down the lateral part of the posterior ventral nerve cord (VNC) to the end of the metasome. (D) In the posterior VNC, a pair of cell bodies was stained with C-AST-like immunolabeling in each of the swimming legs (T2-T6C-AST; T2, white circle; T3, black circle; T4 and T5 in [A]). These somata sent projections into the ventral longitudinal muscles (VLM; anterior part in (C) and see also Fig. 5), where some formed a fascicle along the entire length of the muscle group. Another immunoreactive bipolar neuron had a projection up to the dorsal surface and entered the VNC via the intersegmental dorsal longitudinal nerve in each metasome segment (DLMC-AST; 1st segment shown). Scale bars are 250 μm in (A), 100 μm in (B). The projections in (A) consist of 33, 34, and 33 optical sections each (collected at 4.5μm intervals) while those in (B), (C), and (D) consist of 24, 45, and 25 optical sections (collected at 3 μm intervals).
3.2. Distribution of C-AST-like immunoreactivity in the adult C. finmarchicus nervous system
As stated in Section 2.2.1.1, the distribution of C-AST-like immunoreactivity in the nervous system of C. finmarchicus was mapped using an antibody generated against Manduca sexta-AST-C (Audsley et al., 1998), which is nearly identical in structure to that of pQIRYHQCYFNPISCF (disulfide bridging between the two Cys residues), the only isoform of C-AST thus far identified in crustaceans (Ma et al., 2009b; Stemmler et al., 2010). In our study, fifty-four adults (22 males, 32 females) were labeled with this antibody. Of these animals, 75% of the females and 68% of the males showed clear immunolabeling in the nervous system; incomplete or no labeling was seen in the remaining preparations, likely a result of poor antibody penetration due to an incomplete removal of the dorsal cuticle, digestive tract, and/or musculature overlying the nervous system. The descriptions of the immunoreactivity presented below were compiled using only those preparations that showed clear labeling. It should be noted that, as stated in Section 3.1, the anatomical nomenclature for ganglia and nerves was based on both Lowe’s (1935) description of the anatomy of the C. finmarchicus nervous system, which was derived from a serial reconstruction of light micrographs, and on a data presented by Orcine and Hartline (2010), where acetylated-α-tubulin immunolabeling was used to visualize the axon tracts and neuropil regions of C. finmarchicus central nervous system.
In adult stage C. finmarchicus, numerous reidentifiable structures were routinely labeled by the C-AST antibody. Within the protocerebrum (PC), three pair of somata (PCC-AST; Figs. 1 and 2A-B) were commonly seen in both males and females. These cell bodies were typically 12-16 μm in diameter, and sent axonal projections into the deutocerebrum (DC), where several of the neurites cross and form a midline neuropil, which was characterized by the presence of large, bleb-like varicosities. The PCC-AST neurons also appeared to send projections around the oesophagus, with some small neurites projecting from them into the oesophageal tissue, or possibly, to the labral gland on the ventral surface of the brain. From the oesophageal regions, the PCC-AST projections continued posteriorly in the VNC, traveling in this direction at least to the level of the maxillule. Interestingly, as shown in the individual in Figure 1, a fourth pair of PCC-AST somata was also present (arrow in Fig 1A) in some animals (n =3). These cell bodies were located anterior to the other PCC-AST somata, and were bipolar, extending one axon anteriorly into the frontal nerve, and one posteriorly to an as of yet uncharacterized location. In addition to the PCC-AST projections, several axons in the antennular nerve (dashed arrow in Fig. 2B) also exhibited C-AST-like immunoreactivity; these fibers projected from the antennular nerve posteriorly, from the PC to the DC. The somata from which these axons are derived seem likely to reside in the first antennular segment, possibly from sensory neurons, (see also Fig. 1A), however, our dissection method rarely permitted access of the antibody into appendages (n=2 females), and thus this attribution is currently conjecture. It should be noted that in the female shown in Figure 1, C-AST-like labeling was also present in several fibers located in the tritocerebral commissure, and an anterior and posterior commissure surrounding the mandibular teeth (arrowheads). In addition, in males, there was weak staining in two pair of somata (see schematic, Fig. 5) located at the border of the posterior DC/anterior tritocerebrum (TC). These cell bodies projected posteriorly, around the oesophagus; no immunopositive somata were seen at the DC/TC border in any female. Other than immunopositive fibers that surrounded the female genital opening (arrows in Fig. 2A), the male-specific DC/TC somata were the only difference in staining noted between the sexes anywhere in the C. finmarchicus nervous system.
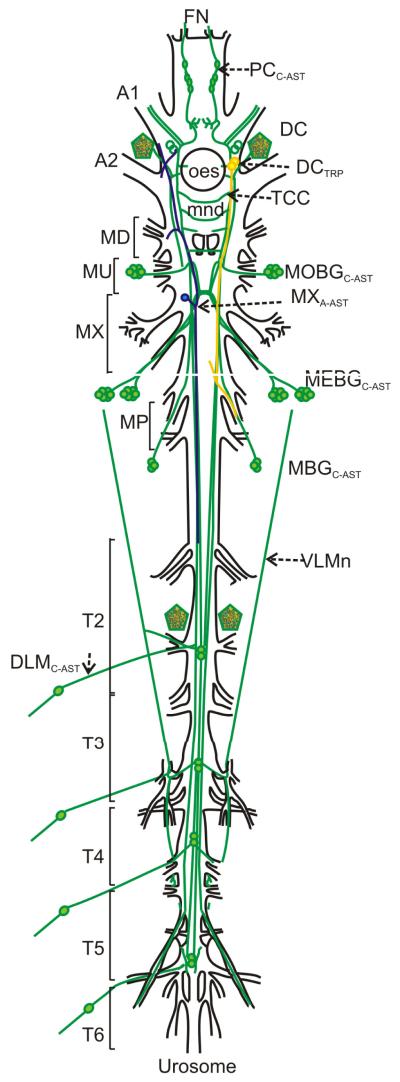
Figure 5.
Schematic representation of the Calanus finmarchicus nervous system showing the locations of C-AST-, A-AST-, and TRP-like immunoreactivity. Dorsal view of the nervous system using acetylated-alpha tubulin as a marker for nerve fiber-containing regions (black lines). C-AST (green) immunopositive cell bodies (circles; open circles indicate male dimorphism in DC) and neurite bundles (lines). Pentagons reveal dense staining anterior to A2 and T2 nerves. Subesophageal neurons that immunolabeled with A-AST (blue) or TRP (gold) are also shown (brain somata not shown). A1, antennular nerve; A2, antennal nerve; DC, deutocerebrum; DLMC-AST, dorsal peripheral neurons; FN, frontal nerve; MBG, maxillipedal basal ganglion; MEBG, maxillar elongated basal ganglion; MOBG, maxillulary oval basal ganglion; MP, maxilliped; MU, maxillule; MX, maxilla; PC, protocerebrum; T2-T6: thoracic neuromeres; TC, tritocerebrum; TCC, tritocerebral commissure; VLMn: ventral longitudinal muscle nerve tract.
Posterior to the TC, several ganglia associated with the VNC contained C-AST-like immunopositive somata. Specifically, in what Lowe (1935) defines as the maxillulary oval basal ganglia, a set of 6-9 somata (MOBGC-AST; Fig. 2B) were routinely labeled by the C-AST antibody. These cell bodies, typically 10-15 μm diameter, had unilateral projections into the VNC, where roughly half traveled for approximately 200 μm anteriorly, innervating the gut or labral gland; the remaining MOBGC-AST axons projected posteriorly to the commissure region formed by neurons originating from the maxillar elongated basal ganglion (MEBGC-AST). Within the MEBGC-AST itself, 6-14 somata (MEBGC-AST; Fig. 2C) exhibited C-AST-like immunoreactivity. These somata, like the MOBGC-AST cell bodies, ranged from about 10-15 μm diameter, and projected axons into the VNC at the level of the maxillar ganglion. These immunopositive fibers either cross the midline immediately upon entering the VNC, or extend anteriorly to the maxillulary ganglion, then loop back posteriorly continuing down the lateral part of the posterior VNC to the end of the metasome. In addition, at least three C-AST-like immunoreactive somata (MBGC-AST), again approximately 10-15 μm in diameter (solid circle in Fig. 2C), were routinely seen in the maxillipedal basal ganglion (MBGC-AST). Axons from the MBGC-AST extended anteriorly to the maxillar commissure region, but could not be followed further. In the posterior VNC, a pair of cell bodies (12-18 μm in diameter) corresponding to each of the swimming leg ganglia (T2-6C-AST; white and black circles in Fig. 2D) exhibited C-AST-like immunolabeling. Axons from these somata send projections into the ventral longitudinal muscles (VLMn, Fig. 5)
In addition to the neurons present in the CNS, several peripherally-located C-AST-like immunopositive structures were identified in adult C. finmarchicus. These included a long neurite or group of neurites that could be visualized on the entire medial portion of the ventral longitudinal muscles (arrow in Fig. 2C); the origin for this fiber tract was the somata in each thoracic segment as described above. Another set of C-AST immunoreactive peripheral somata (DLMC-AST; approximately 15μm diameter), likely sensory neurons due to their bipolar morphology, was found in muscles extending from the ventral body wall up to the dorsal surface in each metasome segment (Fig. 2D; T2 shown). These neurons entered into the VNC through the newly described intersegmental dorsal longitudinal nerve (DLMn; Hartline and Orcine, 2010).
3.3. C-AST labeling in the nervous systems of C3-C5 copepodid developmental stages
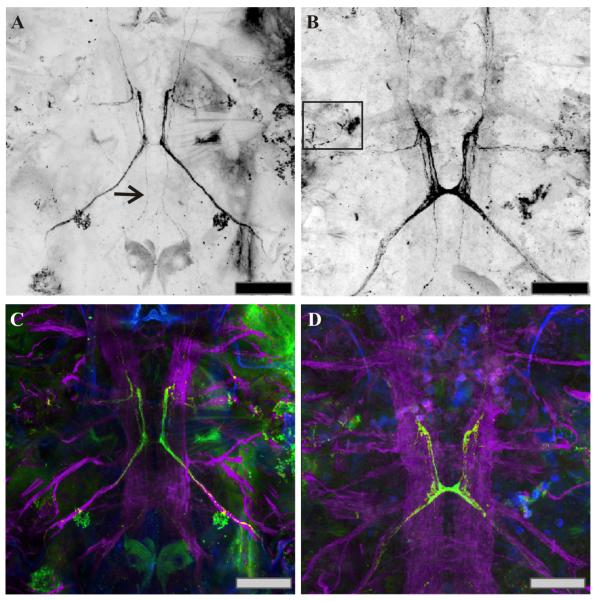
In addition to adult C. finmarchicus, we also examined C-AST-like immunolabeling in several developmental stages of this species. Due to their small size, no attempt was made to examine any embryonic or early-stage (N1-N3) nauplii. Immunohistochemistry was attempted on several late-stage (N4-N6) nauplii, as well as on C1-C2 copepodids. Here, considerable variability was seen between preparations, and due both to the small number of animals that survived the immunolabeling process and potential issues with antibody penetration in those that did (we were unable to remove much of the dorsal cuticle in these tiny animals), the data generated from them has been excluded from this study. Thus, the earliest stage for which we present a description of the distribution of C-AST immunoreactivity is the C3 copepodid (n = 4; Fig. 3C). Here, three pair of PCC-AST somata were labeled by the C-AST antibody in at least some (2 of 4) preparations, as were axonal projections past the maxillar ganglion, a pattern similar to that seen in the adult (at least in 2 of the 4 preparations examined). C-AST-like staining in both the C4 (n = 4; Fig. 3D) and C5 (n = 27; data not shown) copepodids were essentially identical to that seen in the adult and was consistent between preparations at these stages.
Figure 3.
C-type allatostatin (C-AST)-like and acetylated-α-tubulin-like immunoreactivity complemented by DAPI staining, in developing copepodids. (A, C) Anterior end of a C3 copepodid. Axons from the MBGC-AST are present at the C3 stage (arrow). Somata were not obvious in this preparation in any of the lateral ganglia, but were present in other animals (data not shown). (B, D) By C4, the maxillar commissure is well-formed with projections leading both anterior and posterior from this neuromere. The C-AST-like labeled axons have become larger and longer. (C, D) Along with C-AST antibody (green), these preparations were also labeled with the acetylated-α-tubulin antibody (purple) and a DAPI counterstain (blue) to reveal the nervous system and associated cell nuclei. Scale bars: 50 μm. Images are at brightest pixel projections of 15 optical sections taken at 0.67 μm intervals.
3.4. Comparison of C-AST-like labeling with those for other neuropeptides
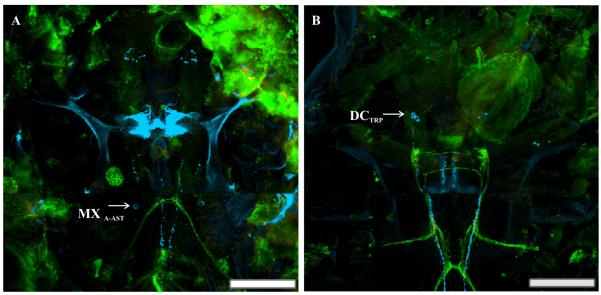
As stated in Section 1, the distributions of A-AST-, PDH- and TRP-like peptides were mapped in the nervous system of C. finmarchicus in two earlier studies (Christie et al., 2008; Sousa et al., 2008). While the PDH antibody, like that for C-AST, was generated using rabbit as a host species, the A-AST and TRP antibodies are mouse and rat monoclonals, respectively, and thus could be paired with the C-AST serum to directly assess the extent of co-localization of these peptides. For both the C-AST/A-AST (Fig. 4A) and C-AST/TRP (Fig. 4B) pairings, no overlap in any structure was noted (n=6 for each pairing). A schematic diagram of the C. finmarchicus nervous system showing the distribution of the C-AST-immunopositive neurons, as well as those labeled by A-AST and TRP antibodies, is presented as Figure 5.
Figure 4.
The relationship between A-type allatostatin (A-AST)-like and tachykinin-related peptide (TRP)-like immunostaining with C-AST. (A) As shown in a previous study (Christie et al., 2008), the A-AST antibody immunolabeled (blue) one pair of somata in the maxillary ganglion (MXA-AST). C-AST-like immunopositive somata and axons (green) showed no overlap with the MXA-AST. (B) TRP-like immunoreactivity (blue) was found in two pair of somata in the deutocerebrum (DCTRP), which send projections posterior through the VNC (Sousa et al., 2008). Again, no overlap of the C-AST-like and TRP-like immunoreactivities were noted; the yellow color shown in this panel is a false-positive colocalization that is a result of the superimposition of single-labeled structures whose X-Y coordinates are the same, but lie at different depths within the tissue (hence the appearance of colocalization in the projected image). Scale bars 100 μm. Images are all from the same preparation at the brightest pixel projections of 11 and 24 optical sections respectively (collected at 1.5 and 2.5 μm intervals).
4. Discussion
4.1. Immunohistochemical evidence for C-AST-like peptides in C. finmarchicus
As stated in the Introduction, the C-AST family of peptides is characterized by a pyroglutamine blocked amino N-terminus, the unblocked C-terminus –PISCF, and a disulfide bridge between two internal Cys residues (Stay and Tobe, 2007), e.g. pQVRFRQCYFNPISCF (Manduca sexta AST-C), the first member of this peptide family to be identified (Kramer et al., 1991). Authentic C-ASTs were long thought to occur only in holometabolous insects (Stay and Tobe, 2007), i.e. ones undergoing a complete metamorphosis. However, with the advent of transcriptome/genome mining, and subsequent mass spectral analyses, authentic C-ASTs have recently been predicted/detected from a broad array of arthropods, including hemimetabolous insects, crustaceans and chelicerates (Ma et al., 2009b; Veenstra, 2009; Weaver and Audsley, 2009; Stemmler et al., 2010). In addition, several C-terminally amidated peptides with structural similarity to the C-ASTs, e.g. SYWKQCAFNAVSCFamide, have recently been identified and shown to be broadly conserved within the Arthropoda (Hummon et al., 2006; Dickinson et al., 2009; Ma et al., 2009b; Veenstra, 2009; Weaver and Audsley, 2009). In fact, Veenstra (2009) suggests that all arthropods possess two C-AST-like peptides, encoded by paralog genes, and, based on structure and function, that the C-AST superfamily is the invertebrate counterpart to the vertebrate somatostatins (Veenstra, 2009).
In crustaceans, the C-AST isoform pQIRYHQCYFNPISCF has been predicted/detected from numerous decapod species (Ma et al., 2009b; Stemmler et al., 2010). Likewise the C-AST-like peptide SYWKQCAFNAVSCFamide has been identified by transcriptome mining/mass spectrometry in a wide array of decapods (Dickinson et al., 2009; Ma et al., 2009b), as well as in two daphnids (Gard et al., 2009; Christie et al., 2010b). While it is clear that the C-ASTs are broadly conserved in members of this arthropod subphylum (Dickinson et al., 2009; Gard et al., 2009; Ma et al., 2009b; Christie et al., 2010b; Stemmler et al., 2010), the cellular distribution of members of this peptide family remained unknown. Here, we used immunohistochemistry to assess the presence and distribution of the C-AST in the copepod C. finmarchicus. As our results detailed, immunoreactivity was found to be widely distributed within the nervous system of this species, strongly supporting the existence of C-AST-like peptides in this animal. Moreover, the immunohistochemical detection of C-AST in C. finmarchicus expands the list of crustaceans for which there is evidence of this peptide group to a second lower crustacean taxon, i.e. the Maxillopoda (the daphnids and the decapods represent the crustacean classes Branchiopoda and Malacostraca, respectively). In addition, our data show that an antibody generated against an insect C-AST isoform (Manduca sexta AST-C) provides a useful tool for investigating the distribution of C-AST-like peptides in other crustacean species.
4.2. The location and appearance of immunopositive structures suggest C-AST functions as both a locally-released paracrine and as a circulating hormonal in C. finmarchicus
In crustaceans, as in many animals, neuropeptides can exert their actions as locally-released autocrines/paracrines or as circulating hormones (e.g. Christie et al., 1995; Marder et al., 1995). Research from many laboratories has shown that, in at least the decapods, most neuropeptides are likely to function as both locally-released transmitters/modulators and as circulating hormones (e.g. Christie et al., 1995; Marder et al., 1995), as they are present in both central neuropil/at neuromuscular junctions and in neuroendocrine organs. In C. finmarchicus, C-AST-like immunoreactivity was detected in fibers that appear to innervate a number of muscle groups and/or possibly the labral gland, as well as in what appear to be regions of central neuropil, suggesting the possibility of local paracrine modulation at these sites. In addition, large bleb-like varicosities were noted in the DC region of the brain, reminiscent of endocrine release terminals, suggesting the possibility of hormonal release. Thus, it seems likely that the C-ASTs in C. finmarchicus too function as both locally-released and circulating modulators of physiology and behavior.
4.3. A modulatory role for C-AST-like peptides in feeding and locomotion
As their name implies, the C-AST family of peptides have been demonstrated to suppress the production of juvenile hormone by the corpora allata in at least some species of insects (Stay and Tobe, 2006; Audsley et al., 2008; Audsley and Weaver, 2009; Weaver and Audsley, 2009). However, given their distribution in insect tissues, it is likely that they are highly pleiotropic, and in many species, may well not be a primary contributor to the inhibition of juvenile hormone production, if they are involved in its regulation at all (Stay and Tobe, 2006; Audsley et al., 2008; Audsley and Weaver, 2009; Weaver and Audsley, 2009). In crustaceans, while limited, recent studies have shown that the C-ASTs are both cardioactive (Dickinson et al., 2009) and modulate the motor output of the foregut (Ma et al., 2009b). In our study, the distribution of C-AST-like immunoreactivity in C. finmarchicus suggests that this group of peptides may be involved in the regulation of feeding, as innervation to both the oesophagus and feeding appendages was noted. Likewise, innervation of the longitudinal muscles of the metasome and sensory input from each thoracic segment suggests that the C-ASTs are likely to contribute to postural control/locomotion in this copepod.
4.4. Sex and stage-specific differences in C-AST expression
In our study, we noted both the sex (in adults) and stage of all individuals that were examined. For adult animals, the vast majority of structures labeled by the C-AST antibody were present in both sexes. This said, there were two structures that exhibited sex-specific staining. First, in females, immunopositive fibers surrounding the genital opening were noted; no immunoreactivity was seen in the genital region in males. In addition, in males, two pair of somata located at the DC/TC border expressed weak C-AST-like labeling. With respect to the former localization, it seems likely that peptide may be involved in a female-specific aspect of reproductive control. Given the paucity of data on the identity of individual neurons in the C. finmarchicus, it is too early to attempt to ascribe function to the putative male-specific labeling present in brain.
A developmental time-course was attempted to assess stage-specific changes in the distribution of C-AST-like peptides in C. finmarchicus. Due to the size of the animals, no immunohistochemistry was attempted on either embryonic or early naupliar (N1-N3) stages. While we did attempt to stain late stage nauplii (N4-N6) and early copepodids (C1-C2), few of these animals survived the immunolabeling process and those that did, were potentially compromised by antibody penetration issues. Thus, interpretable data was obtained only from C3-C5 copepodids. From these animals, it is clear that an adult-like distribution of C-AST expression is at least developing by the C3 copepodid stage and is stable by C4.
Acknowledgements
The authors thank Dr. Daniel Hartline (University of Hawaii at Manoa and MDIBL) for sharing unpublished data with us and for providing critical input to the experimental design of this study. Ms. Marina Karnofsky (Mount Desert Island High School) and Mr. Harrison Kilpatrick (University of Maine, Presque Isle) are thanked for their assistance in data collection and Andrew Peterson (College of the Atlantic) is thanked for his assistance in collecting the animals used in this study. Financial support for this work was provided by: NIH Grant Number P20 RR-016463 from the INBRE Program of the National Center for Research Resources (Patricia Hand, Ph.D., Principal Investigator), the National Institute of Environmental Health Sciences STEER program (NIEHS R25 ES016254), and through institutional funds provided by MDIBL and Denison University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audsley N, Matthews HJ, Price NR, Weaver RJ. Allatoregulatory peptides in Lepidoptera, structures, distribution and functions. J. Insect Physiol. 2008;54:969–980. doi: 10.1016/j.jinsphys.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Audsley N, Weaver RJ. Neuropeptides associated with the regulation of feeding in insects. Gen. Comp. Endocrinol. 2009;162:93–104. doi: 10.1016/j.ygcen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Audsley N, Weaver RJ, Edwards JP. Enzyme linked immunosorbent assay for Manduca sexta allatostatin (Mas-AS), isolation and measurement of Mas-AS immunoreactive peptide in Lacanobia oleracera. Insect Biochem. Mol. Biol. 1998;28:775–784. [Google Scholar]
- Brenneis G, Richter S. Architecture of the nervous system in mystacocarida (Arthropoda, crustacea) - an immunohistochemical study and 3D reconstruction. J. Morphol. 2010;271:169–189. doi: 10.1002/jmor.10789. [DOI] [PubMed] [Google Scholar]
- Christie AE, Hartline N, Ohno P, Lenz PH. Bioinformatic analyses of the publicly accessible crustacean expressed sequence tags (ESTs) reveal numerous novel neuropeptide-encoding precursor proteins, including ones from members of several little studies taxa. Gen. Comp. Endocrinol. 2010b;167:164–178. doi: 10.1016/j.ygcen.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Christie AE, Lenz PH, Hassett RP, Smith CM, Batta Lona P, Ünal E, Bucklin A. Calanus finmarchicus cDNA library: a genomic tool for studies of zooplankton physiological ecology. The Bulletin, MDI Biological Laboratory. 2009;48:112–113. [Google Scholar]
- Christie AE, Skiebe P, Marder E. Matrix of neuromodulators in neurosecretory structures of the crab Cancer borealis. J. Exp. Biol. 1995;198:2431–2439. doi: 10.1242/jeb.198.12.2431. [DOI] [PubMed] [Google Scholar]
- Christie AE, Sousa GL, Rus S, Smith CM, Towle DW, Hartline DK, Dickinson PS. Identification of A-type allatostatins possessing -YXFGI/Vamide carboxy-termini from the nervous system of the copepod crustacean Calanus finmarchicus. Gen. Comp. Endocrinol. 2008;155:526–533. doi: 10.1016/j.ygcen.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stevens JS, Bowers MR, Chapline MC, Jensen DA, Schegg KM, Goldwaser J, Kwiatkowski MA, Pleasant TK, Shoenfeld L, Tempest LK, Williams CR, Wiwatpanit T, Smith CM, Beale KM, Towle DW, Schooley DA, Dickinson PS. Identification of a calcitonin-like diuretic hormone that functions as an intrinsic modulator of the American lobster, Homarus americanus, cardiac neuromuscular system. J. Exp. Biol. 2010a;213:118–127. doi: 10.1242/jeb.037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello AC, Galfre G, Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc. Natl. Acad. Sci. USA. 1979;76:3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson PS, Stemmler EA, Cashman CR, Brennan HR, Dennison B, Huber KE, Peguero B, Rabacal W, Goiney CC, Smith CM, Towle DW, Christie AE. SIFamide peptides in clawed lobsters and freshwater crayfish (Crustacea, Decapoda, Astacidea): a combined molecular, mass spectrometric and electrophysiological investigation. Gen. Comp. Endocrinol. 2008;156:347–360. doi: 10.1016/j.ygcen.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Wiwatpanit T, Gabranski ER, Ackerman RJ, Stevens JS, Cashman CR, Stemmler EA, Christie AE. Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties. J. Exp. Biol. 2009;212:1140–1152. doi: 10.1242/jeb.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducklow HW, Baker K, Martinson DG, Quetin LB, Ross RM, Smith RC, Stammerjohn SE, Vernet M, Fraser W. Marine pelagic ecosystems: the west Antarctic Peninsula. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007;362:67–94. doi: 10.1098/rstb.2006.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, de la Iglesia HO, Christie AE, Li L. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J. Comp. Neurol. 2005;493:607–626. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- Fu Q, Tang LS, Marder E, Li L. Mass spectrometric characterization and physiological actions of VPNDWAHFRGSWamide, a novel B type allatostatin in the crab, Cancer borealis. J. Neurochem. 2007;101:1099–1107. doi: 10.1111/j.1471-4159.2007.04482.x. [DOI] [PubMed] [Google Scholar]
- Gard AL, Lenz PH, Shaw JR, Christie AE. Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea; Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen. Comp. Endocrinol. 2009;160:271–287. doi: 10.1016/j.ygcen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Hill SL, Murphy EJ, Reid K, Trathan PN, Constable AJ. Modelling Southern Ocean ecosystems: krill, the food-web, and the impacts of harvesting. Biol. Rev. Camb. Philos. Soc. 2006;81:581–608. doi: 10.1017/S1464793106007123. [DOI] [PubMed] [Google Scholar]
- Hummon AB, Richmond TA, Verleyen P, Baggerman G, Huybrechts J, Ewing MA, Vierstraete E, Rodriguez-Zas SL, Schoofs L, Robinson GE, Sweedler JV. From the genome to the proteome: uncovering peptides in the Apis brain. Science. 2006;314:647–649. doi: 10.1126/science.1124128. [DOI] [PubMed] [Google Scholar]
- Kastin AJ. Handbook of Biologically Active Peptides. First Edition Academic Press; 2006. [Google Scholar]
- Kramer SJ, Toschi A, Miller CA, Kataoka H, Quistad GB, Li JP, Carney RL, Schooley DA. Identification of an allatostatin from the tobacco hornworm Manduca sexta. Proc. Natl. Acad. Sci. USA. 1991;88:9458–9462. doi: 10.1073/pnas.88.21.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe E. On the anatomy of a marine copepod, Calanus finmarchicus (Gunnerus) Trans. R. Soc. Edinburgh. 1935;58:561–603. [Google Scholar]
- Ma M, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, Smith CM, Towle DW, Christie AE, Li L. Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen. Comp. Endocrinol. 2009a;161:320–34. doi: 10.1016/j.ygcen.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Chen R, Sousa GL, Bors EK, Kwiatkowski MA, Goiney CC, Goy MF, Christie AE, Li L. Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen. Comp. Endocrinol. 2008;156:395–409. doi: 10.1016/j.ygcen.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Gard AL, Xiang F, Wang J, Davoodian N, Lenz PH, Malecha SR, Christie AE, Li L. Combining in silico transcriptome mining and biological mass spectrometry for neuropeptide discovery in the Pacific white shrimp Litopenaeus vannamei. Peptides. 2010 doi: 10.1016/j.peptides.2009.10.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Szabo MT, Jia C, Marder E, Li L. Mass spectrometric characterization and physiological actions of novel crustacean C-type allatostatins. Peptides. 2009b;30:1660–1668. doi: 10.1016/j.peptides.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauchline J. The biology of marine calanoid copepods. Adv. Mar. Biol. 1998;33:1–710. [Google Scholar]
- Marder E, Christie AE, Kilman VL. Functional organization of cotransmission systems: lessons from small nervous systems. Invert. Neurosci. 1995;1:105–112. doi: 10.1007/BF02331908. [DOI] [PubMed] [Google Scholar]
- Martin-Creuzburg D, Wacker A, von Elert E. Life history consequences of sterol availability in the aquatic keystone species Daphnia. Oecologia. 2005;144:362–372. doi: 10.1007/s00442-005-0090-8. [DOI] [PubMed] [Google Scholar]
- Murison LD, Gaskin DE. The distribution of right whales and zooplankton in the Bay of Fundy, Canada. Can. J. Zool. 1989;67:1411–1420. [Google Scholar]
- Orcine M, Hartline DK. Neuroanatomy of a calanoid copepod using acetylated α-tubulin immunohistochemistry. The Bulletin, MDI Biological Laboratory. 2010;49 In press. [Google Scholar]
- Provan J, Beatty GE, Keating SL, Maggs CA, Savidge G. High dispersal potential has maintained long-term population stability in the North Atlantic copepod Calanus finmarchicus. Proc. Biol. Sci. 2009;276:301–307. doi: 10.1098/rspb.2008.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler H, Wanninger A, Høeg JT, Scholtz G. Immunocytochemical studies on the naupliar nervous system of Balanus improvisus (Crustacea, Cirripedia, Thecostraca) Arthropod Struct. Dev. 2008;37:383–395. doi: 10.1016/j.asd.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Smith WO, Jr, Ainley DG, Cattaneo-Vietti R. Trophic interactions within the Ross Sea continental shelf ecosystem. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007;362:95–111. doi: 10.1098/rstb.2006.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa GL, Lenz PH, Hartline DK, Christie AE. Distribution of pigment dispersing hormone- and tachykinin-related peptides in the central nervous system of the copepod crustacean Calanus finmarchicus. Gen. Comp. Endocrinol. 2008;156:454–459. doi: 10.1016/j.ygcen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Stay B, Chan KK, Woodhead AP. Allatostatin-immunoreactive neurons projecting to the corpora allata of adult Diploptera punctata. Cell Tissue Res. 1992;270:15–23. doi: 10.1007/BF00381875. [DOI] [PubMed] [Google Scholar]
- Stay B, Tobe SS. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu. Rev. Entomol. 2007;52:277–299. doi: 10.1146/annurev.ento.51.110104.151050. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Bruns EA, Cashman CR, Dickinson PS, Christie AE. Molecular and mass spectral identification of the broadly conserved decapod crustacean neuropeptide pQIRYHQCYFNPISCF: the first PISCF-allatostatin (Manduca sexta- or C-type allatostatin) from a non-insect. Gen. Comp. Endocrinol. 2010;165:1–10. doi: 10.1016/j.ygcen.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmler EA, Peguero B, Bruns EA, Dickinson PS, Christie AE. Identification, physiological actions and distribution of TPSGFLGMRamide: a novel tachykinin-related peptide from the midgut and stomatogastric nervous system of Cancer crabs. J. Neurochem. 2007;101:1351–1366. doi: 10.1111/j.1471-4159.2007.04520.x. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Cashman CR, Smith CM, Beale KM, Towle DW, Christie AE, Dickinson PS. The peptide pQDLDHVFLRFamide (crustacean myosuppressin) modulates the Homarus americanus cardiac neuromuscular system at multiple sites. J. Exp. Biol. 2009;212:3961–3976. doi: 10.1242/jeb.035741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone GS, Kraus SD, Prescott JH, Hazard KW. Significant aggregations of the endangered right whale, Eubalaena glacialis, on the continental shelf of Nova Scotia. Can. Field Nat. 1988;102:471–474. [Google Scholar]
- Tande K, Miller CB. Preface to: Trans-Atlantic Study of Calanus finmarchicus; Proceedings of a Workshop; Ophelia. 1996. pp. i–ii. [Google Scholar]
- Veenstra JA. Allatostatin C and its paralog allatostatin double C: the arthropod somatostatins. Insect Biochem. Mol. Biol. 2009;39:161–170. doi: 10.1016/j.ibmb.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Wagner A, Benndorf J. Climate-driven warming during spring destabilises a Daphnia population: a mechanistic food web approach. Oecologia. 2007;151:351–364. doi: 10.1007/s00442-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Weaver RJ, Audsley N. Neuropeptide regulators of juvenile hormone synthesis: structures, functions, distributions and unanswered questions. Ann. N.Y. Acad. Sci. 2009;1163:316–329. doi: 10.1111/j.1749-6632.2009.04459.x. [DOI] [PubMed] [Google Scholar]
- Wiebe PH, Beardsley RC, Bucklin A, Mountain DG. Coupled biological and physical studies of plankton populations in the Georges Bank region and related North Atlantic GLOBEC study sites. Deep-Sea Res. II. 2001;48:1–2. [Google Scholar]
- Wishner KF, Schoenherr JR, Beardsley R, Chen C. Abundance, distribution and population structure of the copepod Calanus finmarchicus in a springtime right whale feeding area in the southwestern Gulf of Maine. Cont. Shelf Res. 1995;15:475–507. [Google Scholar]
- Woodhead AP, Stoltzman CA, Stay B. Allatostatins in the nerves of the antennal pulsatile organ muscle of the cockroach Diploptera punctata. Arch. Insect Biochem. Physiol. 1992;20:253–263. doi: 10.1002/arch.940200403. [DOI] [PubMed] [Google Scholar]
- Woodley TH, Gaskins DE. Environmental characteristics of North Atlantic right and fin whale habitat in the lower Bay of Fundy, Canada. Can. J. Zool. 1996;74:75–84. [Google Scholar]