Abstract
Objectives
To compare the trajectory of motor decline, as measured by gait speed and finger tapping speed, between those who developed mild cognitive impairment (MCI) and those who remained cognitively intact. We also sought to determine the approximate time at which decline in motor function accelerated in those who developed MCI.
Design
Longitudinal cohort study
Participants
Subjects were 204 healthy seniors (58% women) from the Oregon Brain Aging Study evaluated for up to 20 years with annual neurologic, neuropsychological and motor examinations.
Main Outcome Measures
The pattern of motor decline with aging was compared using a mixed-effects model with an interaction term for age and clinical diagnosis of mild cognitive impairment. The time prior to diagnosis of MCI when the change in gait or tapping speed accelerates was assessed with a mixed-effects model with a change point for men and women, separately and combined, who developed MCI.
Results
The rates of change with aging of gait speed (p<0.001) and tapping speed in the dominant hand (p<0.003) and non-dominant hand (p<0.001) were significantly different between MCI converters and non-converters. Using a change-point analysis for MCI converters, the decrease in gait speed accelerated by 0.02 m/s/yr (p<0.001) occurring 12.1 years prior to the onset of MCI. An acceleration of gait speed decline occurred earlier in men than women. For tapping speed, the change point occurred after the onset of MCI for both dominant and non-dominant hands when men and women were combined.
Conclusions
Motor decline as indexed by gait speed accelerates up to 12 years prior to MCI. Longitudinal changes in motor function may be useful in the early detection of dementia during pre-clinical stages when the utility of disease-modifying therapies would be greatest.
Introduction
Mild cognitive impairment (MCI) represents an early clinical stage of cognitive impairment, considered distinct from normal aging, with the potential for further progression to Alzheimer's disease (AD) or other dementias.1 Predicting the earliest stages of cognitive impairment has important implications for initiating treatment and monitoring progression of disease. Slowing of motor function is commonly observed in elderly patients and may be more pronounced in older persons with cognitive impairment compared to those who are cognitively intact.2–5 Motor changes may precede the onset of mild cognitive impairment by years.4, 6 Additionally, slower gait speed in those who are cognitively intact at baseline may be predictive of the subsequent onset of cognitive impairment.6–8
Motor changes with aging present with slowing of fine motor movements and mild parkinsonian signs.9, 10 Etiologies are unclear but may include pathological changes caused by neurologic illnesses such as stroke, Parkinson's disease or non-neurologic illnesses.11 These changes are not benign and may predict disability, institutionalization and mortality in community-residing elderly.11–13
Several studies have shown that motor slowing precedes and may predict the onset of cognitive impairment;4, 6–8 however, the time at which this slowing begins in relation to the onset of cognitive impairment is not clear. We used data from a longitudinal aging study to test the hypothesis that persons who develop MCI have a greater rate of decline in motor function as measured by gait and tapping speed than those who remain cognitively intact. We also used a change point statistical model to determine the approximate time at which this change in the rate of decline occurs in relation to the onset of MCI.
Methods
Participants
Subjects were participants in the Oregon Brain Aging Study (OBAS), a longitudinal study of healthy elderly that began in 1989 at the NIA Layton Aging and Alzheimer's Disease Center at the Oregon Health & Science University (OHSU). Study procedures have been previously described.14 Inclusion criteria required that participants be community-dwelling, functionally independent, and free of co-morbid illnesses, have a baseline Mini-Mental State Examination (MMSE) score ≥ 24,15 Clinical Dementia Rating Scale (CDR) = 0,16 and no depression by screening with the Geriatric Depression Scale (GDS).17 Subjects underwent annual medical histories, neurologic examinations and neuropsychological testing. Assessments were performed until death. Between 1989 and 2003, 289 subjects were evaluated and 216 met inclusion criteria and were enrolled. Of those, 204 subject were older than 65 and were included in this analysis. Attrition rates due to loss to follow-up other than death were less than 1% per year. The study and consent forms were approved by the OHSU Institutional Review Board; all subjects signed written informed consent.
Clinical Assessments
Annual evaluations were performed by trained neurologists and geriatric nurse practitioners and included a medical history, mental status exam and standardized neurologic examination. Neurologic examinations were quantified and coded. Inter-rater reliability has been previously reported.14 The MMSE and the Cognistat18 were performed as brief cognitive evaluations. Health assessments consisted of review of medical histories, medication lists, and the modified Cumulative Illness Rating Scale.19 Height and weight were assessed annually. Blood was obtained for determination of APOE-ε4 genotype by DNA extraction and analysis using standard methods.20
Gait speed was assessed by asking participants to walk from a starting point to a marker 15 feet away, turn, and back at a normal casual gait for a total of 30 feet (9.14 meters). Time in seconds was recorded with a stopwatch to the nearest second for two trials and the mean recorded. Tapping was measured by pushing a lever with an attached counter using the index finger of each hand during a 10 second period. Three trials were performed with each hand and the mean value was recorded.
Health conditions or states with the potential to affect mobility were obtained from medical histories and exam. These included heart disease, chronic pulmonary conditions, stroke, Parkinson's disease, cancer, diabetes, major surgeries, musculoskeletal or head injuries, and depression. Presence of depression was based on a score > 11 on the GDS long form17 prior to October 2000 and a score > 4 on the GDS short form21 after that time. These were coded as dichotomous variables, either present or absent. BMI was calculated from height and weight recorded at annual visits.
Cognitive impairment was considered present with a CDR≥0.5. CDR scores were determined by interviews with subjects and collateral informants who provided information on cognitive and functional status. The Cognistat (but not the psychometric battery test scores) were included in the determination of the CDR. The onset of MCI was defined by the first of two consecutive semiannual CDR scores ≥ 0.5 to minimize the possible inclusion of subjects with transient or reversible cognitive impairment. The term “conversion” is used to describe the development of incident MCI during the follow-up period.
Analysis
Characteristics at baseline and presence of health conditions were compared between MCI converters and non-converters using the t-test and Wilcoxon Ranked Sum Test for continuous variables and Pearson Chi-Square test for categorical variables. Longitudinal mixed effects models estimated the patterns of change over time in gait and tapping speeds. First, an interaction term between age and clinical diagnosis was used to test whether the aging pattern for MCI converters differed from non-converters during follow-up. Analyses were adjusted for baseline speed (gait or tapping), years of education, gender (when combining both sexes), and APOE-ε4 genotype. Analyses were also adjusted for the presence of depression and stroke during the entire follow-up in non-converters and prior to conversion in MCI converters in this analysis.
A second analysis investigated whether the annual rate of decline in gait or tapping speed changed at some point relative to clinical diagnosis using a longitudinal mixed effects model with a change point.22–24 The inclusion of a change point in the mixed effects model allows the rates of change to differ before and after the change point. The point of change in the coefficients is relative to the time of diagnosis with MCI, as opposed to age. The model assumes that the timing of the change point relative to MCI diagnosis is common across all subjects. Normality of distribution of outcomes was confirmed by examining normal probability plots. As described above, analyses were adjusted for age, education, gender, APOE-ε4 genotype, baseline speed, stroke, and depression. The analyses were run for men and women separately and combined because of differences in baseline gait and tapping speeds (i.e., men walked or tapped faster than women).
Change point models can be sensitive to a few influential observations. In the gait speed data, six outliers among women and two outliers among men, indicated by DFFITS statistics > 0.2,25 were excluded in the first and mixed effect models and change point models. Exclusion of these observations did not change the main results for gait speed, but improved overall model fit. Therefore, we report the results of change point analysis excluding these influential observations. For tapping speed, DFFIT tests identified no outlying influential cases.
The location of the change point relative to MCI diagnosis was estimated by maximum likelihood using the SAS procedure NLMIXED (SAS Institute, Cary, NC). Separate mixed effects models were fit with the change point at fixed 1-month intervals up to 15 years before and after diagnosis. The model with the highest likelihood was used to summarize the results. We tested whether there was a significant change in the rate of change in outcomes relative to the MCI diagnosis by calculating a 95% confidence interval (CI) around the parameter on the change point term using a likelihood ratio approach. The significance of the other terms in the mixed effects model was determined using a Wald test statistic.26 Standard errors for the parameter estimates were calculated using the conditional variance as proposed previously.22 Significance was taken as p=0.05.
Results
Subject Characteristics
Subject characteristics are summarized in Table 1. Among 204 participants with an average of 9 years of follow-up, 95 (46%) converted to MCI. MCI converters were 4.5 years older (p<0.001), scored 0.2 points lower on the MMSE at baseline (P=0.03), had a longer mean follow-up time (P<0.001), and were more likely to be APOE-ε4 genotype positive (P=0.001) than non-converters (Table 1). There was a significant difference in baseline gait speed between the two groups for women only. Among all health factors assessed (see Methods), only stroke was significantly more frequent in the MCI group prior to the onset of cognitive impairment (P=0.001) and was thus taken into account in subsequent models. Although depression was not significantly more frequent in the MCI group, it was included in the analyses because of its reported association with motor slowing.27
Table 1.
Subject Characteristics. Mean (SD) unless otherwise specified.
| Controls (at baseline) | Converters to MCI (at baseline) | P value* | Converters (at time of conversion) | |
|---|---|---|---|---|
| Sample size, n | ||||
| Combined | 109 | 95 | ||
| Women | 60 | 58 | ||
| Men | 49 | 37 | ||
| Age, years [range] | ||||
| Combined | 79.0 (8.85) [65.2, 100.5] | 83.5 (7.0) [65.1, 98.5] | <0.001 | 89.8 (5.4) [73.1, 99.7] |
| Women | 78.7(8.66) [65.2, 97.4] | 84.7(6.18) [68.6, 98.5] | <0.001 | 90.5 (4.9) [76.1, 99.7] |
| Men | 79.3(9.15) [65.2, 100.5] | 81.6 (7.79) [65.1, 91.8] | 0.21 | 88.8 (6.08) [73.1, 99.4] |
| Education, years | ||||
| Combined | 14.5 (2.7) | 14.7 (2.65) | 0.57 | |
| Women | 14.1 (2.29) | 14.1 (2.66) | 0.99 | |
| Men | 15.0 (3.1) | 15.6 (2.3) | 0.26 | |
| MMSE | ||||
| Combined | 28.3 (1.46) | 28.1 (1.65) | 0.03 | 26.2(2.9) |
| Women | 28.7 (1.20) | 28.0 (1.82) | 0.01 | 26.3 (2.5) |
| Men | 28.3 (1.23) | 28.2 (1.38) | 0.77 | 26.03 (3.5) |
| APOE4, % | ||||
| Combined | 12.6 | 27.7 | 0.001 | |
| Women | 12.7 | 25.9 | 0.08 | |
| Men | 12.5 | 30.6 | 0.04 | |
| stroke, % # | ||||
| Combined | 1.8 | 13.7 | 0.001 | |
| Women | 0 | 15.5 | <0.001 | |
| Men | 4.1 | 10.8 | 0.23 | |
| Depression, % # | ||||
| Combined | 6.4 | 13.7 | 0.08 | |
| Women | 5.0 | 15.5 | 0.06 | |
| Men | 8.2 | 10.8 | 0.68 | |
| Follow-up, years [range] | ||||
| Combined | 8.36 (5.03) [0.1,19.2] | 10.48 (4.0) [2.5,19.3] | 0.001 | |
| Women | 8.4 (5.2) | 10.2 (3.71) | 0.04 | |
| Men | 8.3 (4.85) | 11.0 (4.50) | 0.01 | |
| Time to conversion, years | ||||
| Combined | 6.37 (4.04) | |||
| Women | 5.78 (3.95) | |||
| Men | 7.32 (4.06) | |||
| Gait speed, m/s | ||||
| Combined | 0.96 (0.23) | 0.91(0.24) | 0.10 | 0.78(0.23) |
| Women | 0.94 (0.24) | 0.84(0.03) | 0.03 | 0.78(0.25) |
| Men | 1.00 (0.21) | 1.01(0.04) | 0.79 | 0.78(0.20) |
| Tapping speed dominant, taps/second | ||||
| Combined | 3.87 (0.8) | 3.77 (0.85) | 0.40 | 3.86(0.95) |
| Women | 3.53 (0.71) | 3.49(0.80) | 0.72 | 3.64(0.97) |
| Men | 4.29 (0.77) | 4.21(0.73) | 0.6 | 4.23(0.79) |
| Tapping speed non-dominant, taps/second | ||||
| Combined | 3.63 (0.65) | 3.62 (0.71) | 0.89 | 3.64(0.79) |
| Women | 3.41 (0.57) | 3.38(0.65) | 0.76 | 3.48(0.73) |
| Men | 3.91 (0.65) | 3.99(0.64) | 0.59 | 3.90(0.83) |
Comparison between control group and converters at baseline
Frequency during entire follow-up period for non-converters and until conversion in converters.
Gait speed
There was a significant decline of gait speed over time in the mixed effects model of 0.013m/s/yr (p<0.001) for all participants, demonstrating the effect of age on gait speed. MCI converters had a further decline of 0.01m/s/yr than non-converters (p<0.0001).
In the change point model, profile likelihood values showed a clear peak 12.1 years (145 months) prior to the MCI diagnosis. On average, the MCI subjects' rate of decrease in gait speed accelerated by 0.023 m/s/yr (p<0.001) approximately 12 years prior to diagnosis (Table 3). The upper limit of the confidence interval could not be observed in our current data since the maximum follow-up duration observed prior to the MCI conversion was 16.3 years, too short to observe the upper limit in the change point (i.e., left censoring).
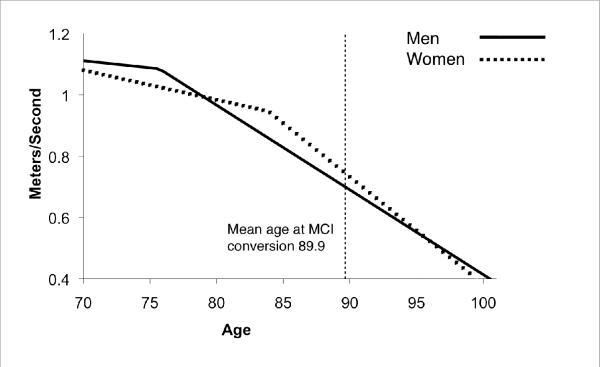
When the model was run separately for men and women (Table 2), we found that for men, the MCI subjects' rate of decrease in gait speed accelerated by 0.023 m/s/yr (p<0.001) at 14.2 years (95% CI: 8.7, unknown) prior to MCI diagnosis. For women, the MCI subjects' rate of decrease in gait speed accelerated by 0.025 m/s/yr (p<0.001) at 6.0 years (95%CI: 4.6, 9.5 yrs) prior to MCI diagnosis. Figure 1 shows an example of gait speed trajectory relative to the time of MCI conversion if the subject converted to MCI at age 89.9, the average age of conversion among this cohort.
Table 2.
Results of change point mixed model analysis*
| Men and Women | Men only | Women only | |
|---|---|---|---|
| Walking Speed | |||
| Rate of annual change prior to change point in MCI group (m/s/yr) | −0.005 (−0.010, 0.0004) P=0.07 | −0.004 (−0.014, 0.004) P=0.33 | −0.009 (−0.015, −0.003) P=0.002 |
| Additional rate of change after change point in MCI group (m/s/yr) | −0.023 (−0.029, −0.017) P<0.001 | −0.023 (−0.033, −0.012) P<0.001 | −0.025 (−0.033, −0.017) P<0.001 |
| Change point location (years) in relation to MCI diagnosis** | 12.1 (8.1, UNK)*** | 14.2 (8.7, UNK)*** | 6.0 (4.6, 9.5) |
| Number of finger with dominant hand | |||
| Rate of annual change prior to change point in MCI group (taps/second) | −0.03 (−0.04, −0.02) P<0.001 | −0.01 (−0.04, 0.08) P=0.21 | −0.03 (−0.05, 0.02) P<0.001 |
| Additional rate of change after change point in MCI group (taps/second) | −0.18 (−0.27, −0.09) P<0.001 | −0.04 (−0.08, −0.01) P=0.008 | −0.13 (−0.22, −0.04) P=0.004 |
| Change point location (years) in relation to MCI diagnosis* | −3.75 (−4.66, −1.66) | 5.91 (−6.50, 10.0) | −3.00 (−4.91, −0.16) |
| Number of finger with non-dominant hand | |||
| Rate of annual change prior to change point in MCI group (taps/second) | −0.03 (−0.03, −0.02) P<0.001 | −0.04 (−0.05, −0.02) P<0.001 | −0.01 (−0.02, −0.002) P=0.10 |
| Additional rate of change after change point in MCI group (taps/second) | −0.15 (−0.19, −0.09) P<0.001 | −1.04 (−1.63, −0.45) P=0.001 | −0.11 (−0.15, −0.08) P<0.001 |
| Change point location (years) in relation to MCI diagnosis* | −2.66 (−4.33, 0.25) | −6.16 (−6.83, 7.66) | −0.41 (−2.88, 1.75) |
Results are rate of change or year (95% CI)
Positive values indicate that a change point occurred prior to MCI conversion, and negative values indicate a change point occurred after the MCI conversion
UNK (Unknown) based on currently available information due to left censoring.
Figure 1.
Change point example for gait speed of MCI converters in relation to the average age of MCI conversion in men and women.
Although the change point analysis is modeled relative to MCI diagnosis, we wished to further test that the change point was not also present in non-converters as a result of a specific age. Therefore, we also examined the change point among non-converters relative to the age of 89.9 years, the average age of conversion in MCI converters. No change point for non-converters was found.
Tapping
There was a significant decline of tapping speed over time in the mixed effects model of 0.02 taps/s/yr (p<0.0001) and 0.01 taps/s/yr (p<0.002) for the dominant and non-dominant hands, respectively, for all participants. This shows the effect of age on tapping speed. MCI converters had a further decline of 0.02 taps/s/yr (p<0.003) for the dominant hand and 0.03 taps/s/yr (p<0.0001) for the non-dominant hand compared to non-converters.
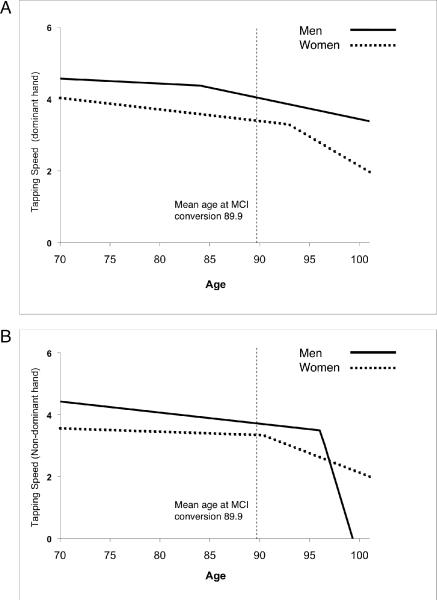
Change point analysis of tapping speed showed that change-points occurred after MCI onset (indicated by negative values) with the confidence interval including 0 for non-dominant hand and male dominant hand, suggesting that tapping speed changes close to or after the time of MCI conversion (Table 2; Figure 2).
Figure 2.
Change point example for tapping speed for MCI converters in men and women. A) dominant hand; B) non-dominant hand.
Discussion
Our results show a difference in rates of change in gait and tapping speed with aging between MCI converters and non-converters. Further, a change point was identified where acceleration in the decline of gait speed occurs approximately 12 years prior to MCI onset in a combined analysis for men and women. Change points occurred approximately 14 years prior to MCI onset in men and approximately 6 years for women. Although change points were found with tapping, this occurred after the onset of cognitive impairment in most analyses (the association of this change with the onset of later dementia was not assessed in our current analysis). This suggests that change in the rate of decline in gait speed may be a sensitive marker of cognitive changes distinct from the general motor slowing demonstrated with tapping speed.
Men and women were found to have different change points. There was no significant difference in follow-up time or time to MCI conversion to account for these differences. One possible explanation is that there is a difference in baseline gait speed in women between converters and non-converters, suggesting that the change point may have occurred in women prior to the start of this study. It is possible we may have found an earlier change point for women if we had a larger sample with longer duration of follow-up. Another possibility is that the different change points may be attributed to underlying gender-specific physiological differences.
Several studies have examined the prediction of baseline gait speed and other motor signs to the future development of cognitive impairment6, 7, 28 or dementia8, 29 using survival analysis or linear regression analysis. We are not aware of other studies that have prospectively examined the rates of change in gait speed or other motor signs and their relationship to incident MCI. We used up to 20 years of data to determine rates of motor changes and to identify the earliest time at which these changes occur in relation to clinical findings of cognitive impairment.
The sensitivity of gait changes to early cognitive changes may be best understood if gait is viewed as a complex cognitive task.30–32 Gait requires an interplay of attention, executive function and visuospatial function, as well as the motor processing functions of the motor cortex, basal ganglia and cerebellum. Therefore, the same mechanisms that underlie decline in cognitive functioning may be associated with decline in gait. Gait speed change may thus be a bellwether of the efficiency of the central integration of multiple cognitive domains needed for this complex task. Decline in gait speed may also be viewed as part of a larger construct of physical frailty in the elderly. Physical frailty is common in the elderly and includes measures of gait speed, strength, body composition and fatigue and is associated with incident dementia and AD pathology.33
The underlying pathophysiology behind motor decline is not clear. Motor slowing and parkinsonism have been shown to be related to periventricular white matter changes.34–36 There may also be a relationship between gait dysfunction and the presence of neurofibrillary tangles in the substantia nigra37 and markers of Alzheimer's disease pathology in the frontal lobes and basal ganglia.38, 39
Our study limitations include: The change point model requires large samples and long-term follow-up and may be difficult to generalize to individuals due to inter-individual variability. This model does not allow for time-varying covariates which limits the ability to assess the contributions of health conditions that develop during follow-up. We were unable to determine the upper confidence limits of the change point in gait speed for men and the combined analysis due to left censoring. This may be due to the need for a larger sample or longer follow-up. Additionally, the change point is calculated relative to the age of MCI onset and the use of alternate criteria to define the age of MCI onset could move the change point either earlier or later than the values reported here.
Despite these limitations, there are several strengths to our study. We used longitudinal data with up to 20 years of follow-up for some participants. Standardized, validated testing measures were used in the evaluations. Our participants were generally healthy with no major comorbid illnesses at baseline and a low rate of development of intercurrent illnesses, which were accounted for in subsequent analyses.
This study complements findings from other studies in this cohort showing accelerated cognitive decline on neuropsychological testing 3 to 4 years prior to MCI and accelerated expansion of ventricular volumes 2 years prior to the onset of MCI.23, 24 Future studies may compare these different methods for assessing disease progression to determine which is the more sensitive measure predicting the onset of cognitive impairment. The use of annual data may be complemented by use of more continuous, home-based gait monitoring. This allows for more frequent and ecologically representative assessment of gait speeds which suggest that there are differences in the variance of daily acquired gait speeds between MCI and normal subjects.40 These findings have important implications for identifying cognitive impairment at the earliest pre-clinical stages when initiation of disease-modifying therapies may be most beneficial.
Acknowledgements
The authors thank the research volunteers and family members for their invaluable donation to research, and the faculty and staff of the NIA Layton Aging and Alzheimer's Disease Center.
This study was supported by the Department of Veterans Affairs; National Institute on Aging (P30 AG008017, R01 AG024059); National Center for Research Resources (NCRR), and K01AG023014.
References
- 1.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001 Dec;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Schupf N, Manly J, Marder K, Tang MX, Mayeux R. Association between mild parkinsonian signs and mild cognitive impairment in a community. Neurology. 2005 Apr 12;64(7):1157–1161. doi: 10.1212/01.WNL.0000156157.97411.5E. [DOI] [PubMed] [Google Scholar]
- 3.Boyle PA, Wilson RS, Aggarwal NT, et al. Parkinsonian signs in subjects with mild cognitive impairment. Neurology. 2005 Dec 27;65(12):1901–1906. doi: 10.1212/01.wnl.0000188878.81385.73. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006 Dec;63(12):1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 5.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008 Jul;56(7):1244–1251. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998 May;50(5):1496–1498. doi: 10.1212/wnl.50.5.1496. [DOI] [PubMed] [Google Scholar]
- 7.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002 Apr;59(4):601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 8.Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA. Gait slowing as a predictor of incident dementia: 6-yaer longitudinal data from the Sydney Older Persons Study. J Neurol Sci. 2005 Mar 15;229–230:89–93. doi: 10.1016/j.jns.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Bennett DA, Beckett LA, Murray AM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996 Jan 11;334(2):71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol. 2005 Feb;62(2):297–302. doi: 10.1001/archneur.62.2.297. [DOI] [PubMed] [Google Scholar]
- 11.Verghese J, Levalley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006 Feb;54(2):255–261. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Schneider JA, Beckett LA, Evans DA, Bennett DA. Progression of gait disorder and rigidity and risk of death in older persons. Neurology. 2002 Jun 25;58(12):1815–1819. doi: 10.1212/wnl.58.12.1815. [DOI] [PubMed] [Google Scholar]
- 13.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007 Jan;55(1):11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaye JA, Oken BS, Howieson DB, Howieson J, Holm LA, Dennison K. Neurologic evaluation of the optimally healthy oldest old. Arch Neurol. 1994 Dec;51(12):1205–1211. doi: 10.1001/archneur.1994.00540240049015. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 Nov;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 17.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 18.Kiernan RJ, Mueller J, Langston JW, Van Dyke C. The Neurobehavioral Cognitive Status Examination: a brief but quantitative approach to cognitive assessment. Ann Intern Med. 1987 Oct;107(4):481–485. doi: 10.7326/0003-4819-107-4-481. [DOI] [PubMed] [Google Scholar]
- 19.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995 Feb;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 20.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990 Mar;31(3):545–548. [PubMed] [Google Scholar]
- 21.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. The Haworth Press, Inc.; NY: 1986. pp. 165–173. [Google Scholar]
- 22.Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Stat Med. 2000 Jun 15–30;19(11–12):1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Carlson NE, Moore MM, Dame A, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008 Mar 11;70(11):828–833. doi: 10.1212/01.wnl.0000280577.43413.d9. [DOI] [PubMed] [Google Scholar]
- 24.Howieson DB, Carlson NE, Moore MM, et al. Trajectory of mild cognitive impairment onset. J Int Neuropsychol Soc. 2008 Mar;14(2):192–198. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- 25.Neter J, Wasserman W, Kutner MH. Applied linear statistical models: regression, analysis of variance, and experimental designs. 3rd ed. Irwin; Homewood, IL: 1990. [Google Scholar]
- 26.Casella GBR. Statistical Inference. 2nd ed. Duxbury Press; Belmont, CA: 2001. [Google Scholar]
- 27.Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am J Psychiatry. 1997 Jan;154(1):4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- 28.Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009 Jun 23; doi: 10.1093/ageing/afp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007 Sep;78(9):929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006 Mar;20(2):215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 31.Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson's disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006 Oct–Dec;32(4):411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander NB, Hausdorff JM. Guest editorial: linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2008 Dec;63(12):1325–1328. doi: 10.1093/gerona/63.12.1325. [DOI] [PubMed] [Google Scholar]
- 33.Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008 Aug 12;71(7):499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benson RR, Guttmann CR, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002 Jan 8;58(1):48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- 35.Onen F, Feugeas MC, Baron G, et al. Leukoaraiosis and mobility decline: a high resolution magnetic resonance imaging study in older people with mild cognitive impairment. Neurosci Lett. 2004 Jan 30;355(3):185–188. doi: 10.1016/j.neulet.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 36.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008 Jul 8;71(2):108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006 Jan;59(1):166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 38.Gearing M, Levey AI, Mirra SS. Diffuse plaques in the striatum in Alzheimer disease (AD): relationship to the striatal mosaic and selected neuropeptide markers. J Neuropathol Exp Neurol. 1997 Dec;56(12):1363–1370. doi: 10.1097/00005072-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Suva D, Favre I, Kraftsik R, Esteban M, Lobrinus A, Miklossy J. Primary motor cortex involvement in Alzheimer disease. J Neuropathol Exp Neurol. 1999 Nov;58(11):1125–1134. doi: 10.1097/00005072-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Hayes TL, Abendroth F, Adami A, Pavel M, Zitzelberger TA, Kaye JA. Unobtrusive assessment of activity patterns associated with mild cognitive impairment. Alzheimers Dement. 2008 Nov;4(6):395–405. doi: 10.1016/j.jalz.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]