Abstract
In Pavlovian overshadowing, a stimulus that predicts a biologically important event reduces conditioning to another, equally predictive stimulus. We tested the effects of an opioid antagonist and dopamine agonist on the ability of a salient white-noise to overshadow a less salient light. Rats were conditioned to fear a light or a noise-light compound using a mild footshock. Compound-conditioned rats trained under the saline vehicle revealed significant overshadowing of the light by the noise. This overshadowing effect was significantly attenuated in animals trained under the opioid antagonist naltrexone, consistent with an opioid-mediated negative feedback model of conditioning. In line with predictions made by negative feedback type models, we failed to obtain overshadowing with few trials, suggesting that the processes underlying conditioning during initial trials do not contribute to the opioid-dependent Pavlovian overshadowing obtained in our preparation. Lastly, we compared the involvement of dopamine-mediated and opioid-mediated processes in overshadowing by conditioning animals under the partial dopamine (DA) D1 receptor agonist SKF 38393 or the opioid antagonist naltrexone. Both naltrexone and SKF 38393 were found to attenuate overshadowing, however, the behavioral profiles produced by each pharmacological manipulation were distinct. Collectively, these studies demonstrate an important role for both opioid and dopamine mediated-processes in multiple-trial overshadowing.
Keywords: Overshadowing, opioids, fear, error-correction, negative feedback, dopamine
When a discrete stimulus (conditional stimulus [CS]) is paired with a biologically significant stimulus (unconditional stimulus [US]), an organism will learn an association between the two, as exhibited by subsequent, conditional responding (CR) to the CS alone (Pavlov, 1927). For example, if a light presentation is followed by a brief footshock, an animal will subsequently display fear-related behaviors in response to that light. However, if an additional cue, such as a noise stimulus, is presented in conjunction with the light, the degree to which that light can be fear conditioned is reduced (Mackintosh, 1971). In other words, the noise overshadows the light during conditioning. Like Kamin’s blocking effect (Kamin, 1968; 1969) – wherein conditioning of a stimulus is blocked by being presented in compound with a previously conditioned stimulus, overshadowing seems to rest on the degree to which the US is surprising.
This idea of US-surprisingness was elegantly captured by Rescorla and Wagner (1972), whom formulated a mathematical model to describe the course of conditioning. According to their model, the less predictable (i.e. more surprising) a US is, the more power it has as a reinforcer. The Rescorla-Wagner model states:
Where ΔV refers to the change in associative strength between a particular CS and US on a given trial. The term α is a learning rate parameter determined by the salience of the CS and accounts for the more rapid learning that accrues to attention-grabbing CS’s. The term λ refers to US intensity and VΣ refers to the associative strength of all stimuli present on that trial. Put simply, learning about a CS on a given trial is determined by how surprising the US is on that trial. Thus, surprise is essentially an error term – the difference between the predicted and the actual value of a reinforcing stimulus on a given conditioning trial (λ − VΣ). It is this element of surprise, or prediction error, which regulates conditioning.
A strength of the Rescorla-Wagner model is that it can be used to perform calculations that describe the course of conditioning on a trial-by-trial basis. In particular, the model is excellent at predicting US-limited phenomena such as blocking and overshadowing, because the surprise term (λ − VΣ) can clearly be lowered for a blocked or overshadowed stimulus because the other cue in the compound contributes to VΣ. In the case of overshadowing, the more salient CS and the US build an association early on in conditioning, rapidly bringing the error term (λ − V) to near zero and thereby limiting further learning at later trials for either CS. Consequently, the Rescorla-Wagner model makes the prediction that if there weren’t US limitations on conditioning (VΣ), then eventually all CS’s could fully condition to asymptote.
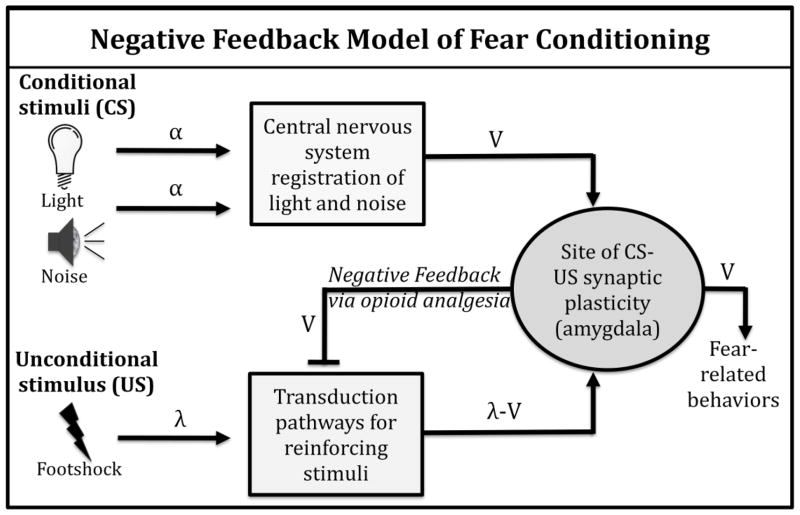
Current knowledge of the neural mechanisms responsible for Rescorla-Wagner-like calculations makes empirical investigations of its predictions quite feasible (Fanselow, 1986, 1998; Kim, Krupa, & Thompson, 1998). For example, pairing an initially neutral CS with an aversive foot-shock-US conditions an endogenous opioid-mediated analgesic response (Fanselow & Baackes, 1982; Fanselow & Bolles, 1979a). Thereafter, this conditional analgesia modulates the painful impact of the shock-US, thereby diminishing the effectiveness of the US as a reinforcer and providing negative feedback on the acquisition of fear. In essence, this “negative feedback model” (Figure 1) provides a neural mechanism by which calculations of the Rescorla-Wagner kind are automatically performed and US-limited phenomena are predicted (Fanselow, 1981, 1998). In a similar vein, Schull (1979) described how the acquisition of a conditional opioid response could provide an “opponent process” that antagonized a US’s ability to condition.
Figure 1.
Schematic diagram of the negative feedback model of Pavlovian fear conditioning. The model describes how Rescorla-Wagner calculations may be made (the symbols adjacent to the arms refer to the Rescorla-Wagner model). The diagram displays how input from two CS’s (e.g. as in overshadowing) and input from the US converge within a single brain site (e.g. amygdala in fear conditioning; cerebellum in eyeblink conditioning) to ultimately produce conditional responses. For fear conditioning, the model depicts a conditional analgesia that feeds back onto the structure that registers the noxious US to regulate conditioning. Similar operations occur within an anatomical distinct circuit for eyeblink conditioning (see Fanselow, 1998). Figure based on Bolles & Fanselow, 1980; Fanselow, 1986; 1998.
The discovery of negative feedback-type mechanisms in other Pavlovian preparations has helped to support their ubiquity. For example, negative-feedback type mechanisms have been established in eyeblink conditioning via GABAergic inhibitory feedback onto olivary neurons conveying airpuff-US information to the cerebellum (Kim, et al., 1998). Further support for this view comes from findings that specific groups of neurons fire in proportion to predicted magnitudes of the error signal (Kim, et al., 1998; see Schultz, 2006 for review). Systematic studies from Schultz and colleagues found that in appetitive conditioning, midbrain dopamine neurons fire in a manner generally consistent with the formation of a prediction error for signaling reward (e.g. Fiorillo, Newsome, & Schultz, 2008; Fiorillo, Tobler, & Schultz, 2003; Hollerman & Schultz, 1998; Tobler, Dickinson, & Schultz, 2003; Tobler, Fiorillo, & Schultz, 2005).
One benefit of such models is that they are readily testable. For instance, in fear learning, conditional analgesia is mediated by endogenous opioids, which can be blocked by opioid antagonists. Indeed, a number of fear conditioning studies have done just that. The administration of an opioid antagonist has been shown to eliminate blocking (Fanselow & Bolles, 1979a; McNally, Pigg, & Weidemann, 2004; Schull, 1979), attenuate unblocking (McNally, et al., 2004), lift the limits on conditional asymptotes (Young & Fanselow, 1992), eliminate Hall-Pearce negative transfer (Young & Fanselow, 1992), and prevent over-expectation of fear (McNally, et al., 2004).
While a number of studies have offered strong support for the role of endogenous opioids in regulating US-limited Pavlovian phenomena such as blocking, none have determined whether or not they play a role in Pavlovian overshadowing. Given that overshadowing, like blocking, can be thought of as due to US-limitations, it should similarly be abolished by administration of an opioid antagonist. However, because overshadowing, unlike blocking, may allow for direct sensory competition between novel CS’s, it may be regulated by distinguishable mechanism(s). Thus, in the experiments presented below, we investigated whether or not overshadowing, like blocking, could be explained by an endogenous opioid-mediated negative feedback circuit. In Experiment 1, we tested the effects of the opioid antagonist, naltrexone, on eight-trial overshadowing. Experiments 2 and 3 investigated whether one-trial or two-trial overshadowing could provide a possible, “pre” conditional analgesia, contribution to overshadowing. Lastly, Experiment 3 investigated whether dopamine-mediated, like opioid-mediated processes, contribute to overshadowing.
Experiment 1
The aim of Experiment 1 was to reproduce the classic overshadowing effect in our fear conditioning preparation and determine if it could be blocked by administration of an opioid antagonist. Rats were injected with the opioid antagonist naltrexone (i.p., 7mg/kg) or the saline vehicle prior to conditioning. This dose was selected because of its use in prior work on fear-induced analgesia (Fanselow & Baackes, 1982; Helmstetter & Fanselow, 1987). Animals were conditioned to fear a noise-light compound (overshadowing groups), a light alone (light control groups) or simply received unsignaled shocks (no-CS control groups). All groups were then tested to the light CS to assess overshadowing (saline groups) and its blockade (naltrexone groups).
Method
Subjects
The subjects were 48 naïve, adult male Long-Evans rats, initially weighing 270–300 g, purchased from Harlan (Indianapolis, IN). After arrival, rats were individually housed in the Herbert L. Washington Vivarium in the Psychology Department at the University of California, Los Angeles (Los Angeles, CA) and kept on a 12-hour light/dark cycle. Animals were given a one-week adjustment period prior to the start of the experiment, with daily handling (one-two minutes per rat per day), to habituate them to the experimenter, and access to food and water ad libitum. The procedures used in this experiment were in accordance with policy set by the Division of Laboratory Animal Medicine and approved by the Animal Research Committee at the University of California, Los Angeles.
Apparatus
Conditioning and testing were conducted in two distinct sets of 8 identical observation chambers (Lafayette Instruments, North Lafayette, IN). Chamber sets were situated in separate, isolated rooms. A video camera mounted on the opposing wall of the chambers enabled the experimenter to record and observe behavior in an adjacent room during the entire experiment. All chambers were constructed of Plexiglas (back wall and front door) and aluminum (side walls) with shockable grid flooring. The grids were wired to a shock generator and scrambler (Med Associates, St. Albans, VT) for the delivery of the foot-shock US. A speaker was mounted on the side of one wall to enable the delivery of the auditory CS.
Two distinct “contexts” were used – one for conditioning (“A”) and one for testing (“B”). Using a novel context B for the light test allowed us to control for any baseline contextual fear conditioned to A during training. These were counterbalanced across groups. The contexts were differentiated by chamber-shape, illumination, odor, cleaning solution, background noise, and transport. All chambers were cleaned with a 10 % bleach solution following each day of behavioral testing.
Context A
Chambers measured 28 × 21 × 22 cm. The grid floor consisted of 18 rods (4mm diameter) spaced 1.5 cm apart (center-to-center) on a flat plane. One panel of fluorescent lights on the ceiling provided illumination. Removable pans were sprayed with simple green to provide a distinct odor. Each chamber was cleaned with a 5% sodium hydroxide solution between squads. Background noise was given by a high-speed fan (60 dB, A-scale) positioned on the floor across the chambers. Animals were transported to the context in squads of 8 in their own homecages, which were slid onto hanging racks mounted to a portable cart and covered with a white sheet.
Context B
Chambers measured 28 × 21 × 22 cm. A staggered grid floor, made up of 17 rods (4mm diameter) arranged as two rows, spaced 1 cm apart vertically (rods in each row were spaced 2.6 cm apart horizontally), constituted the flooring. A triangular roof was created using opaque, plastic inserts that sloped inwards at the ceiling. A panel of red fluorescent lights on the ceiling provided a dim, red illumination (30 W). Removable pans were sprayed with a thin film of imitation coconut solution (11%) to provide a distinct odor. Each chamber was cleaned with a 1 % acetic acid solution between squads. There was no background noise. Animals were transported to the context in squads of 8 in two large, square, black plastic tubs divided in four with a black plastic insert. The tubs had bedding covering the floors and were placed on a distinct cart for transport.
Drugs
Naltrexone hydrochloride (Sigma Chemical, St. Louis, MO) was dissolved in 0.9% saline to obtain a concentration of 7 mg/ml, which was injected at a volume of 1 ml/kg to provide a 7 mg/kg dose. This systemic dose has previously been shown to block conditional analgesia (Fanselow & Baackes, 1982; Helmstetter & Fanselow, 1987). The 0.9% saline vehicle was used for control intraperitoneal (i.p.) injections.
Procedure
Table 1 gives an outline of the behavioral training procedure and groups. In the conditioning phase (day 1), rats were transported from the vivarium to the laboratory in squads of 8. They were first brought into a sound-attenuating “injection” room where they were either injected with naltrexone or saline. Ten minutes later, rats were transported to and placed in experimental chambers, which were located in a nearby sound-attenuating room (context “A”). Following a three-minute acclimation period, rats in the light conditioning groups received eight light CS presentations (two, 95-watt roomlights flashing on/off every .5 sec for 30 sec), which co-terminated with a foot-shock US (2 sec, 0.8 mA). Rats in the compound conditioned groups received the same pairings, but with the addition of a white-noise CS (80 dB) presented concurrently with the light CS to form a compound. Background noise was provided by a small fan (60 dB) positioned on the floor of context A. There was no background noise in context B. Rats in the no-CS groups simply got the same 8 foot-shocks but they were unsignaled by any discrete CS. The inter-trial interval (ITI) for all the groups was four minutes. One minute following the final foot-shock, animals were transported back to the vivarium. The following day, rats were tested for fear to the light CS. To avoid confounding light-fear with any baseline contextual fear, animals were transported to a novel context (“B”) for testing. After a three-minute acclimation period in context B, rats were tested for freezing to one presentation of the light CS (30 seconds). No injections were given on test day.
Table 1.
Experimental Design
| Conditioning | Test | ||||
|---|---|---|---|---|---|
| CS Type | Drug | Trials | |||
| Light | X | Saline | X | 8 | Light? |
| Compound | Naltrexone | ||||
| No CS | |||||
Note. Animals were conditioned to fear a light CS, compound light-noise CS or trained in the absence of an explicit CS (No CS group). Eight-trial conditioning proceeded under the influence of the opioid antagonist naltrexone or the saline vehicle. The CS type and drug condition were manipulated using a 3 × 2 factorial design. The following day, animals were tested to one presentation of the light CS.
Data Analysis
Behavior during the entire experiment was recorded onto videotape by cameras mounted on the wall opposite the experimental chambers. Freezing behavior (an index of fear defined by the absence of all movement other than that required for respiration) was subsequently scored by an observer blind to experimental conditions. Each animal was assessed for freezing behavior (binary/yes or no) every 8 seconds. Raw scores were then transformed into a freezing percentage for each animal.
Freezing data were statistically analyzed using two-way (CS-type × Drug) between-subjects analyses of variance (ANOVAs). A repeated measures (trial) mixed two-way ANOVA was used to analyze conditioning. The single light presentation at test was analyzed using a two-way (CS-type × Drug) ANOVA. Post-hoc tests were performed following significant findings. The level of significance used for all analyses was p < .05.
Results
Four rats were removed from the analyses because they did not receive the footshock US. As a result, the Light-Naltrexone group had n=6, both No CS groups had n=7, and the remaining groups had n=8. The mean (± SEM) freezing percentages to the CS during each conditioning trial are shown in Figure 2 for animals trained under saline vs. naltrexone (collapsed across CS-type conditioned during acquisition (A) as well as broken down by CS type (B–D)).
Figure 2.
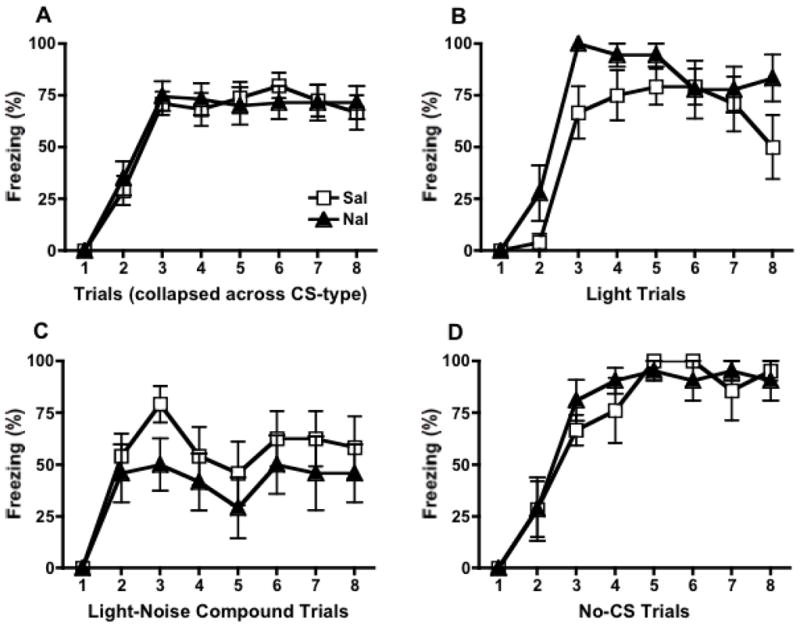
Fear acquisition. Animals were trained under the influence of naltrexone or the saline vehicle. Mean (+/− SEM ) percent freezing to each respective CS during eight trials of conditioning is shown. Data collapsed across the type of CS that was used for conditioning reveals no significant overall effect of drug on acquisition (A). Although there was a significant effect of CS-type, all animals showed significant fear acquisition regardless of whether they were trained with the Light CS (B), the Light-Noise compound CS (C) or without any discrete CS (D). Acquisition for each respective CS was not significantly different for animals trained under saline compared to naltrexone.
All animals were able to acquire fear regardless of drug condition. The repeated measures mixed two-way ANOVA revealed no significant difference between animals trained under saline compared to naltrexone, F(1, 42)=0.01, ns (Figure 2A). A main effect of CS-type, F(2, 23)=5.12, p<.05 was found, demonstrating significant asymptotic differences between groups (Figure 2B–D). No significant CS-type × drug interaction was found, F(2,38)=2.45, ns). Post-hoc tests (Tukey’s Multiple Comparisons Test) revealed a significant difference (p < .05) between the Compound and No-CS groups (Figure 2C–D). However, these acquisition data do not distinguish context and CS influences on performance. Furthermore, they reflect freezing as sampled during three very different circumstances (to the compound CS, the light-CS, or to the context for the no CS group), which may produce differences in overall behavior (i.e. more orienting behavior in Compound CS groups, which would negatively influence freezing). Thus, these data should simply be taken to indicate that both drug and saline-control groups were able to acquire fear.
The mean (± SEM) percentages of freezing during the 30-second light test are shown in Figure 3. A two-way ANOVA (CS-type × drug) revealed a highly significant main effect of the CS-type trained during conditioning, F(2, 38) = 60.89, p < 0.0001 and a significant CS-type × drug interaction, F(1,38) = 3.598, p < 0.05. Post hoc analyses (Bonferroni) indicated a significant difference between compound-trained and light-trained animals conditioned under saline (p < 0.001), establishing the basic overshadowing effect in our control groups. This overshadowing effect was attenuated by naltrexone, as animals from the same respective groups conditioned under naltrexone failed to show a significant overshadowing effect. These analyses also revealed a significant effect of drug for animals in the compound conditioned groups (p < .05), but not for animals in either the light-trained or no-CS trained groups. This suggests that administration of naltrexone on its own does not support fear conditioning.
Figure 3.
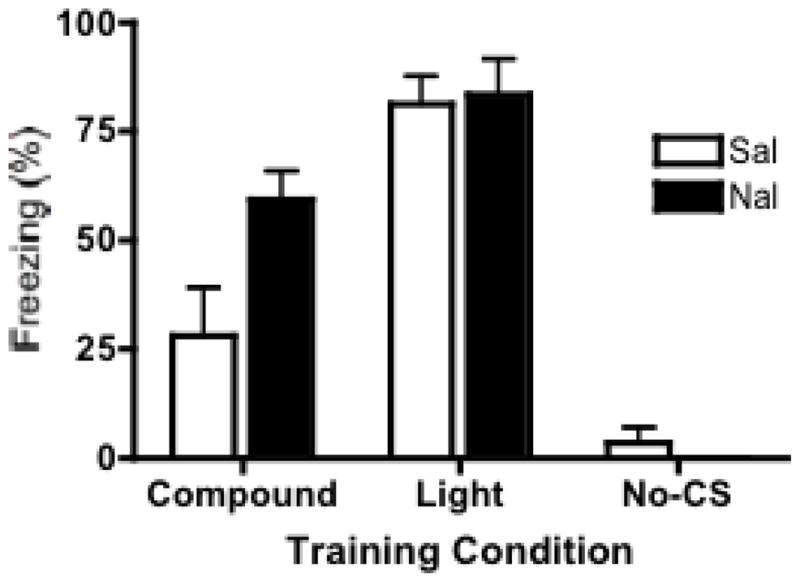
Light fear test. Mean (+/− SEM) percent freezing during test phase, in which animals received one presentation of the light CS. Pavlovian overshadowing is shown in the saline trained animals, as there was a significant difference between compound-conditioned vs. light-conditioned animals. This overshadowing was attenuated by naltrexone, as there was no significant difference between compound and light-conditioned animals, although a non-significant residual amount of overshadowing remained.
Discussion
This experiment established the overshadowing effect in our fear conditioning preparation, as saline control rats trained with a noise-light compound froze significantly less to a test of the light than did rats trained with just the light. In addition, the data show that administration of the opioid antagonist naltrexone significantly rescued responding to the light in compound-conditioned animals, preventing significant overshadowing of the light by the noise. These results support the idea that naltrexone reduces the competition that normally occurs between concurrently presented CS’s in overshadowing by blocking the analgesic component of the CR that limits the US’s ability to support conditioning. The attenuation of this competition due to a blockade of opioid-regulated negative feedback is consistent with the idea that endogenous opioids play a significant role in calculating the error signal that normally drives Pavlovian learning (Bolles & Fanselow, 1980; McNally, et al., 2004).
Nevertheless, one could raise the alternative interpretation that naltrexone may not actually affect overshadowing proper, but rather, may be aversive in its own right, thereby increasing conditioning to any CS. This could explain why animals trained with the compound CS froze more to the light if they were trained under naltrexone as opposed to saline. However, this implies that animals trained with the light CS should also show enhanced freezing to the light. While this was not observed, the lack of a difference between the light-trained groups could be due to a ceiling effect. One line of evidence against this is that no-CS animals trained under naltrexone do not show an enhancement in freezing. Although this was not demonstrated in a naltrexone paired discrete CS, there is substantial evidence showing that naltrexone does not influence conditioning unless there is a cue predicting shock (Fanselow & Bolles, 1979a, 1979b). Additionally, if naltrexone could support conditioning directly, it should enhance freezing during shock-free tests of fear; which does not occur (Fanselow, 1981, 1986; Helmstetter & Fanselow, 1987).
Although the significant overshadowing of the lights by the noise seen in saline trained animals was prevented in animals trained under naltrexone, one could argue that a residual – though non-significant – amount of overshadowing remains unaccounted for in naltrexone trained animals. One possibility is that this residual overshadowing is a result of insufficient opioid antagonism, however, the relatively high dose of naltrexone we used suggests that this was likely not the case. Another possibility is that there may exist additional mechanism(s) that work in conjunction with a negative feedback mechanism to explain overshadowing.
Experiment 2 attempts to address the issue of residual overshadowing, as well as the ceiling effect in the light-conditioned groups, by reducing the number of trials used in conditioning. We examined whether or not overshadowing occurs in a single compound trial in our preparation. In one-trial overshadowing, both CS’s are still novel and so should not elicit any conditional analgesia. Thus, any overshadowing that occurs during this trial could not be caused by opioid-mediated negative feedback. Furthermore, because opioid antagonists have no enhancing effect on one-trial contextual fear conditioning (Fanselow & Bolles, 1979a), one-trial overshadowing shouldn’t be affected by naltrexone. Thus, Experiment 2 tested whether the mechanism behind one-trial overshadowing might explain residual overshadowing unaccounted for by opioid-mediated negative feedback.
Experiment 2
The overshadowing effect can be acquired with just one conditioning trial (James & Wagner, 1980; Mackintosh, 1971; Mackintosh & Reese, 1979). This one-trial overshadowing effect suggests that there exists direct competition between conditional stimuli before they even become predictors of the US (either because of differences in salience, biological relevance, attention grabbing capabilities, immediate environmental factors, etc). Unlike the overshadowing that occurs with multiple trials, one-trial overshadowing cannot be explained in terms of US-surprisingness because the US is fully surprising on the first trial. Similarly, Rescorla-Wagner type US limitations or a negative feedback model also fail to account for one-trial overshadowing, as these models require at least two CS-US pairings to account for overshadowing. Thus, the presence of one-trial overshadowing suggests the action of other processes in overshadowing, such as the direct interaction between CS’s. Therefore, in addition to replicating the findings of Experiment 1, Experiment 2 attempted to establish the existence of one-trial overshadowing in our preparation and determine the effects of naltrexone on it.
We hypothesized that naltrexone would have no effect on one-trial overshadowing because it should work directly on negative feedback (where more than one trial is required). On the other hand, if naltrexone works simply by enhancing the perceived intensity of the shock, then naltrexone might facilitate conditioning during any given trial (Fanselow & Bolles, 1979b). Experiment 2 followed a procedure very similar to that used for Experiment 1, only with the addition of one-trial overshadowing groups and the elimination of the no-CS control groups. Rats were injected with either the drug naltrexone (i.p., 7mg/kg) or the vehicle saline before conditioning to the noise-light compound or the light alone for either one or eight trials. All animals were then tested to the light CS in the absence of any drug.
Method
Subjects, Apparatus, and Drugs
The subjects were 56 naïve, adult male Long-Evans rats, initially weighing 270–300 g, purchased and maintained in the same way as described in Experiment 1. Conditioning chambers and distinct “contexts” were the same as used in Experiment 1. Drug manipulations, volume, concentration and dosage were identical to Experiment 1.
Procedure
Table 2 gives an outline of the behavioral procedure used. The experimental design and groups are similar to Experiment 1, except that the No-CS groups were omitted, and one-trial overshadowing groups were added for each condition. Animals were transported in squads of 7. The groups were removed from experimental chambers and returned to their homecages one minute following the termination of the last trial.
Table 2.
Experimental Design
| Conditioning | Test | ||||
|---|---|---|---|---|---|
| CS Type | Drug | Trials | |||
| Light | X | Saline | X | 1 | Light? |
| Compound | Naltrexone | 8 | |||
Note. Animals were conditioned to fear a light CS or compound light-noise CS. One-trial or eight-trial conditioning proceeded under the influence of the opioid antagonist naltrexone or the saline vehicle. The CS type, drug condition and number of trials were manipulated using a 2 × 2 × 2 factorial design. The following day, animals were tested to one presentation of the light CS.
Data Analysis
Behavior was measured, assessed and analyzed as in Experiment 1. CS-type × Drug analyses of variance (ANOVAs) on freezing percentages to the single light presentation during test were performed for 1-trial and 8-trial groups respectively. Post-hoc tests were performed following significant findings. The level of significance used for the analyses was p < .05.
Results
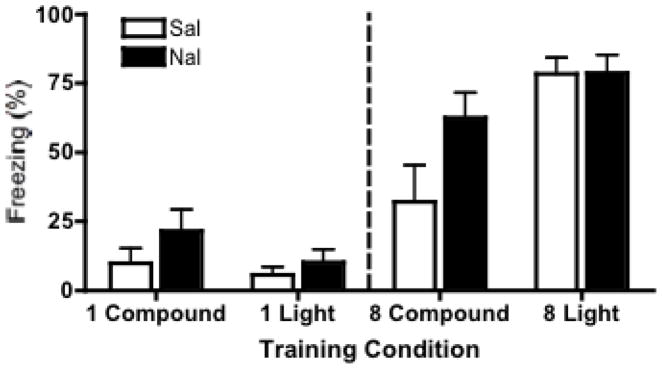
The mean (± SEM) percentages of freezing during the 30-second light test are shown in Figure 4. Each of the eight conditions had an n=7. Experiment 1’s finding – the overshadowing effect and its attenuation by naltrexone – was replicated here for animals receiving 8 CS-US trials. For the 8-trial groups, there was a significant main effect of CS-type F(1,24) = 11.66, p < .01. Post-hoc analyses (Bonferroni) found that amongst saline-treated groups, compound conditioned animals froze significantly less to the light than light conditioned animals (p < .01), revealing significant overshadowing in saline-controls. This difference was not significant for animals trained under naltrexone, replicating naltrexone’s prevention of significant overshadowing. In addition, animals in the compound conditioned groups froze significantly more (p < .05) to the light if they had been trained with naltrexone compared to saline.
Figure 4.
Light fear test. Mean (+/− SEM) percent freezing during the light test phase in which animals received 1 presentation of the light CS. No one-trial Pavlovian overshadowing (left panels) was exhibited in the saline trained animals. Significant overshadowing and its attenuation by naltrexone were replicated in the eight-trial training procedure (right panels).
Animals conditioned with one trial failed to show any significant effect of CS-type F(1,24)=2.12, ns, drug F(1,24)=2.25, ns, or an interaction between the two F(1,24)=.43, ns. Failure to obtain one-trial overshadowing suggests that any process that causes one-trial overshadowing (e.g. James & Wagner, 1980) does not contribute to overshadowing in our preparation.
Discussion
Experiment 2 replicates the findings of Experiment 1 – supporting the role of endogenous opioid-mediated conditional analgesia in the regulation of Pavlovian fear. While evidence for one-trial overshadowing would support a direct interaction between conditional stimuli and suggest a CS-centered mechanism underlying overshadowing, Experiment 2 failed to detect one-trial overshadowing under the conditions used here. Thus, it seems unlikely that conditioning during the first trial contributes to the significant overshadowing effect we see with eight trials.
In addition, we found that naltrexone did not increase conditioning in any one-trial groups; thus, it seems unlikely that naltrexone directly supports fear conditioning to CS’s with which it is paired. This is not to say that naltrexone is not aversive in its own right, as there are substantial data indicating that opioid antagonists supports place aversion learning (e.g. Mucha & Iversen, 1984; Mucha, van der Kooy, O’Shaughnessy, & Bucenieks, 1982). However, such aversion is not sufficient to support the conditioning of fear such that CS’s come to elicit the species-specific defensive reactions that are the hallmark of fear responding (Bolles, 1970). Thus, we can conclude that naltrexone is doing more than just acting aversively to increase freezing levels. Rather, it seems to be working directly on the conditioning of stimuli that would otherwise be limited by the amount of conditioning the US could support.
Experiment 3
In contrast to US-limited models of conditioning, a number of theories stress the importance of CS-associability in conditioning. For example, both Mackintosh (1975) and Pearce & Hall (1980) (although for different reasons) suggest that conditioning is driven by the amount of attention a CS is able to garner, where the allocation of attention is a function of the extent to which the CS provides a unique prediction of the US. Thus, according to such models, Pavlovian effects such as blocking and overshadowing are the result of a lack of attention paid to a blocked or overshadowed CS.
Several researchers have proposed that these attentional processes are regulated by dopamine (DA). For example, both blocking and overshadowing were disrupted by administration of the indirect DA agonist amphetamine (Crider, Solomon, & McMahon, 1982; O’Tuathaigh, et al., 2003; Ohad D, Lubow RE, Weiner I, & J, 1987). The DA D1 receptor is likely responsible for these effects, as the selective DA D1 agonist SKF 38393 also attenuates overshadowing (O’Tuathaigh & Moran, 2002). Importantly, these studies used relatively few conditioning trials (e.g. two), raising the possibility that dopamine-mediated processes are important early on in conditioning. However, these studies leave open whether such processes continue to modulate conditioning as trials increase (and presumably negative-feedback processes begin to exert more control).
The focus on dopamine-mediated processes in conditioning – and their success in accounting for Pavlovian phenomenon – led us to examine whether these factors may contribute to overshadowing in our preparation as well. In particular, we were interested in whether SKF 38393, would block overshadowing with eight trials in the same way that it has been found to do so with two trials (O’Tuathaigh & Moran, 2002). Thus, we investigated the effects of both SKF 38393 and naltrexone on two-trial and eight-trial overshadowing. This allowed us to determine within a single experiment whether overshadowing could be similarly attenuated by opioid or dopamine mediated processes, or whether these processes contribute differentially to overshadowing produced by multiple (8) compared to few (2) trials. respectively.
Method
Subjects, Apparatus, and Drugs
Subjects were 80 naïve, adult male Long-Evans rats, initially weighing 270–300 g, maintained in the same way as described in Experiment 1. Conditioning chambers and distinct “contexts” were the same as used in Experiment 1. Opioid antagonism was achieved using naltrexone, as described in Experiment 1. Dopamine agonism was achieved using the selective DA D1 agonist SKF 38393 in a concentration of 5mg/kg (as described in O’Tuathaigh & Moran, 2002). Control groups received injections of the saline vehicle. Drug administration and volume was as described in Experiment 1.
Procedure
Table 3 gives an outline of the behavioral procedure used. Animals were transported in squads of 4 (transport as described in Experiment 1). Prior to behavioral conditioning, rats were injected with naltrexone, SKF 38393, or the saline vehicle (drug administration as described in Experiment 1). Animals were then conditioned with two or eight CS-US pairings, using either a compound noise-light CS or a light CS. One minute following conditioning, animals were transported back to their homecages.
Table 3.
Experimental Design
| Conditioning | Test | ||||
|---|---|---|---|---|---|
| CS Type | Drug | Trials | |||
| Light | X | Saline | X | 2 | Light? |
| Compound | Naltrexone SKF 38393 |
8 | |||
Note. Animals were conditioned to fear a light CS or compound light-noise CS. Two-trial or eight-trial conditioning proceeded under the influence of the opioid antagonist naltrexone, the saline vehicle or the partial dopamine (D1) agonist SKF 38393. The CS type, drug condition and number of trials were manipulated using a 2 × 3 × 2 factorial design. The following day, animals were tested to one presentation of the light CS.
Data Analysis
Behavior was measured, assessed and analyzed as in Experiment 2. CS-type × Drug analyses of variance (ANOVAs) on freezing percentage to the single light test (30 seconds) were performed for animals trained with 2 trials and 8 trials. Post hoc analyses were performed following any significant findings. The level of significance used for analyses was p < .05.
Results
Each group’s mean (± SEM) freezing percentage to a test of the light CS is shown in Figure 5. One rat fell ill during conditioning, resulting in the following group sizes: for the 2 trial condition there were n=8 for each group trained with compound-CS trials, and n=6 for each group trained with light-CS trials. For the 8 trial condition there were n=4 in both naltrexone-trained groups, n=9 for the compound-CS saline group, n=7 for the Light-CS saline group, n=7 for the compound-CS SKF group and n=6 for the Light-CS SKF group.
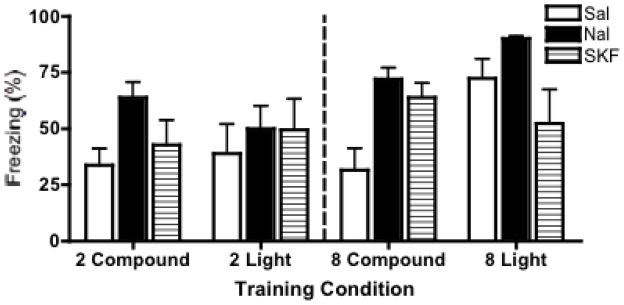
Figure 5.
Light fear test. Mean (+/− SEM) percent freezing during test phase in which animals received one test presentation of the light CS. No significant Pavlovian overshadowing was observed with two trials (left panels), making it difficult to interpret any effect of drug. Significant overshadowing is shown in the eight-trial training procedure (right panels), as saline treated animals froze significantly less in the compound compared to the light conditioned groups. The overshadowing effect was attenuated (albeit differently) by both naltrexone and SKF 38393.
A two-way (CS-type × Drug) ANOVA on freezing percentages for animals trained with two trials revealed no significant effect of the CS-type conditioned F(1,36)=.01, ns, drug F(2,36)=1.97, ns, or an interaction F(2,36)=.60, ns. In particular, there was no significant overshadowing observed in animals, as the difference between compound, as opposed to light conditioned saline groups was not significant. Thus, similar to Experiment 2, we were not able to get sufficient overshadowing with few trials, making it difficult to draw any conclusions about drug effects on overshadowing with few trials, despite a trend for increased freezing to the light in naltrexone-treated, compound-conditioned animals after just two conditioning trials.
For animals trained with eight trials, a two-way (CS-type × Drug) ANOVA revealed a significant effect of drug, F(2,31) = 3.835, p < .05 and a significant CS-type × Drug interaction, F(2, 31) = 4.03, p < .05. Post hoc analyses (Bonferroni) revealed significant overshadowing of the light by the noise, as saline animals froze significantly more to the light if they had been light-conditioned as opposed to compound-conditioned (p < .01). As in Experiments 1 and 2, overshadowing was again attenuated if animals were trained under naltrexone, as there was no significant difference in freezing to the light between naltrexone-trained compound and light conditioned animals. Again, animals conditioned with the compound CS froze significantly more to the light if they were trained under naltrexone as opposed to saline (p < .05).
Interestingly, the statistical pattern of results seen for animals trained under naltrexone was also found for animals trained under SKF 38393. Post hoc analyses (Bonferroni) revealed that the significant overshadowing seen in saline controls was also eliminated if animals were trained under SKF 38393, as SKF 38393 treated animals showed no significant difference between those conditioned with the compound CS and those conditioned with the light CS. In addition, compound conditioned SKF 38393 treated animals froze significantly more to the light than their saline controls (p < .05). However, it should also be noted that in 8-trial, compound conditioned animals, rats treated with SKF 38393 froze significantly less than rats treated with naltrexone (p < .05), suggesting that while both drugs attenuate overshadowing, they may be doing so in different ways.
Discussion
Similar to the one-trial conditioning conducted in Experiment 2, we found that overshadowing in our preparation did not occur with two trials. These data suggest that the processes responsible for overshadowing take several trials to emerge, which points to the importance of error-correction processes over direct sensory interactions. Interestingly, the increase in freezing to the light obtained when compound-conditioned animals were trained under naltrexone was already quite apparent after two trials of conditioning. This contrasts with the effect of naltrexone on compound-conditioned animals trained with one trial (Experiment 2). This difference between one-trial and two-trial conditioning under naltrexone is consistent with a negative feedback model of conditioning, wherein conditional analgesia – and presumably its blockade by naltrexone – is elicited by the CS starting the second trial of conditioning (Fanselow & Bolles, 1979a).
Experiment 3 also replicated (for the third time) eight-trial overshadowing and its attenuation by naltrexone. In addition, we found that eight-trial overshadowing was prevented by the DA D1 agonist SKF 38393. On the one hand, this reduction in overshadowing by either naltrexone or SKF 38393 is consistent with the idea that both drugs could act on a common, error-correction type mechanism to prevent overshadowing. Such a view would be in line with research showing that in reward systems, dopaminergic neurons are modulated by opioids (see Wise, 2002; 2004 for review). However, the different behavioral patterns seen in animals treated with naltrexone compared to SKF 38393 suggests that while both drugs attenuated overshadowing, it is more likely they are doing so through distinct mechanisms. In particular, overshadowing is reduced in naltrexone-trained animals because animals in the compound conditioned group increase freezing to the light at test. Conversely, the reduction in overshadowing seen in SKF 38393-trained animals seems to be driven by a combination of increased freezing to the light in compound-conditioned animals and decreased freezing to the light in light-conditioned animals. Thus, SKF 38393 may be exerting an effect on conditioning proper (e.g. by affecting attentional processes), in which case overshadowing would be affected as well.
These data offer definitive support for an opioid-mediated mechanism underlying overshadowing. Additionally, they suggest a possible role for dopaminergic processes in overshadowing, however, additional research to investigate the nature of these processes is warranted.
General Discussion
Collectively, the experiments presented here find an important role for the opioid-regulation of Pavlovian overshadowing, as administration of the opioid antagonist, naltrexone was repeatedly shown to attenuate overshadowing with eight conditioning trials. Additionally, overshadowing in our preparation was only seen to emerge after multiple conditioning trials. Thus, overshadowing seems to share important similarities with Kamin blocking, as both develop after a CS has the opportunity to predict the US and both can be blocked by opioid antagonism (Fanselow & Bolles, 1979a; McNally, et al., 2004).
The negative feedback model of fear conditioning explains US-limited Pavlovian phenomenon such as blocking and overshadowing in terms of an endogenous-opioid mediated conditional analgesia that provides negative feedback onto the acquisition of fear. This conditional analgesia dampens the nociceptive impact of the US, thereby resulting in a reduction in the reinforcement power a US has to support conditioning. In Rescorla-Wagner terminology, conditional analgesia is the property of VΣ, which thereby allows it to reduce the reinforcing signal (λ−VΣ) in comparison to the actual reinforcer (λ). Because conditional analgesia has an opioid component (see Bolles & Fanselow, 1980; Fanselow, 1986 for reviews), this negative feedback circuit is sensitive to opioid-blockade. Thus, the attenuation of overshadowing by opioid antagonism shown here is consistent with the idea that US-limited phenomena in Pavlovian conditioning are regulated by an opioid-based negative feedback circuit.
Although overshadowing was repeatedly and reliably attenuated by naltrexone, the prevention of overshadowing was not as complete as that seen in the prevention of blocking (e.g. McNally, et al., 2004). Presumably, this may be due to inherent differences between the phenomena of overshadowing compared to the phenomenon of blocking. While overshadowing and blocking share deep similarities and indeed, are often grouped together as examples of US-limited phenomenon, they may also be distinct in other, non-US-based respects. That is, blocking occurs because one CS is given extra, prior training with the US, and so a blocking CS has a conditioning history with the reinforcer. An overshadowing CS does not have this explicit conditioning history, and thus draws more heavily on its natural salience (Mackintosh, 1971).
Experiments 2 and 3 tested whether overshadowing could be explained (in part) by sensory competition between CS’s early on in conditioning. However, our failure to obtain significant one or two-trial overshadowing suggests that the processes underlying these effects are unlikely to account for overshadowing – at least in our preparation.
Lastly, we investigated whether dopamine-mediated processes could also account for overshadowing in our preparation. Experiment 3 revealed that like naltrexone, the DA D1 agonist SKF 38393 prevented eight-trial overshadowing. However, the exact way in which SKF 38393 attenuates overshadowing compared to naltrexone is unclear. One possibility is that DA D1 receptors could function by modulating stimulus selection and determining stimulus saliency (O’Tuathaigh & Moran, 2002). Thus, agonism of these receptors could enhance attention and hence conditioning of less salient cues. This contrasts with a role for opioids in modulating overshadowing at the US-side of conditioning and is consistent with the difference in behavioral profiles we demonstrate in animals treated with naltrexone compared to SKF 38393.
The overshadowing data presented here – together with previous studies looking at physiological mechanisms that behave according to error-correction rules – suggest that error correction drives learning and responding across a variety of paradigms and circuitry. Tracking relationships in the environment and orchestrating behavior accordingly is imperative for an animal’s survival. For this reason, error-driven phenomenon such as overshadowing, are key because they enable an animal to condition best to salient cues and ignore cues that may be irrelevant. Not only are error-correction processes ubiquitous, they are vital to the successful continued existence of an organism. The data presented here provide evidence that such processes are mediated by endogenous opioids.
We have described how these data have implications for the mechanisms underlying overshadowing – the systems regulating the selection of one stimulus over another during conditioning. However, these findings can also be applied to recent data showing that when one structure within the fear circuitry is compromised, another structure compensates (e.g. Maren, 1999; Wiltgen et al. 2006; Ponnusamy et al., 2007; Poulos et al., 2009). Thus, one can think of the brain as selecting one particular “salient” circuit over another to support fear conditioning (Fanselow, 2010). In this sense, circuits, like stimuli, compete with one another such that the more salient circuit/stimulus overshadows the less salient circuit/stimulus. We posit that the basic findings obtained here to account for stimulus selection, may similarly explain circuit selection (Fanselow, 2010; Zelikowsky & Fanselow, in press). Investigation of the role of opioids and dopamine in the regulation of circuit competition and circuit overshadowing offer a promising direction for future research.
Acknowledgments
This work was supported by NIH Grants RO1-MH62122 and P50-DA005010 awarded to MSF.
We thank Daniel Pham and Ariel Badger for research assistance with behavioral testing.
References
- Bolles RC. Species-specific defence reactions and avoidance learning. Psychological review. 1970;77:32–48. [Google Scholar]
- Bolles RC, Fanselow MS. A Perceptual-Defensive-Recuperative Model of Fear and Pain. Behavioral and Brain Sciences. 1980;3(2):291–301. [Google Scholar]
- Crider A, Solomon PR, McMahon MA. Disruption of selective attention in the rat following chronic d-amphetamine administration: relationship to schizophrenic attention disorder. Biol Psychiatry. 1982;17(3):351–361. [PubMed] [Google Scholar]
- Fanselow MS. Naloxone and Pavlovian fear conditioning. Learning & Motivation. 1981;12:398–419. [Google Scholar]
- Fanselow MS. Conditioned fear-induced opiate analgesia: a competing motivational state theory of stress analgesia. Ann N Y Acad Sci. 1986;467:40–54. doi: 10.1111/j.1749-6632.1986.tb14617.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Pavlovian conditioning, negative feedback, and blocking: mechanisms that regulate association formation. Neuron. 1998;20(4):625–627. doi: 10.1016/s0896-6273(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends Cogn Sci. 2010;14(1):7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Baackes MP. Conditioned fear-induced opiate analgesia on the formalin test: Evidence for two aversive motivational systems. Learning and Motivation. 1982;13:200–221. [Google Scholar]
- Fanselow MS, Bolles RC. Naloxone and Shock-Elicited Freezing in the Rat. Journal of Comparative and Physiological Psychology. 1979a;93(4):736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Bolles RC. Triggering of the Endorphin Analgesic Reaction by a Cue Previously Associated with Shock - Reversal by Naloxone. Bulletin of the Psychonomic Society. 1979b;14(2):88–90. [Google Scholar]
- Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nat Neurosci. 2008;11(8):966–973. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Fanselow MS. Effects of naltrexone on learning and performance of conditional fear-induced freezing and opioid analgesia. Physiol Behav. 1987;39(4):501–505. doi: 10.1016/0031-9384(87)90380-5. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1(4):304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- James JH, Wagner AR. One-Trial Overshadowing - Evidence of Distributive Processing. Journal of Experimental Psychology-Animal Behavior Processes. 1980;6(2):188–205. [PubMed] [Google Scholar]
- Kamin LJ. ‘Attention-like’ processes in classical conditioning. In: Jones MR, editor. Miami Symposium on the Prediction of Behavior: Aversive stimulation. Miami: University of Miami Press; 1968. pp. 9–33. [Google Scholar]
- Kamin LJ. Predictability, surprise, attention, and conditioning. In: MChurch BCR, editor. Punishment and aversive behavior. New York: Appleton-Century-Crofts; 1969. pp. 9–31. [Google Scholar]
- Kim JJ, Krupa DJ, Thompson RF. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science. 1998;279(5350):570–573. doi: 10.1126/science.279.5350.570. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. An analysis of overshadowing and blocking. Quarterly Journal of Experimental Psychology. 1971;23:118–125. doi: 10.1080/14640747108400245. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological review. 1975;82:276–298. [Google Scholar]
- Mackintosh NJ, Reese B. One-Trial Overshadowing. Quarterly Journal of Experimental Psychology. 1979;31(Aug):519–526. [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19(19):8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Blocking, unblocking, and overexpectation of fear: a role for opioid receptors in the regulation of Pavlovian association formation. Behav Neurosci. 2004;118(1):111–120. doi: 10.1037/0735-7044.118.1.111. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology (Berl) 1984;82(3):241–247. doi: 10.1007/BF00427782. [DOI] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243(1):91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CM, Moran PM. Evidence for dopamine D(1) receptor involvement in the stimulus selection task: overshadowing in the rat. Psychopharmacology (Berl) 2002;162(3):225–231. doi: 10.1007/s00213-002-1107-1. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CM, Salum C, Young AM, Pickering AD, Joseph MH, Moran PM. The effect of amphetamine on Kamin blocking and overshadowing. Behav Pharmacol. 2003;14(4):315–322. doi: 10.1097/01.fbp.0000080416.18561.3e. [DOI] [PubMed] [Google Scholar]
- Ohad D, Lubow RE, Weiner I, JF The effects of amphetamine on blocking. Psychobiology. 1987;15:137–143. [Google Scholar]
- Pavlov IP. In: Conditioned Reflexes. Anrep GV, translator. Oxford University Press; 1927. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87(6):532–552. [PubMed] [Google Scholar]
- Ponnusamy R, Poulos AM, Fanselow MS. Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience. 2007;147(4):919–927. doi: 10.1016/j.neuroscience.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Li V, Sterlace SS, Tokushige F, Ponnusamy R, Fanselow MS. Persistence of fear memory across time requires the basolateral amygdala complex. Proc Natl Acad Sci U S A. 2009;106(28):11737–11741. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Prokasy WF, editor. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Schull J. A conditioned opponent theory of Pavlovian conditioning and habituation. In: Bower GH, editor. Psychology of learning and Motivation. New York: Academic Press; 1979. pp. 57–90. [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23(32):10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26(20):5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Young SL, Fanselow MS. Associative regulation of Pavlovian fear conditioning: unconditional stimulus intensity, incentive shifts, and latent inhibition. J Exp Psychol Anim Behav Process. 1992;18(4):400–413. doi: 10.1037//0097-7403.18.4.400. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Fanselow MS. Conditional analgesia, negative feedback & error correction. Oxford University Press; In press. [Google Scholar]